Abstract
RNA replication enzymes are multi-subunit protein complexes whose activity can be modulated by other viral and cellular factors. For genotype 1b Hepatitis C virus (HCV), the RNA-dependent RNA polymerase (RdRp) subunit of the replicase, NS5B, has been reported to interact with the HCV Core protein to decrease RNA synthesis (Kang et al., 2009). Here we used a cell-based assay for RNA synthesis to examine the Core–NS5B interaction of genotype 2a HCV. Unlike the 1b NS5B, the activity of the 2a NS5B was stimulated by the Core protein. Using the bimolecular fluorescence complementation assay, the 2a Core co-localized with 2a NS5B when they were transiently expressed in cells. The two proteins can form a coimmunoprecipitable complex. Deletion analysis showed that the N-terminal 75 residues of 2a Core were required to contact 2a NS5B to modulate its activity. The C-terminal transmembrane helix of 2a NS5B also contributes to the interaction with the 2a Core. To determine the basis for the differential effects of the Core–RdRp interaction, we found that the 2a RdRp activity was enhanced by both the 1b Core and 2a Core. However, the 1b NS5B activity was slightly inhibited by either Core protein. The replication of the 2a JFH-1 replicon was increased by co-expressed 2a Core while the genotype 1b Con1 replicon was not significantly affected by the corresponding Core. Mutations in 2a NS5B that affected the closed RdRp structure were found to be less responsive to 2a Core. Finally, we determined that RNA synthesis by the RdRps from genotypes 2a, 3a and 4a HCV were increased by the Core proteins from HCV of genotypes 1–4. These results reveal another difference between RNA syntheses by the different genotype RdRps and add additional examples of a viral structural protein regulating viral RNA synthesis.
Keywords: Hepatitis C virus, Core protein, RNA-dependent RNA polymerase, Genotype-specific interaction, De novo initiated RNA synthesis
1. Introduction
The positive-strand HCV RNA genome contains a single open reading frame of approximately 9600 nucleotides flanked by nontranslated regions (NTRs) that are important for viral RNA translation and replication. The HCV polyprotein precursor is processed by viral or host proteases into at least 10 viral proteins: Core, E1, E2, P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B. Most, if not all, of the HCV proteins are multifunctional for both viral processes and processes affecting the physiology of the host cell (Moradpour and Penin, 2013). The Core protein comprises the nucleocapsid that assembles around the HCV genomic RNA. E1 and E2 are envelope glycoproteins that mediate virus entry. Nonstructural proteins NS3, NS4A, NS4B, NS5A and NS5B contribute activities required for HCV RNA replication, including the formation of a replication complex on the host endoplasmic reticulum (ER) membrane or lipid droplets (Moradpour et al., 2003; Moradpour and Penin, 2013). NS5B, the RNA-dependent RNA polymerase, is the catalytic core of the replication complex, and its activity can be modulated by interactions with the cellular membrane or with viral and cellular proteins (Kang et al., 2009; Piccininni et al., 2002; Quezada and Kane, 2009; Shirota et al., 2002; Watashi et al., 2005; Weng et al., 2010).
The structure of NS5B resembles a closed right hand, containing palm, fingers and thumb subdomains with an RNA channel encircled by the three domains (Ago et al., 1999; Bressanelli et al., 1999; Lesburg et al., 1999). The C-terminal 21 residues of NS5B serve as a transmembrane anchor (Schmidt-Mende et al., 2001). NS5B lacking this sequence retains RdRp activity in biochemical reactions and inside cells, but has different RNA synthesis activity and susceptibility to inhibitors (Ranjith-Kumar et al., 2011; Vo et al., 2004).
At least 7 major genotypes and more than 80 subtypes of HCV have been described (Kuiken et al., 2008). The different HCV geno-types have as little as 65% genetic sequence homology. The HCV 2a RdRp is catalytically more active than the 1b RdRp, and the two proteins have distinct inhibition profiles with several inhibitors (Kneteman et al., 2009; Ramirez et al., 2013, 2014). The crystal structure of the genotype 2a RdRp exists in a more compact, closed conformation than that of the 1b RdRp, in part due to additional interactions between the [Δ]1 loop and the thumb subdomain (Ago et al., 1999; Simister et al., 2009).
NS5B associates with other HCV proteins, including Core (Kang et al., 2009; Piccininni et al., 2002; Quezada and Kane, 2013; Shirota et al., 2002; Uchida et al., 2002; Zhang et al., 2005). The 1b Core protein can bind recombinant 1b NS5B resulting in the inhibition of RNA-dependent RNA synthesis in vitro (Kang et al., 2009). The co-expression of Core will also inhibit subgenomic 1b replicon replication in Huh 7 cells (Kang et al., 2009; Uchida et al., 2002). For the human norovirus and the mouse norovirus, their capsid proteins increased the activities of their respective RdRps (Subba-Reddy et al., 2012). The difference between the interactions of the norovirus proteins and the genotype 1b proteins prompted us to examine further the effects of the HCV Core–RdRp interaction.
Using a cell-based reporter assay that can examine RNA synthesis by NS5B proteins from multiple genotypes of HCV (Ranjith-Kumar et al., 2011), we found that the genotype 2a Core protein (designated 2aCore) can enhance NS5B activity in a concentration-dependent manner. Expression of the 2aCore at low concentrations increased HCV RNA replication of the 2a replicon. These results suggest that, unlike the 1bCore, the 2aCore is a positive modulator of HCV RNA replication. Finally, activities of the NS5B proteins from genotypes 3a, and 4a HCV were also enhanced by co-expression with their Core proteins.
2. Materials and methods
2.1. Constructs and mammalian cell culture
The plasmid pUNO-hRIG was from InvivoGen (San Diego, CA). phRL-TK was from Promega. IFN-β-Luc plasmid was a kind gift from Dr. R. Lin, Lady Davis Institute for Medical Research, Montreal, Canada. The IFN-β-Luc plasmid contains the firefly luciferase cDNA driven by the IFN-β promoter. The pRL-TK plasmid expresses Renilla reniformis luciferase that is driven by the herpes simplex virus thymidine kinase (TK) promoter and was used to monitor efficiency of transfection. The plasmid expressing GII.4 Norovirus VP1 (GenBank accession number DQ658413) was from Subba-Reddy et al. (2011). cDNAs of the coat proteins from the West Nile Virus (WNV; GenBank accession number HQ596519.1) and the Classic Swine Fever Virus (CSFV; AF326963.1) were custom synthesized (Biobasics Inc.) and inserted into pUNO vector. The construction of various NS5B plasmids was reported by Ranjith-Kumar et al. (2011). HCV 2a Core, E1, E2, P7, NS2, NS4B and NS5A were amplified with specific primers from the JFH-1 infectious clone (AB047639.1) and cloned into the pUNO vector. DNA sequences for the HCV Cores from 1a (AF011753.1), 1b (AJ238799.1), 3a (ADF97231.1) and 4a (AFU76703.1) genotypes were chemically synthesized (Biobasics Inc.) and cloned into the pUNO vector. Mutants with single-residue mutations were generated by site-directed mutagenesis using the Quickchange kit (Agilent Technologies). Deletion mutants of the 2aCore were generated by PCR amplification of desired DNA sequences followed by cloning into pUNO vector. To make the constructs YFPN-2a5B and YFPN-2aΔ21 for the bimolecular fluorescence complementation assay, YFP residues 1–154 were amplified using PCR and inserted in front of each of the NS5B coding sequences respectively with a linker sequence of ‘RSIAT’ in between. Constructs YFPC-2aCore and YFPC-2aC76-191 were constructed in the same manner except that they contained the coding sequence for YFP residues 155–258 and a linker with a 17-amino acid sequence of RPACKIPNDLKQKVMNH. All constructs were confirmed to have the correct sequence by DNA sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
HEK 293T cells were from the American Type Culture Collection and were cultured as described in Ranjith-Kumar et al. (2011). Huh7.5 cells were cultured as described in Vaughan et al. (2012).
2.2. Cell-based 5BR assay
The 5BR assay was performed as per Ranjith-Kumar et al. (2011). A standard assay was performed in 293T cells or Huh7.5 cells that were transfected with Lipofectamine 2000 reagent (Invitrogen) mixed with plasmids pUNO-hRIG (0.5 ng), IFN-β-Luc (15 ng), phRLTK (5 ng), each respectively expressing the RIG-I receptor, a firefly luciferase reporter, and a Renilla luciferase whose activity can act as a transfection control. Where combinations of plasmids were transfected, the pUNO vector was used to equalize the amount of plasmids in each transfection. The cells were incubated for 24 h to allow gene expression. Luciferase activity was assayed using the Dual-Glo Luciferase Assay System reagents (Promega) and quantified using the FLUOstar OPTIMA Plate Reader (BMG Labtech).
2.3. Co-immunoprecipitation
HEK 293T cells grown in 6-well plates were transfected with a mixture of plasmids expressing HA-2a5B (1 μg) and c-Myc-2aCore plasmid (300 ng) using Lipofectamine 2000 (Invitrogen). The cells were harvested 24 h post-transfection, washed with PBS, and treated with lysis buffer (20 mM Tris–HCl, pH 7.5, 5 mM EDTA, 100 mM NaCl, protease inhibitor cocktail (Roche), and 1% NP-40) for 30 min on ice. Cell lysates were clarified by centrifugation at 16,000 × g for 30 min at 4 °C. 5% of total cell lysates were saved to analyze for protein expression. Immunoprecipitation was performed with anti-Core antibody (Santa Cruz Biotechnology) and protein A/G magnetic beads (Thermoscientific) according to the manufacturer's protocol. The immunoprecipitated protein complexes on magnetic beads were denatured by addition of 1 Laemmli buffer and heated to 95 °C for 2 min. ×
2.4. Western blots
Protein samples were separated using a 4–12% SDS-PAGE and then transferred onto PVDF membranes (Invitrogen). The membranes were incubated with blocking buffer (5% skim milk (w/v) in 1× PBST) for 20 min, followed by a 2 h incubation in fresh blocking buffer containing antibodies that recognize the target proteins. The anti-HA antibody is from Abcam; c-Myc antibody is from Santa Cruz Biotechnology; anti-FLAG is from Sigma and anti-Core is from Thermo Scientific. Western blots were developed using the ECL-plus or ECL-advance Western blotting detection system (Amersham Biosciences) and imaged with a ChemiDoc XRS+ system (BioRad).
2.5. Bimolecular fluorescence complementation
HEK 293T cells at 60% confluence were transfected with plasmids expressing the N-and C-terminal portions of the yellow fluorescent protein, YFPN and YFPC. After transfection, the cells were incubated at 37 °C for 24 h followed by a 3 h incubation prior to analysis with confocal microscopy or flow cytometry. Confocal microscopy used cells grown on glass coverslips that were washed once with 1× PBS, and fixed in 4% (w/v) paraformaldehyde in PBS for 15 min at room temperature. The coverslips were washed three times with PBS and then mounted on glass slides with anti-fade mounting medium (Invitrogen). Micrographs were acquired with a Leica TCS SP5 confocal inverted-base microscope with a 63× oil objective lens. Flow cytometry used cells grown in 6-well plates and collected by trypsinization and centrifugation. The cells were washed three times with and re-suspended in 1 × PBS to a final concentration of 2 × 107 cells/ml. Samples were analyzed by a FACS Calibur flow cytometer (Beckton Dickinson). 50,000 cells were analyzed per group. Graphics and data analysis used the FlowJo software (Tree Star Inc.).
2.6. Subcellular fractionation
Subcellular fractionation was performed as described in Ranjith-Kumar et al. (2011). HEK 293T cells which had been transfected for 24 h to express the desired proteins were harvested and washed with 1× PBS. A portion of the cells was saved for analysis of protein expression. The rest of cells were permeabilized with buffer containing 0.019% digitonin in the presence of protease inhibitor (P8340, Sigma–Aldrich). After centrifugation at 15,000 × g for 15 min at 4 °C, the supernatant was collected and the resulting pellet was washed with 1× PBS and resuspended in buffer containing 0.5% Triton X-100 and protease inhibitors. The sample was then centrifuged at 15,000 × g for 15 min at 4 °C to separate soluble and insoluble materials. The insoluble material was washed once with 1× PBS and suspended in 1× Laemmli sample buffer. All samples were subjected to SDS-PAGE in the presence of reducing agent, transferred to PVDF membrane, and probed with monoclonal antibody specific to the c-Myc tag that was added to the NS5B protein (Santa Cruz Biotechnology).
2.7. Replicon assay and quantitative RT PCR
For electroporation, trypsin-mobilized Huh7.5 cells were washed twice with and suspended in ice-cold Cytomix (10 mM K2HPO4/KH2PO4, pH 7.6; 120 mM KCl; 0.15 mM CaCl2; 25 mM HEPES; 2 mM EGTA; 5 mM MgCl2) at 1 × 107 cells/ml. 200 μl of the cell suspension was mixed with 5 μg of in vitro transcribed HCV replicon RNA together with 200 ng of the plasmids to express either Core or the pUNO vector. The samples were pulsed at 270 V, 96 mF in an electroporation cuvette with a 2 mm gap (Bio-Rad Gene-pulser). The cells were allowed to recover for 10 min at room temperature before dilution in complete medium. The cells were seeded into 6-well plates at 1.25 × 106 to 2.0 × 106 cells/well. The total volume of culture medium was adjusted to 2 ml with the addition of extra media, as necessary. The cells were harvested over a time course to quantify the relative abundance of HCV RNA copies using RT-PCR. cDNAs were reversed transcribed from total RNA with 9-nt primers that had random sequences by using the MMLV reverse transcription enzyme (New England Biolabs). The quantitative real-time PCR used primers specific to the HCV 5′ NTR and HiGreen qPCR master mix (Thermo scientific). All experiments were performed independently three times, and data were plotted as means and one standard error of the means.
2.8. Gel-based RNA synthesis assay
The gel-based RNA synthesis assay was performed as per Ranjith-Kumar et al. (2011). The reaction mixture contains 1 pmol of RNA template and 0.04 μM of recombinant protein in 20 μl RNA synthesis buffer (20 mM sodium glutamate, pH 8.2, 20 mM NaCl, 4 MgCl2, 12.5 mM dithiothreitol, 0.5% (v/v) Triton X-100, 250 nM α-[P32]-CTP (Amersham, Inc.), 200 μM GTP, 100 μM ATP and 100 μM UTP). The reactions were incubated at 30 °C for 60 min and stopped by the addition of 2× sample buffer and heating at 90 °C for 3 min. The products were separated by electrophoresis in polyacrylamide gels containing 7.5 M urea. The gels were wrapped in plastic, and radiolabel was quantified using a Typhoon imager (GE Healthcare). The intensity of the radioactive bands was quantified using Image J software.
3. Results
3.1. Core modulates NS5B activity
We seek to determine whether the co-expression of genotype 2a HCV proteins can affect the activity of 2a5B using the 5BR assay. In this assay, HEK 293T cells transiently transfected to express 2a5B will synthesize RNAs that will serve as ligands for the innate immune receptor, RIG-I. Ligand-bound RIG-I molecules then activate signal transduction to produce luciferase reporter proteins driven by the interferon beta (IFNβ) promoter. 2a NS3-4A was not tested in this assay since its protease activity cleaves IPS-1 and short circuits signaling by RIG-I (Li et al., 2005). All other HCV proteins were expressed with an N-terminal c-Myc tag except NS5A, which we had previously made with an HA tag (Ranjith-Kumar et al., 2011). NS5A was detected less efficiently in Western blots due to the lower efficiency of antibody to HA epitope, but it is clear that all proteins were expressed (Fig. 1A). The E1 was detected as multiple bands, likely due to the presence of multiple glycosylated forms and/or degradation (Lanford et al., 1993). The expression of 2a5B or RIG-I were not affected by the co-expressed 2a HCV viral proteins (Fig. 1B).
Fig. 1.
Effect of JFH-1 viral proteins on RNA synthesis activity by the JFH-1 NS5B. (A) Western blot result on the expression of the JFH-1 viral proteins in transiently-transfected HEK 293T cells. NS5B(GAA) is a version of NS5B that had its two catalytic aspartic acids in Motif C mutated to alanine. The Western blot was probed with goat anti-HA antibody to detect NS5A and mouse anti-c-Myc antibody to detect the other proteins. (B) Accumulation of 2a5B and RIG-I were unaffected when they were co-expressed with JFH-1 viral proteins. Each transfection contains plasmids denoted above the blot with the amount specified. The Western blot was probed with mouse anti-FLAG antibody to detect 2a NS5B and RIG-I proteins. “Vec.” denotes the empty pUNO vector used to express the proteins listed above. (C) Results from the 5BR assay on the effects of JFH-1 proteins on RNA synthesis by the WT JFH-1 NS5B. Ratio in the vertical axis denotes the units of firefly luciferase driven from the IFN-β promoter relative to the Renilla luciferase driven from the thymidine kinase promoter. The cells were transfected to express the constructs denoted below the horizontal axis. Each bar represents the means of three independent trials, and the error bars show the standard deviation. The reading of each experiment is denoted on top of each bar. Asterisk denotes a p value of <0.05 (n = 3) in the Student T test.
In the 5BR assay, the 2a NS5A did not affect NS5B activity (Ranjith-Kumar et al., 2011). Furthermore, co-expression of 2aCore increased 2a5B activity reproducibly by more than 100% above the activity of the 2a5B co-expressed with the empty vector (Fig. 1C). An active site mutant of 2a5B was unaffected by the co-expression of 2aCore, consistent with RNA synthesis by NS5B being important for the read-out from the 5BR assay. Notably, the enhancement of 2a5B activity by 2aCore differs from a previous report of the inhibitory effect of 1bCore on 1b5B (Kang et al., 2009).
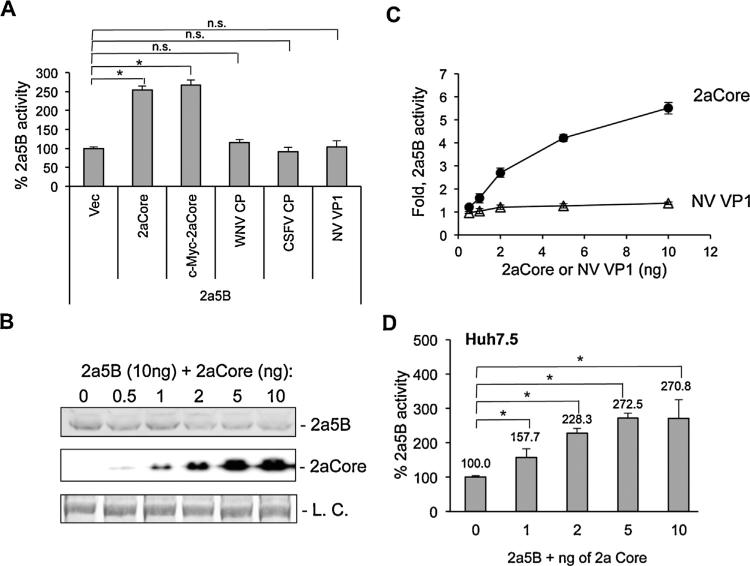
To understand whether the enhancement of NS5B is specific to the HCV Core protein, capsid proteins from the closely related West Nile Virus (WNV) and Classic Swine Fever Virus (CSFV) and the distantly related norovirus (NV) were tested for their effect to HCV NS5B activity. In the 5BR assay, only the 2aCore was able to enhance the activity of the 2a RdRp (Fig. 2A). The ability of 2aCore to enhance 2a NS5B activity was not affected by the presence of an epitope tag (Fig. 2A), and hence in the following experiments, the 2aCore contained a Cyc tag was used unless stated otherwise. To further test whether the difference is due to a variation in the amount of protein expressed, we transfected a range of plasmid concentrations expressing the 2aCore or the norovirus capsid protein VP1 along with a constant amount of the plasmid expressing 2a5B. 2aCore was readily detected with 1 ng of transfected plasmid, and its accumulation increased with the amount of plasmid transfected into cells, demonstrating that the concentration of transfected plasmid could be used to regulate 2aCore accumulation (Fig. 2B). 2a5B activity increased in concert with the increase of 2aCore protein levels (Fig. 2C). However, co-expression of the human norovirus capsid protein VP1 did not enhance 2a5B activity (Fig. 2C). These results demonstrate that the interaction between 2aCore and 2a5B is likely species-specific. Importantly, the dose-dependent enhancement of 2a5B activity by 2aCore is also observed with Huh7.5 cells (Fig. 2D).
Fig. 2.
Dose dependent enhancement of 2a5B activity by 2aCore. (A) 5BR assay in HEK 293T cells suggesting species-specific interaction of 2a5B with 2aCore. 10 ng of empty vector or plasmids to express 2aCore, c-Myc-2aCore, WNV CP (strain 99), CSFV CP (strain Eystrup) or NV VP1 (strain GII.4) were co-transfected with 10 ng of 2a5B plasmid. (B) Western blot results of 2a5B and 2aCore levels in HEK 293T cells co-transfected with a constant level of 2a5B plasmid (10 ng per well of 5 × 105 cells) and increasing amount of 2aCore plasmids. pUNO vector was used to maintain constant amounts of DNA for each experiment. Protein expression level was analyzed 24 h after transfection with Western blots. Both 2a5B and 2aCore were detected with mouse anti-c-Myc antibody. The loading control (L.C.) was a protein band on the membrane used to detect 2a5B and 2aCore. (C) 5BR activity of 2a5B was enhanced by 2aCore in a concentration dependent manner, but not by VP1, the major capsid protein from human Norovirus. Each data point was analyzed in triplicate and the results normalized to the sample with 0 ng 2aCore. (D) 2aCore can enhance 2a5B activity in a dose-dependent manner in Huh7.5 cells. An asterisk denotes P < 0.05, and n.s. denotes P > 0.05, n = 3.
It must be noted that cells transfected with more than 10 ng of the 2aCore plasmid had reduced accumulation of 2a5B (Fig. 2B). Core has previously been shown to reduce the translation of some proteins (Li et al., 2003). To minimize this effect in subsequent experiments, the plasmid expressing full-length Core was limited to 10 ng or less.
3.2. HCV-796 inhibits the 2aCore–2a5B interaction
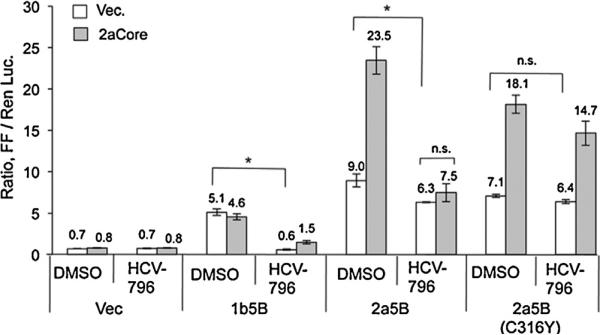
We seek to use the NS5B-specific inhibitor to discern whether 2aCore enhances 2a5B activity through acting directly on 2a5B polymerase or non-directly, acting on other cellular factors. HCV non-nucleoside inhibitors (NNI) active against the genotype 1b NS5B were screened for their ability to inhibit 2a5B in the 5BR assay. HCV-796, which acts at the catalytic pocket of the 1b NS5B (Hang et al., 2009), was found to cause a modest reduction in 2a5B activity by 33–51% when present at 10 μM (Fig. 3). The ability of HCV-796 to inhibit 2a5B is consistent with a previous report that HCV-796 can down regulate HCV 2a replicon replication (Roelandt et al., 2012). We tested a C316Y mutation, which was shown to confer HCV-796 resistance in 1b5B (Howe et al., 2008), to determine whether HCV-796 acts at a comparable site within 2a5B. As shown in Fig. 3, the 2a5B C316Y mutant was not inhibited by HCV-796. Interestingly, in the presence of HCV-796, the activity of 2a5B was no longer enhanced by 2aCore, whereas 2a5B C316Y maintained the interaction with 2aCore even in the presence of HCV-796. Since HCV-796 acts specifically on NS5B, these results suggest that 2aCore enhances 2a5B activity through an interaction with 2a5B.
Fig. 3.
Effect of HCV-796 to 2a5B–2aCore interaction in the 5BR assay. HCV-796 was dissolved in 100% DMSO. DMSO or HCV-796 was added into the medium 4 h post transfection. The final concentration of HCV-796 is 10 μM and DMSO is 0.5%. The cells were assayed 24 h after drug addition. Asterisk denotes P < 0.05, and n.s. denotes P > 0.05, n = 3.
3.3. The 2aCore can bind 2a5B
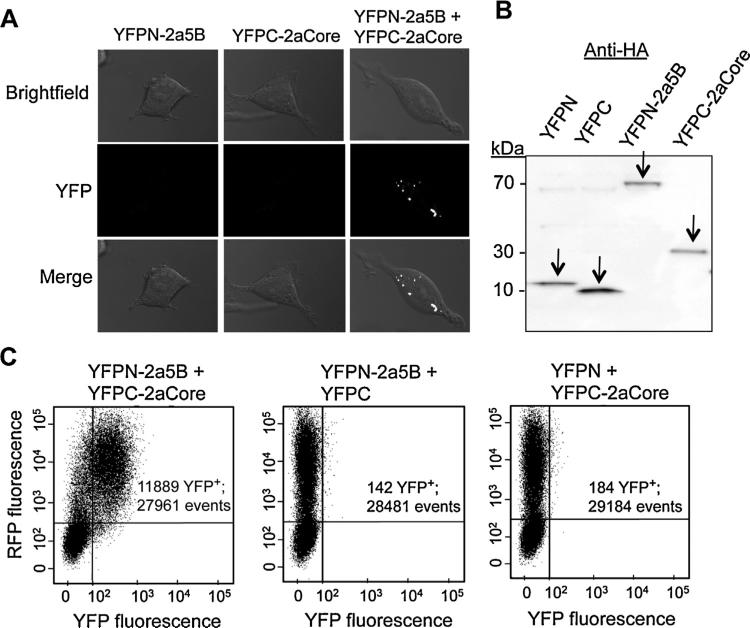
To examine 2aCore interaction with 2a5B in cells, we used a bimolecular fluorescence complementation (BiFC) assay (Hu et al., 2002). Briefly, constructs were made to express NS5B or Core fused to either the N-terminal portion or the C-terminal portion of the yellow fluorescence protein (YFPN or YFPC, respectively). Should NS5B interact with Core, the N-terminal and C-terminal halves of YFP would be brought together to reconstitute YFP. Cells transfected to express only one of the two constructs (YFPN-2a5B or YFPC-2aCore) had no detectable YFP fluorescence, as expected (Fig. 4A). Cell expressing both YFPN-2a5B and YFPC-2aCore exhibited yellow fluorescence, indicating that 2a5B is physically near 2aCore (Fig. 4A). The expression of YFPN-2a5B, YFPC-2aCore, YFPN and YFPC was comparable, as can be seen in the Western blot (Fig. 4B). Fluorescence-activated cell sorting (FACS) was used to quantify the number of cells that harbor reconstituted yellow fluorescence. The cells were also co-transfected to express the red fluorescence protein (RFP) to indicate the efficiency of transfection. 43% of the cells expressing RFP also expressed YFP when the cells were transfected with YFPN-2a5B and YFPC-2aCore (Fig. 4C). In contrast, cells expressing YFPN-2a5B or YFPC-2aCore with YFPC or YFPN, respectively, had background levels of YFP fluorescence. These results are in keeping with a direct interaction occurring between the 2aCore and the 2a5B proteins.
Fig. 4.
2a5B interacts with 2aCore. (A) Confocal microscopy images showing the interaction between 2a5B and 2aCore. 2a5B and 2aCore were tagged at their N- or C-termini with truncated yellow fluorescent protein (YFP). The cells were transfected to express the constructs denoted to the left of each row. Vectors expressing the N- or C-terminal domains of YFP alone with each corresponding fusion construct were used as negative controls. Bimolecular complementation of YFP fluorescence is indicated by the YFP signal. Individual and merged images of YFP and bright field images are shown. (B) Western blot showing the expression of the constructs YFPN, YFPC, YFPN-2a5B and YFPC-2aCore used in BiFC assay. All of the bands are the expected masses. (C) Quantification of BiFC signal by FACS. For each FACS plot, cells were transfected to express the constructs as denoted. A construct expressing intact RFP was co-transfected to indicate transfection efficiency. Horizontal and vertical axes each represent the intensity of YFP and RFP signal. “YFP+ denotes cell events that are positive for YFP fluorescence.
3.4. 2aCore domains needed to modulate 2a5B activity
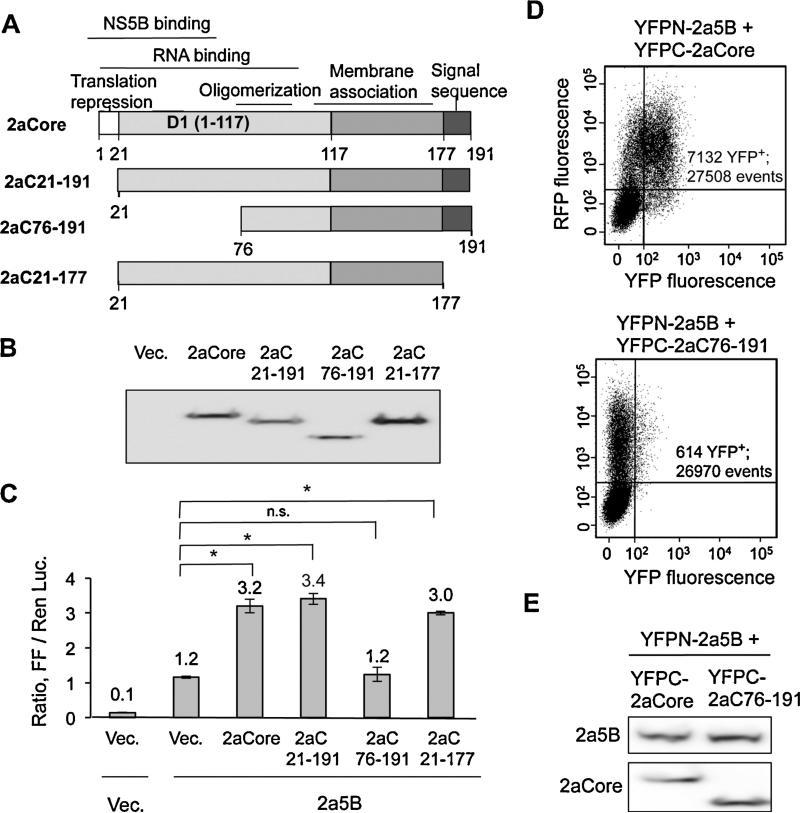
We sought to map the region in 2aCore required to enhance 2a5B activity. Core is a 191 amino acid protein with several functional domains (Fig. 5A). The C-terminal portion of 2aCore contains an endoplasmic reticulum-targeting signal that is cleaved by cellular signal peptide peptidase to form a mature core protein named P21 (Fig. 5A). Mature Core contains a highly positively charged N-terminal domain 1 (D1) involved in recruiting viral RNAs to the site of viral particle morphogenesis and to provide subunit contacts to encapsidate HCV RNA (Fromentin et al., 2007). The N-terminal 20-residues of D1 could repress cap-dependent translation and (Hope and McLauchlan, 2000; Li et al., 2003). Residues 1–75 in D1 of genotype 1b Core were reported to interact with the 1b NS5B in vitro (Kang et al., 2009). Finally, the C-terminal 60 residues of D1 can associate with lipid droplets and membranes (Hope and McLauchlan, 2000).
Fig. 5.
Domain in 2aCore that modulates 2a5B activity. (A) Schematic of 2aCore and its truncations. The functions assigned to the domains of 2aCore are shown about the schematic. The numbers denote the N- and C-terminal residues that are present in the truncated proteins. (B) Expression of WT and truncated 2aCore detected using Western blot. All plasmids expressing WT or truncated Cores were transfected at 10 ng/well except for 2aC21-191 which was transfected at 100 ng/well due to its accumulation being below that of the others. pUNO was used to compensate for the amount of DNA where needed. (C) Effects of 2aCore truncations on the ability to enhance 2a5B activity in the 5BR assay. 2a5B was co-expressed with the 2aCore constructs that are denoted below the horizontal axis. Asterisk denotes P < 0.05, and n.s. denotes P > 0.05, n = 3. (D) Analysis of the interaction between 2a5B and 2aC76-191 by FACS. Results from FACS analysis of cells that exhibited yellow fluorescence due to the interaction of WT 2a5B and 2aCore or WT 2a5B and 2aC76-191. The constructs were assessed using bimolecular fluorescence complementation. RFP expression was used as a transfection control. The quantification of the FACS events positive for YFP versus the total events enumerated is shown. (E) The expression of the proteins in cells subjected to FACS analysis, as analyzed by Western blots. The plasmids transfected in each sample are indicated on top of the blots. Anti-HA antibody was used to detect YFPN-2a5B and YFPC-2aCore.
Core constructs with one or more motifs deleted were tested for effects on 2a5B activity using the 5BR assay. 2aC21-191 lacks the N-terminal 20 residues that can repress protein translation; 2aC21-177 removes further the C-terminal endoplasmic reticulum (ER) localization signal; 2aC76-191 has its N-terminal 75 residues deleted. All constructs were expressed at similar protein levels (Fig. 5B). Expression of 2aC21-191 enhanced 2a5B activity to levels comparable to WT 2aCore (Fig. 5C), indicating that Core's enhancement of 2a5B activity is independent of the residues that mediate translational repression. Similarly, 2aC21-171, the mature form of 2aC21-191 was competent to enhance 2a5B. However, 2aC76-191 lacking the first 75 residues in D1 failed to enhance 2a5B activity. Constructs with more extensive truncations in D1 and D2 accumulated to low levels, suggesting that they are unstable (data not shown).
We sought to determine whether 2aC76-191 which had lost the ability to enhance 2a5B activity was also affected for interaction with 2a5B in the BiFC assay. Cells expressing YFPN-2a5B and YFPC-2aC76-191 were reduced by about 10-fold for the complemented YFP fluorescence when compared to cells expressing YFPN-2a5B and YFPC-2aCore (Fig. 5D). Comparable protein expression in aliquots of the cells analyzed by FACS was confirmed using Western blots (Fig. 5E). These results indicate that the first 75 residues of 2aCore are required to interact with 2a5B in cells. Notably, the comparable region in the 1bCore was reported to interact with 1b5B (7). Altogether, the results from the NS5B inhibitor HCV-796, the BiFC results, and those with truncations of the 2aCore suggest that physical interaction between 2a5B and 2aCore likely regulates 2a5B activity in the 5BR assay.
3.5. Membrane association of 2a5B is required for its interaction with 2aCore
We tested a truncation that removes the C-terminal membrane helix of 2a5B, 2aΔ21, for interaction with 2aCore. 2aΔ21 was readily solubilized with 0.019% digitonin in membrane-enriched fractions (Fig. 6A). Both 2aΔ21 and 2a5B were competent for RNA synthesis activity in the 5BR assay (Fig. 6B). However, only 2a5B could enhance RNA synthesis in the presence of 2aCore in a concentration-dependent manner (Fig. 6C).
Fig. 6.
The 2aCore does not enhance RNA synthesis by 2a5B lacking the C-terminal transmembrane helix. (A) 2a5B and 2aΔ21 are present in different subcellular fractions. The images are from Western blots performed with HEK 293T cell lysates that express 2a5B or 2aΔ21 with N-terminal Myc epitopes. The cellular fractionation protocol was as described in the Materials and Methods. Input, digitonin, TX-100, and pellet denote, respectively, the total cell lysate, cellular fraction that is solubilized by digitonin, cell material solubilized with Triton X-100, and the detergent insoluble materials. (B) Activity of 2a5B and 2aΔ21 in the 5BR assay. Asterisk denotes P < 0.05, and n.s. denotes P > 0.05, n = 3. (C) A comparison of the effects of 2aCore on the activities of 2a5B and 2aΔ21 using the 5BR assay. Each data point represents the mean of three independent samples and one standard deviation of the mean is indicated by the bracket. The transfections were done as described in Fig. 2A. (D) Co-immunoprecipitation (Co-IP) assay to analyze the interactions between 2a5B or 2aΔ21 and 2aCore. HEK 293T cells expressing c-Myc tagged 2aCore together with FLAG tagged 2a5B or 2aΔ21 were solubilized with buffer containing 1% Triton X-100 and the lysates were subjected to immunoprecipitation (IP) with anti-c-Myc. The input and immunoprecipitated materials were probed in Western blots (WB) with antibodies specific to c-Myc and FLAG epitopes that recognize 2aCore and 2a5B. (E) BiFC analysis comparing the interaction between the Core protein and full-length 2a5B or 2aΔ21. The left panel summarizes the percentage of cells positive for YFP fluorescence using FACS. The cells were transfected 300 ng of YFPC-2a5B or YFPC-2aΔ21 plasmids were co-transfected with 150, 100 or 50 ng of YFPN-2aCore plasmids. The Western blot shows the expression of YFPC-2a5B and YFPC-2aΔ21 in the samples analyzed for YFP fluorescence. Both YFPC-2a5B and YFPC-2aΔ21 were detected with anti-HA antibody.
Next, we determined whether the loss of the C-terminal membrane helix affected 2aNS5B–2aCore interaction. Western blots of materials co-immunoprecipitated with 2aCore revealed that 2a5B is in a complex with 2aCore, but 2aΔ21 was not (Fig. 6D). When YFPN-2aΔ21 was co-expressed with varying amount of YFPC-2aCore, 2aΔ21 was less able to drive the reconstitution of YFP as compared to YFPN-2a5B, despite that YFPN-2aΔ21 was expressed at a much higher level than YFPN-2a5B (Fig. 6E). Thus, the C-terminal membrane helix and/or membrane localization of 2a5B can impact interaction with 2aCore.
3.6. 2aCore expression in trans can stimulate 2a replicon replication
To examine the effects of 2aCore on HCV RNA synthesis in a more complete HCV RNA replication system, we co-expressed 2aCore in trans with the HCV JFH-1 subgenomic replicon that lacked Core. Briefly, in vitro transcripts of the JFH-1 replicon RNAs were electro-porated into Huh7.5 cells together with plasmids expressing 2aCore or empty vector. The transfected cells were collected at intervals from 0 to 96 h for quantitative RT-PCR of HCV RNAs and for Western blots to detect 2aCore (Fig. 7). The amount of the total HCV RNA decreased at 24 h post-transfection (hpt) due to elimination of nonfunctional transcripts generated during in vitro transcription. At 60 and 96 hpt, the HCV replicon RNAs transfected with pUNO-2aCore were approximately 10-fold higher than replicon transfected with the empty pUNO vector (Fig. 7A). Enhancement of JFH-1 replicon RNA by co-expressed 2aCore was observed in three independent experiments. 2aCore accumulation was detected by 36 hpt (Fig. 7B). In contrast, the Con1 replicon was not enhanced by the co-expressed 1bCore (Fig. 7C and D). These results are consistent with our results from the 5BR assay and suggest that expression of 2aCore in trans of the replicon can stimulate the JFH-1 RNA replication.
Fig. 7.
JFH-1 replication in Huh7.5 cells can be enhanced by co-expressed Core. (A and C) Time course of HCV JFH-1 or Con-1 replicon RNA accumulation in the absence or presence of Core. HCV RNA copy numbers were obtained by quantitative reverse transcription PCR and normalized to the copy numbers of the GAPDH from the same samples. To allow analysis of the change in copy number, the results from 0 h after transfection were normalized to 100 for each of the experiments. (B and D) Western blots showing the expression of 1bCore and 2aCore in replicon cells co-transfected with the plasmids denoted above each image. Loading controls (L.C.) are included below the blots.
3.7. RdRp conformation affects the modulation of RNA synthesis by Core
In the 5BR assay, the full-length NS5B is consistently more active than its C-terminally truncated counterparts (Fig. 5B). In the 5BR assay, detection of the RNA products of NS5B uses the innate immune receptor, RIG-I, which preferentially recognizes de novo initiated and uncapped RNAs [14]. We wondered whether mem brane association by 2a5B and its subsequent interaction with Core could facilitate de novo initiated RNA synthesis by the HCV RdRp and result in a greater abundance of de novo initiated RNAs.
The conformation of NS5B is known to modulate de novo initiated RNA synthesis. The ‘closed’ conformation of 2a5B contributes to more efficient de novo initiated RNA synthesis (Simister et al., 2009). We tested the effects of the 1bCore (Con1 strain) on 1b5B and 1bΔ21 activity using the 5BR assay. The enhancement of either 1b5B or 1bΔ21 activity by 1bCore was not observed although the proteins were expressed at high levels (Fig. 8A and B). These results are consistent with our analysis of the replication of the 1b Con1 replicons in the presence of 1bCore (Fig. 7C). To elucidate whether NS5B or Core was responsible for the differential regulation of NS5B RNA synthesis from genotype 1b and 2a, we co-expressed 1bCore with the 2a5B and observed a dramatic enhancement of RNA synthesis. The 1b5B was unaffected by the co-expression of 2aCore (Fig. 8C). Furthermore, co-expression of the 2a5B along with the 1bCore resulted in a several fold increase in 2a5B activity. These results clearly demonstrate that NS5B determines responsiveness to the Core in the 5BR assay.
Fig. 8.
Interaction between the Core and genotype 1b HCV NS5B. (A) Western blot results on the accumulation of the genotype 1b NS5B (1b5B) in the presence of increasing amounts of 1bCore). L.C. denotes loading control. (B) 5BR assay result for RNA synthesis by full-length 1b5B and C-terminally truncated 1b NS5B (1bΔ21) in HEK 293T cells expressing increasing amounts of 1bCore. (C) Inter-genotypic interaction between Core and NS5B. The results were from the 5BR assay. The 5BR results in panels B and C are consistent in more than three independent experiments.
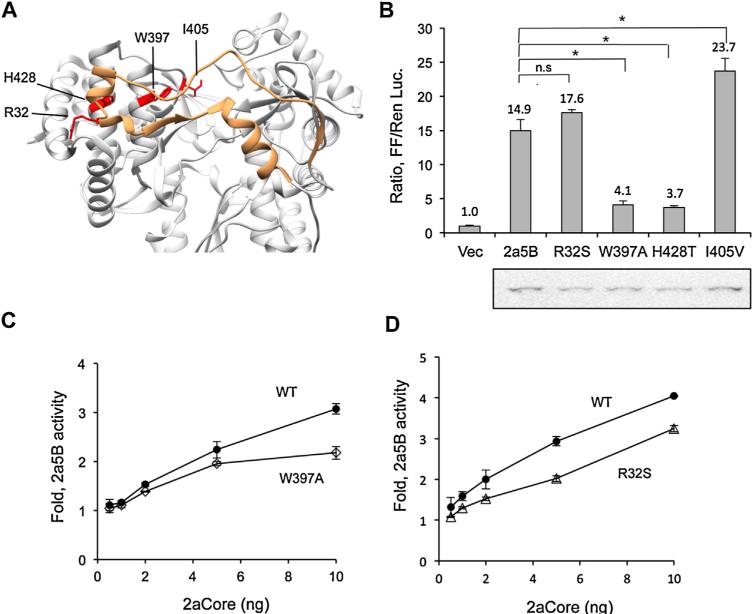
Given that the closed conformation of the 2a5B is correlated with de novo-initiated RNA synthesis, we introduced mutations in 2a5B that should decrease the interactions needed to form the closed conformation (Fig. 9A). Mutations were made in the Δ1 loop, which extends from the fingers subdomain to contact the thumb subdomain (Chinnaswamy et al., 2008), near the so-called lowaffinity GTP binding site in the thumb subdomain that has also been reported to affect de novo RNA synthesis of the 1b NS5B (Cai et al., 2005; Schmitt et al., 2011; Scrima et al., 2012). W397A Substitutions of R32S, W397A, H428T and I405V were engineered into 2a5B and tested for their RNA synthesis in the absence of 2aCore. W397A, and H428T had reduced activity while the I405V mutant increased activity while the R32S mutant was not reduced for 2a5B activity. We selected mutants W397A and R32S for additional analysis in the presence of increasing concentration of the 2aCore protein. The expression of W397A and R32S were also adjusted to be comparable to that of WT 2a5B. However, mutants W397A and R32S had reduced enhancement of RNA synthesis by 2aCore relative to WT 2a5B under all concentrations of Core expressed (Fig. 9A and D). These results suggest that even small perturbations of the NS5B conformation will affect the response to Core protein.
Fig. 9.
Mutations that affect the 2a5B “closed” conformation can influence the modulatory effects of 2aCore. (A) Crystal structure of 2a5B (PDB: 3I5K) displayed as ribbons. Orange: the Δ1 loop. The residues changed from WT are colored red. (B) 5BR assay analyzing the activity of the 2a5B mutants. Western blot results showing the expression of the respective proteins is below the 5BR plot. To achieve similar protein expression, 10, 20, 7, 13 and 20 ng of plasmids expressing WT, R32S, W397A, H428T and I405V were transfected into HEK293T cells and the level of RNA synthesis assessed using the 5BR assay. pUNO vector were used to compensate for the amount of DNA where needed. (C and D) Comparison of the effects of the 2aCore on the activities of the 2a5B WT, W397A and R32S. The results were obtained with the 5BR assay. The amount of WT, W397A and R32S plasmids used in each transfection was 10, 7, and 20 respectively to achieve comparable levels of protein expression. Asterisk denotes P < 0.05, and n.s. denotes P > 0.05, n = 3.
3.8. NS5B from HCV genotypes 3a and 4a are stimulated by Core
Given that the Core–NS5B interaction differed between geno-type 1b and 2a HCV, we sought to determine whether Core proteins from other HCV genotypes could modulate the activities of the homotypic NS5B proteins. Multiple genotypes of HCV NS5B were documented to be active in the 5BR assay [14]. However, we had yet to determine whether the different Core proteins are expressed. The Core from genotypes 3a and 4a were found to accumulate to lower levels in HEK 293T cells than they did from 1a, 1b and 2a HCV (Fig. 10A). However, all of the Cores except for that from genotype 1b enhanced the activities of their homotypic NS5Bs by up to twofold in HEK 293T cells (Fig. 10B). Consistent results were observed with all of the NS5B proteins and their homotypic cores in Huh7.5 cells. Notably, in both cell lines, the 1a Core had more modest regulatory activity when compared to those Cores from 2a, 3a, or 4a genotypes (Fig. 10B and C).
Fig. 10.
NS5Bs from two additional HCV genotypes are enhanced by Core proteins. (A) Western blot result showing the expression of Core from HCV of 1a, 1b, 2a, 3a and 4a genotypes in transiently-transfected HEK 293T cells. (B) Effects of Core in various concentrations on the activities of corresponding NS5Bs in the 5BR assay. The transfections were as described in Fig. 2B. (C) NS5B–Core interaction of HCV 1a, 1b, 2a, 3a and 4a genotypes in the 5BR assay using Huh7.5 cells. Asterisk denotes P < 0.05, and n.s. denotes P > 0.05 (n = 3) in the Student T test. (D) Summary of the intra- and inter-genotypic effect of Core and NS5B co-expressed in the 5BR assay. 25 ng of 1a5B, 1b5B or 10 ng of 2a5B, 3a5B and 4a5B plasmids were tested in the presence of 10 ng Core plasmids. Differing amounts of the plasmids expressing the NS5B proteins was used to allow comparable levels of expression of NS5B to Core. ‘0’ denotes no effect; ‘−’ denotes an inhibition of less than 30% and ‘+’, ‘++’ and ‘+++’ denote an increase in the NS5B activity of 1.5-, 2- or greater than 3-fold, respectively. All results were from the 5BR assay performed in HEK 293T cells.
Inter-genotypic interactions between Core and NS5B were then examined in HEK 293T cells. The majority of NS5Bs from different HCV genotypes were modulated by heterologous Core proteins in a manner similar to that of their own Core (Fig. 10D). These results indicate that the 2a, 3a, and 4a NS5B all share the same property of interaction with their Core proteins while the genotype 1 NS5B does not.
4. Discussion
In this study, we sought to elucidate whether the HCV Core protein can regulate HCV RNA synthesis. Using a cell-based assay to test pairwise interactions of HCV proteins with Core, we demonstrated in both HEK 293T and Huh7.5 cells that the activity of 2a NS5B was enhanced by co-expression of the Core protein to comparable levels (Figs. 1 and 2). Using HEK 293T cells, we found that in the presence of NS5B specific inhibitor HCV-796, 2a5B was not stimulated by 2aCore (Fig. 3). The D1 region in the N-terminal 76 residues of Core is required to interact with NS5B (Fig. 5). The 2a5B interacts with the 2aCore in cells (Figs. 4 and 5) in a manner that requires NS5B association with cellular membrane, given that the deletion of the C-terminal membrane interaction sequence abolished the effects of interaction with Core (Fig. 6). The replication of the JFH-1 subgenomic replicon can be enhanced by the co-expression of 2aCore while the genotype 1b Con1 replicon was not affected or slightly inhibited by the 1bCore, demonstrating differences in the requirements for genotypes 1b and 2a HCV RNA synthesis (Fig. 7). Furthermore, NS5B contains the determinants to interact with Core (Fig. 9). Finally, we demonstrate that NS5B from genotypes 2a, 3a and 4a HCV can interact with both of the corresponding Core proteins to increase RNA synthesis in our cell-based assay, but that genotype 1 HCV RdRps have either reduced or no obvious interactions with their Core proteins (Fig. 10).
Similar to other viral coat proteins, HCV Core protein is multi-functional and not only serves as the structural building block of the HCV virion, but also regulates multiple signaling pathways and affects cellular and viral gene expression (de Lucas et al., 2005; Hosui et al., 2003; Li et al., 2003; Ni and Cheng Kao, 2013). The result that the 2aCore mutant lacking the N terminal translational regulation motif can modulate 2a5B in a manner similar to WT 2aCore rules out translational regulation as being responsible for the modulatory effects. Moreover, the observation that HCV-796 prevents 2aCore from stimulating NS5B's 5BR activity strongly argues that 2aCore acts on 2a5B directly to regulate NS5B's RNA synthesis. These results are also confirmed by the bi-molecular fluorescence complementation assay and the co-immunoprecipitation of Core and NS5B. Finally, there is some species-specific interaction between the HCV Core and NS5B since the capsid proteins of three other closely or distantly related viruses, WNV, CSFV and NV, did not affect 2a5B's 5BR activity. The simplest explanation for all of the observations is that Core will interact with NS5B in a species-specific manner.
The seven HCV genotypes differ in replicative capacity, immuno-logic escape, drug efficacy, and host cell interaction (Blackard and Sherman, 2007; Weng et al., 2010). Our work suggests that the genotype 1b and 2a HCVs differ in the interaction between the Core and NS5B proteins. 2aCore can enhance the RNA synthesis activity of NS5B and the replication of JFH-1 subgenomic replicon that lacks the structural proteins. In contrast, the 1b Core has been reported to cause a repression of the 1b NS5B activity (Uchida et al., 2002). We observed only a modest inhibitory effect of the 1b Core in both the 5BR and the replicon replication assays. This could be due to the lower levels of Core proteins expressed in our system. We intentionally kept the expression of Core low because its high level expression can also cause an oxidative stress that can induce anti-viral responses (Yano et al., 2009), as well as inhibit the accumulation of the NS5B protein. We have also demonstrated that NS5A from the 1b genotype negatively affects RNA synthesis by the 1b NS5B while 2a NS5A does not (Ranjith-Kumar et al., 2011). All of these different factors could contribute to distinct properties of 2a and 1b HCV replication and susceptibility to inhibitors.
The 2a JFH-1 virus causes fulminant hepatitis while the geno-type 1b HCV has a higher propensity to cause persistent infections (Bruno et al., 2007; Kato et al., 2001). This difference in pathogenesis is at least partially attributed to the higher level of RNA synthesis by the 2a HCV. Our observation about the ability of the Core protein to interact with the 2a NS5B and cause higher levels of RNA replication could contribute to the higher levels of JFH-1 RNA synthesis during infection.
We speculate that 2aCore aids in RNA synthesis by 2a NS5B through overcoming the rate limiting step in HCV RNA synthesis: de novo initiation. Consistent with this notion, several mutations in the JFH-1 NS5B that should render the polymerase to be in a more open conformation, debilitating them for de novo initiated RNA synthesis, also decreased the effects of Core on NS5B activity (Fig. 9). We have also screened mutants of 1b NS5B for changes that allowed response to the 1b Core protein. The majority of the mutants had no effect, but two with changes on the side of the polymerase that contacts the membrane had moderately increased response to 1bCore (data not shown). At present, we do not know whether these residues affect the conformation of the polymerase or de novo initiation. It is interesting, however, that the same region of the 1b and 2a Cores (residues 21–75) are both required to interact with their respective RdRps, with distinct outcomes.
Finally, it has become an emerging theme that viral structural proteins will regulate the activities of the nonstructural proteins (Kao et al., 2011). In addition to the HCV Core protein, the capsid proteins of the Rubella Virus, noroviruses and brome mosaic virus all exhibit regulatory effects on RNA synthesis (Subba-Reddy et al., 2012; Tzeng et al., 2006; Yi et al., 2009). Given that the capsid protein is one of the few proteins of a positive-strand RNA virus to enter the cell with the viral genomic RNA, it seems logical to have the Core regulate RNA synthesis when the virus needs to produce its RNAs to high levels. Furthermore, the capsid-associated protein of rotavirus, a virus with a double-stranded RNA genome that replicates in association with the virion, has been shown to regulate the activities of the rotavirus RdRp (McDonald et al., 2009). Similar interaction in the nucleoprotein and polymerase of influenza virus, a virus with a negative-stranded multipartite genome, has been reported (Marklund et al., 2012). These results suggest that capsid modulation of viral RdRp activity is a commonly shared property for viral RNA synthesis.
Acknowledgements
We thank B. Fan for help and consultation with the HCV replicon assays and C. Hassel at the IUB flow cytometry core facility for the assistance with FACS analyses. This work was funded by the National Institute of Allergy and Infectious Diseases for grant 1RO1AI073335.
References
- Ago H, Adachi T, Yoshida A, Yamamoto M, Habuka N, Yatsunami K, Miyano M. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure. 1999;7(11):1417–1426. doi: 10.1016/s0969-2126(00)80031-3. [DOI] [PubMed] [Google Scholar]
- Blackard JT, Sherman KE. Hepatitis C virus coinfection and superinfection. J. Infect. Dis. 2007;195(4):519–524. doi: 10.1086/510858. [DOI] [PubMed] [Google Scholar]
- Bressanelli S, Tomei L, Roussel A, Incitti I, Vitale RL, Mathieu M, De Francesco R, Rey FA. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl. Acad. Sci. U.S.A. 1999;96(23):13034–13039. doi: 10.1073/pnas.96.23.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007;46(5):1350–1356. doi: 10.1002/hep.21826. [DOI] [PubMed] [Google Scholar]
- Cai Z, Yi M, Zhang C, Luo G. Mutagenesis analysis of the rGTP-specific binding site of hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 2005;79(18):11607–11617. doi: 10.1128/JVI.79.18.11607-11617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaswamy S, Yarbrough I, Palaninathan S, Kumar CT, Vijayaraghavan V, Demeler B, Lemon SM, Sacchettini JC, Kao CC. A locking mechanism regulates RNA synthesis and host protein interaction by the hepatitis C virus polymerase. J. Biol. Chem. 2008;283(29):20535–20546. doi: 10.1074/jbc.M801490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas S, Bartolome J, Carreno V. Hepatitis C virus core protein down-regulates transcription of interferon-induced antiviral genes. J. Infect. Dis. 2005;191(1):93–99. doi: 10.1086/426509. [DOI] [PubMed] [Google Scholar]
- Fromentin R, Majeau N, Laliberte Gagne ME, Boivin A, Duvignaud JB, Leclerc D. A method for in vitro assembly of hepatitis C virus core protein and for screening of inhibitors. Anal. Biochem. 2007;366(1):37–45. doi: 10.1016/j.ab.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang JQ, Yang Y, Harris SF, Leveque V, Whittington HJ, Rajyaguru S, Ao-Ieong G, McCown MF, Wong A, Giannetti AM, Le Pogam S, Talamas F, Cammack N, Najera I, Klumpp K. Slow binding inhibition and mechanism of resistance of non-nucleoside polymerase inhibitors of hepatitis C virus. J. Biol. Chem. 2009;284(23):15517–15529. doi: 10.1074/jbc.M808889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope RG, McLauchlan J. Sequence motifs required for lipid droplet association and protein stability are unique to the hepatitis C virus core protein. J. Gen. Virol. 2000;81(Pt 8):1913–1925. doi: 10.1099/0022-1317-81-8-1913. [DOI] [PubMed] [Google Scholar]
- Hosui A, Ohkawa K, Ishida H, Sato A, Nakanishi F, Ueda K, Takehara T, Kasahara A, Sasaki Y, Hori M, Hayashi N. Hepatitis C virus core protein differently regulates the JAK-STAT signaling pathway under interleukin-6 and interferon-gamma stimuli. J. Biol. Chem. 2003;278(31):28562–28571. doi: 10.1074/jbc.M210485200. [DOI] [PubMed] [Google Scholar]
- Howe AY, Cheng H, Johann S, Mullen S, Chunduru SK, Young DC, Bard J, Chopra R, Krishnamurthy G, Mansour T, O'Connell J. Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796. Antimicrob. Agents Chemother. 2008;52(9):3327–3338. doi: 10.1128/AAC.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell. 2002;9(4):789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- Kang SM, Choi JK, Kim SJ, Kim JH, Ahn DG, Oh JW. Regulation of hepatitis C virus replication by the core protein through its interaction with viral RNA polymerase. Biochem. Biophys. Res. Commun. 2009;386(1):55–59. doi: 10.1016/j.bbrc.2009.05.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CC, Ni P, Hema M, Huang X, Dragnea B. The coat protein leads the way: an update on basic and applied studies with the Brome mosaic virus coat protein. Mol. Plant Pathol. 2011;12(4):403–412. doi: 10.1111/j.1364-3703.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Furusaka A, Miyamoto M, Date T, Yasui K, Hiramoto J, Nagayama K, Tanaka T, Wakita T. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J. Med. Virol. 2001;64(3):334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- Kneteman NM, Howe AY, Gao T, Lewis J, Pevear D, Lund G, Douglas D, Mercer DF, Tyrrell DL, Immermann F, Chaudhary I, Speth J, Villano SA, O'Connell J, Collett M. HCV796: a selective nonstructural protein 5B polymerase inhibitor with potent anti-hepatitis C virus activity in vitro, in mice with chimeric human livers, and in humans infected with hepatitis C virus. Hepatology. 2009;49(3):745–752. doi: 10.1002/hep.22717. [DOI] [PubMed] [Google Scholar]
- Kuiken C, Hraber P, Thurmond J, Yusim K. The hepatitis C sequence database in Los Alamos. Nucleic Acids Res. 2008;36(Database issue):D512–D516. doi: 10.1093/nar/gkm962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford RE, Notvall L, Chavez D, White R, Frenzel G, Simonsen C, Kim J. Analysis of hepatitis C virus capsid, E1, and E2/NS1 proteins expressed in insect cells. Virology. 1993;197(1):225–235. doi: 10.1006/viro.1993.1583. [DOI] [PubMed] [Google Scholar]
- Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat. Struct. Biol. 1999;6(10):937–943. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- Li D, Takyar ST, Lott WB, Gowans EJ. Amino acids 1-20 of the hepatitis C virus (HCV) core protein specifically inhibit HCV IRES-dependent translation in HepG2 cells, and inhibit both HCV IRES- and cap-dependent translation in HuH7 and CV-1 cells. J. Gen. Virol. 2003;84(Pt 4):815–825. doi: 10.1099/vir.0.18697-0. [DOI] [PubMed] [Google Scholar]
- Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. U.S.A. 2005;102(49):17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund JK, Ye Q, Dong J, Tao YJ, Krug RM. Sequence in the influenza A virus nucleoprotein required for viral polymerase binding and RNA synthesis. J. Virol. 2012;86(13):7292–7297. doi: 10.1128/JVI.00014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Tao YJ, Patton JT. The ins and outs of four-tunneled Reoviridae RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 2009;19(6):775–782. doi: 10.1016/j.sbi.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D, Gosert R, Egger D, Penin F, Blum HE, Bienz K. Membrane association of hepatitis C virus nonstructural proteins and identification of the membrane alteration that harbors the viral replication complex. Antiviral Res. 2003;60(2):103–109. doi: 10.1016/j.antiviral.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F. Hepatitis C virus proteins: from structure to function. Curr. Top. Microbiol. Immunol. 2013;369:113–142. doi: 10.1007/978-3-642-27340-7_5. [DOI] [PubMed] [Google Scholar]
- Ni P, Cheng Kao C. Non-encapsidation activities of the capsid proteins of positive-strand RNA viruses. Virology. 2013;446(1–2):123–132. doi: 10.1016/j.virol.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccininni S, Varaklioti A, Nardelli M, Dave B, Raney KD, McCarthy JE. Modulation of the hepatitis C virus RNA-dependent RNA polymerase activity by the non-structural (NS) 3 helicase and the NS4B membrane protein. J. Biol. Chem. 2002;277(47):45670–45679. doi: 10.1074/jbc.M204124200. [DOI] [PubMed] [Google Scholar]
- Quezada EM, Kane CM. The hepatitis C virus NS5A stimulates NS5B during in vitro RNA synthesis in a template specific manner. Open Biochem. J. 2009;3:39–48. doi: 10.2174/1874091X00903010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada EM, Kane CM. The stimulatory mechanism of hepatitis C virus NS5A protein on the NS5B catalyzed replication reaction in vitro. Open Biochem. J. 2013;7:11–14. doi: 10.2174/1874091X01307010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Li YP, Jensen SB, Pedersen J, Gottwein JM, Bukh J. Highly efficient infectious cell culture of three HCV genotype 2b strains and sensitivity to lead protease, NS5A, and polymerase inhibitors. Hepatology. 2013 doi: 10.1002/hep.26660. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Li YP, Jensen SB, Pedersen J, Gottwein JM, Bukh J. Highly efficient infectious cell culture of three hepatitis C virus genotype 2b strains and sensitivity to lead protease, nonstructural protein 5A, and polymerase inhibitors. Hepatology. 2014;59(2):395–407. doi: 10.1002/hep.26660. [DOI] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Wen Y, Baxter N, Bhardwaj K, Cheng Kao C. A cell-based assay for RNA synthesis by the HCV polymerase reveals new insights on mechanism of polymerase inhibitors and modulation by NS5A. PLoS ONE. 2011;6(7):e22575. doi: 10.1371/journal.pone.0022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelandt P, Obeid S, Paeshuyse J, Vanhove J, Van Lommel A, Nahmias Y, Nevens F, Neyts J, Verfaillie CM. Human pluripotent stem cell-derived hepatocytes support complete replication of hepatitis C virus. J. Hepatol. 2012;57(2):246–251. doi: 10.1016/j.jhep.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Schmidt-Mende J, Bieck E, Hugle T, Penin F, Rice CM, Blum HE, Moradpour D. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 2001;276(47):44052–44063. doi: 10.1074/jbc.M103358200. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Scrima N, Radujkovic D, Caillet-Saguy C, Simister PC, Friebe P, Wicht O, Klein R, Bartenschlager R, Lohmann V, Bressanelli S. A comprehensive structure-function comparison of hepatitis C virus strain JFH1 and J6 polymerases reveals a key residue stimulating replication in cell culture across genotypes. J. Virol. 2011;85(6):2565–2581. doi: 10.1128/JVI.02177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima N, Caillet-Saguy C, Ventura M, Harrus D, Astier-Gin T, Bressanelli S. Two crucial early steps in RNA synthesis by the hepatitis C virus polymerase involve a dual role of residue 405. J. Virol. 2012;86(13):7107–7117. doi: 10.1128/JVI.00459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y, Luo H, Qin W, Kaneko S, Yamashita T, Kobayashi K, Murakami S. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J. Biol. Chem. 2002;277(13):11149–11155. doi: 10.1074/jbc.M111392200. [DOI] [PubMed] [Google Scholar]
- Simister P, Schmitt M, Geitmann M, Wicht O, Danielson UH, Klein R, Bressanelli S, Lohmann V. Structural and functional analysis of hepatitis C virus strain JFH1 polymerase. J. Virol. 2009;83(22):11926–11939. doi: 10.1128/JVI.01008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Reddy CV, Goodfellow I, Kao CC. VPg-primed RNA synthesis of norovirus RNA-dependent RNA polymerases by using a novel cell-based assay. J. Virol. 2011;85(24):13027–13037. doi: 10.1128/JVI.06191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Reddy CV, Yunus MA, Goodfellow IG, Kao CC. Norovirus RNA synthesis is modulated by an interaction between the viral RNA-dependent RNA polymerase and the major capsid protein, VP1. J. Virol. 2012;86(18):10138–10149. doi: 10.1128/JVI.01208-12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tzeng WP, Matthews JD, Frey TK. Analysis of rubella virus capsid protein-mediated enhancement of replicon replication and mutant rescue. J. Virol. 2006;80(8):3966–3974. doi: 10.1128/JVI.80.8.3966-3974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Hino N, Yamanaka T, Fukushima H, Imanishi T, Uchiyama Y, Kodama T, Doi T. Hepatitis C virus core protein binds to a C-terminal region of NS5B RNA polymerase. Hepatol. Res. 2002;22(4):297–306. doi: 10.1016/s1386-6346(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Vaughan R, Fan B, You JS, Kao CC. Identification and functional characterization of the nascent RNA contacting residues of the hepatitis C virus RNA-dependent RNA polymerase. RNA. 2012;18(8):1541–1552. doi: 10.1261/rna.031914.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo NV, Tuler JR, Lai MM. Enzymatic characterization of the full-length and C-terminally truncated hepatitis C virus RNA polymerases: function of the last 21 amino acids of the C terminus in template binding and RNA synthesis. Biochemistry. 2004;43(32):10579–10591. doi: 10.1021/bi049773g. [DOI] [PubMed] [Google Scholar]
- Watashi K, Ishii N, Hijikata M, Inoue D, Murata T, Miyanari Y, Shimotohno K. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol. Cell. 2005;19(1):111–122. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Weng L, Hirata Y, Arai M, Kohara M, Wakita T, Watashi K, Shimotohno K, He Y, Zhong J, Toyoda T. Sphingomyelin activates hepatitis C virus RNA polymerase in a genotype-specific manner. J. Virol. 2010;84(22):11761–11770. doi: 10.1128/JVI.00638-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Ikeda M, Abe K, Kawai Y, Kuroki M, Mori K, Dansako H, Ariumi Y, Ohkoshi S, Aoyagi Y, Kato N. Oxidative stress induces anti-hepatitis C virus status via the activation of extracellular signal-regulated kinase. Hepatology. 2009;50(3):678–688. doi: 10.1002/hep.23026. [DOI] [PubMed] [Google Scholar]
- Yi G, Letteney E, Kim CH, Kao CC. Brome mosaic virus capsid protein regulates accumulation of viral replication proteins by binding to the replicase assembly RNA element. RNA. 2009;15(4):615–626. doi: 10.1261/rna.1375509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cai Z, Kim YC, Kumar R, Yuan F, Shi PY, Kao C, Luo G. Stimulation of hepatitis C virus (HCV) nonstructural protein 3 (NS3) helicase activity by the NS3 protease domain and by HCV RNA-dependent RNA polymerase. J. Virol. 2005;79(14):8687–8697. doi: 10.1128/JVI.79.14.8687-8697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]