Abstract
A growing body of evidence suggests that studying cell biology in classical two-dimensional formats, such as cell culture plasticware, results in misleading, non-physiological findings. For example, some aspects of cancer biology cannot be observed in 2D, but require 3D culture methods to recapitulate observations in vivo. Therefore, we developed a microsphere-based model to permit 3D cell culture incorporating physiological extracellular matrix components. Bio-electrospraying was chosen as it is the most advanced method to produce microspheres, with THP-1 cells as a model cell line. Bio-electrospraying parameters, such as nozzle size, polymer flow rate, and voltage, were systematically optimized to allow stable production of size controlled microspheres containing extracellular matrix material and human cells. We investigated the effect of bio-electrospraying parameters, alginate type and cell concentration on cell viability using trypan blue and propidium iodide staining. Bio-electrospraying had no effect on cell viability nor the ability of cells to proliferate. Cell viability was similarly minimally affected by encapsulation in all types of alginate tested (MVM, MVG, chemical- and food-grade). Cell density of 5 × 106 cells ml-1 within microspheres was the optimum for cell survival and proliferation. The stable generation of microspheres incorporating cells and extracellular matrix for use in a 3D cell culture will benefit study of many diverse diseases and permit investigation of cellular biology within a 3D matrix.
Keywords: bio-electrospraying, cell encapsulation, 3D cell culture, cellular kinetics, biological models
1. Introduction
Extensive work in the field of cell biology, and cancer biology in particular, [1] has aimed to develop three-dimensional (3D) cell culture models. It is an emerging concept that growing cells on 2D substrates does not mimic cellular biology in vivo.[2] Cell-cell interactions take place in three dimensions, and cell-matrix interactions regulate cellular survival and signalling pathways.[3] Furthermore, the mechanical properties of the matrix regulates cellular gene expression.[4] Consequently, results from 2D culture may be physiologically unrepresentative and even misleading. Development of a 3D cell culture model incorporating human cells and extracellular matrix (ECM) would permit studies in a more physiological context. Within such 3D models the ECM, cell interactions with the ECM and ECM destruction can be investigated, all of which are virtually impossible in 2D culture systems. The breadth and depth of understanding in all fields of biology that could be enhanced when addressed by a 3D cell culture system incorporating extracellular matrix should not be underestimated.
Cell encapsulation is most often used to immunoprotect an allogeneic or xenogenic cellular payload after in vivo transplantation. The most common reason for transplantation of encapsulated cells is to reverse a disease state, and more recently for tissue engineering.[5] However, growing cells in a 3D encapsulated environment, such as hydrogel microspheres, can modify cellular behaviour, for example increasing protein secretion,[6] or changing gene expression.[7] Consequently, cells grown within 3D hydrogel microspheres yield results that are more representative of in vivo observations.
Diverse polymers and processes have been utilised to encapsulate cells in hydrogel microspheres. The simplest of these techniques is droplet generation using a narrow orifice nozzle. The polymer of choice is extruded through a nozzle thus forming droplets, the droplets subsequently fall into a crosslinking bath where they become crosslinked hydrogel microspheres. Use of gravity alone to generate droplets results in large diameter microspheres after crosslinking.[8] Many manifestations have been utilised to allow smaller diameter microspheres to be produced, for example; coaxial air flow,[9] vibrating jet break-up,[10] and the rotating jet break-up and microfluidic methods.[11]
Bio-electrospraying (BES) uses an electric field to assist droplet generation from the nozzle. An electrostatic potential is generated between the tip of the nozzle and the gelation bath. As the electrostatic voltage applied increases, droplet size decreases.[12] Multiple variables can modify droplet size, such as polymer flow rate, nozzle diameter, and voltage;[13] however, a systematic study of the complex effect of changing these interdependent parameters has not been carried out. BES may not affect the viability of distinct cell types,[14] and indeed whole microscopic organisms have been encapsulated using this technique.[15] In addition, BES is simple in operation, has high encapsulation efficiency, and allows for sterile preparation of encapsulated cells.[16]
Alginate is very commonly used as a cell encapsulation polymer due to biocompatibility, ease of crosslinking, and availability of purified polymer. Alginate is a linear block copolymer comprised of β-D-mannuronic (M) acid and α-L-guluronic (G) acid residues.[17] The residues form three types of block: homopolymeric MM- and GG-blocks and alternating MG-blocks.[18] Gels formed from alginate with differing M/G ratios have different properties; Ca2+ ions selectively bind homopolymeric GG-blocks and thus alginates with a low M/G ratio (high G content) form stronger gels than alginates with high M/G ratio (high M content).[17] Gels formed from low M/G ratio alginate shrink less than gels formed from high M/G ratio alginates and thus form larger microspheres.[19]
We aimed to develop a 3D cell culture model system whereby cellular content and extracellular matrix composition can be accurately regulated. To this end, optimisation of numerous BES parameters was undertaken using alginate as the encapsulation matrix and THP-1 (a human monocytic cell line[20]) as the model cell. Parameters affecting the microsphere size, including voltage, flow rate and nozzle size were systematically investigated. We hypothesized that cell viability may be dependent upon gel strength and therefore the effects of different types of alginate and the addition of collagen on microsphere size and cell viability were studied.
2. Results and Discussion
2.1. Optimizing parameters for reproducible microsphere generation
Optimisation of BES parameters was carried out by varying the applied voltage and flow rate of food-grade alginate solution for each of three different nozzle diameters; 0.6 mm, 0.4 mm and 0.17 mm i.d. These nozzle sizes were chosen to produce a range of droplet sizes between 900 and 450 μm in diameter. When no voltage was applied large droplets formed at the nozzle, which then set within the collecting bath to form large microspheres of 2811.96 ± 21 μm (0.6 mm i.d. nozzle), 2566.46 ± 92 μm (0.4 mm i.d. nozzle), and 2073.13 ± 15 μm (0.17 mm i.d. nozzle) in diameter (Figure 1a). The size of microspheres formed without an applied voltage was dependent upon the size of the nozzle used to generate them. As the inner diameter of the needle increased, the bead size increased (p < 0.005). Similar observations have been made by other researchers using electrospraying to produce microspheres.[21]
Figure 1.
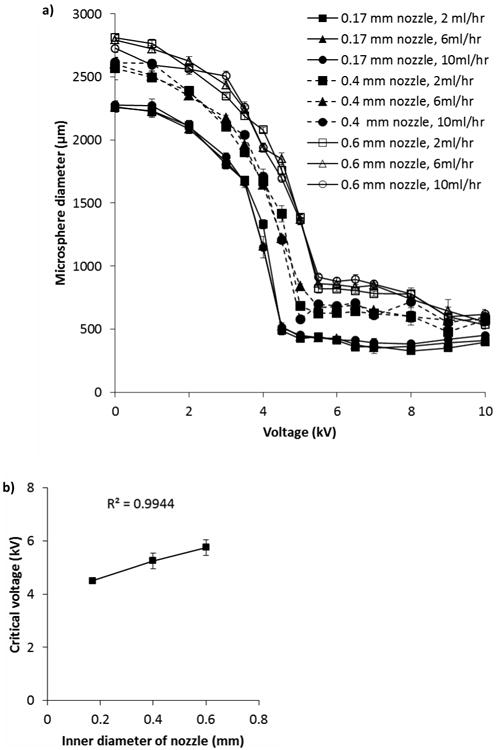
a) Effect of voltage, flow rate and nozzle size on diameter of microspheres. b) Lowest (critical) voltage needed to produce smallest microspheres is related to inner diameter of the nozzle used. Alginate concentration was 1.5% Manugel DMB alginate solvated in water. Data points are mean of three independent replicates where 30 microspheres were measured for each replicate. Error bars represent standard deviation.
As the applied voltage supplied was increased, a decrease in microsphere diameter was observed, until a point when no further increase in applied voltage resulted in smaller microspheres, referred to as the critical voltage [22] (Figure 1a,b). The lowest voltage needed to produce the smallest diameter microsphere is linearly related to the diameter of the nozzle used (Figure 1b). Therefore, this linear ratio can be used to determine the minimum voltage required to produce microspheres in the jetting mode without extended empirical optimisation. When using a 0.17 or 0.4 mm i.d. nozzle to produce microspheres, no decrease in microsphere diameter was observed above the critical voltage. However, when using a 0.6 mm i.d. nozzle, increasing applied voltage above ∼7 kV led to a decrease in observed microsphere diameter. Other groups also observe no change in microsphere diameter above the critical voltage.[12, 16b] By using much higher voltages than used in this and prior studies and an impulse electrostatic microsphere generator, Lewińska et al. observed decreasing microsphere diameter between 10 and 22 kV.[22]
Values collected were not normally distributed so ANOVA could not be carried out; therefore log transformed data was analysed instead. As the flow rates of alginate varied, no effect of flow rate on microsphere diameter was observed (p = 0.981). Several groups have observed that as flow rate is decreased, there is a decrease in microsphere diameter.[12, 16b, 21a] This effect appears to become more noticeable when a wide range of flow rates are explored. For instance, examining the effect of flow rates in the range of ∼ 6 – 72 ml hr-1,[12] 1 – 12 ml hr-1,[16b] and ∼ 6 – 126 ml hr-1,[21a] allowed a decrease in microsphere size to be observed, whereas flow rates in the range of ∼ 0.1 – 6 ml hr-1[23] did not highlight this effect. The effect is also more pronounced when higher viscosity solutions or more concentrated alginate solutions are used.[16b] Despite the fact that lower flow rates produce microspheres with small diameters, production rates are also lower and so higher flow rates may be more advantageous from this point of view. Using a smaller diameter nozzle at higher flow rates to produce smaller microspheres may be a good compromise.
The effect of sodium alginate concentration on ease of BES operation was explored by varying the applied voltage and alginate flow rate whilst using food-grade alginate solutions at two different concentrations; 3% and 1.5%. Parameter maps were produced (Figure 2a,b) to visualise areas of optimal operation. BES with 1.5% sodium alginate solution became unstable in the area shown; unstable is used here to indicate unstable jet formation resulting in alginate solution collecting on the ring electrode. However, when 3% sodium alginate solution was used, an unstable state was reached at much lower applied voltages than when using 1.5% alginate solution. In addition, spherical microspheres were not produced; instead “teardrops” (Figure 2c) and “tadpoles” (Figure 2d) formed. Non-spherical alginate shapes have been reported previously,[24] although at higher concentrations of alginate than used here (5 and 8% compared with 3% reported here). The differences in observations may be due to the viscosity of the alginate solutions used.
Figure 2.
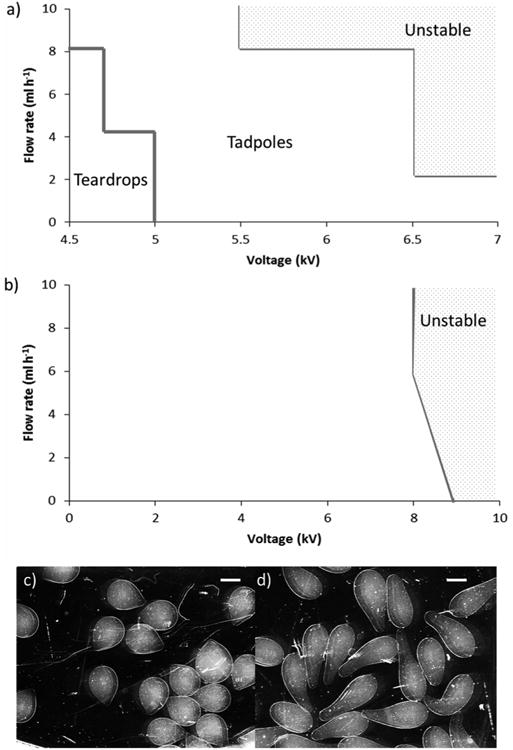
Observed outcomes when electrospraying a) 3% Manugel DMB alginate and b) 1.5% Manugel DMB alginate using 0.6 mm inner diameter nozzle for various combinations of flow rate and applied voltage. Unstable is used here to indicate unstable jet formation resulting in alginate solution collecting on the ring electrode. c) Teardrop-shaped microspheres and d) tadpole-shaped microspheres observed when electrospraying 3% Manugel DMB. Scale bar 1000 μm.
2.2 Effect of solvent on microsphere formation
Stable cone-jet formation in BES is dependent upon using low conductivity solvents.[25] As the cells to be encapsulated are combined and maintained with the polymer and solvent solution, it is important that the solvent promotes cell viability and does not damage them. Low conductivity organic solvents, such as dichloromethane, acetone, and acetonitrile, commonly used in BES, are highly toxic to cells. Several alternative solvents which are used during measurement of electrical properties of cells were chosen based upon conductivity. All solvents were also isotonic to minimise osmotic stress to the cells.
Each of the alternative solvents was used to solvate food-grade sodium alginate at 1.5%. Each of the solutions was introduced into the Encapsulator through a 0.4 mm i.d. nozzle at a flow rate of 10 ml hr-1, with an applied voltage of ∼7 kV. These BES parameters were chosen as microspheres of the required size were produced and a stable cone-jet was formed. Microspheres were produced from all solutions tested, with the exception of dielectrophoresis buffer (Figure 3). Droplets of alginate solution formed when dielectrophoresis buffer was used as a solvent; however, gel microspheres were not formed when droplets fell into a setting bath composed of 100 mm CaCl2 in HBSS. Formation of gel microspheres with dielectrophoresis buffer required the collecting bath to also be composed of dielectrophoresis buffer, containing 100 mm CaCl2. Microspheres made using internal electrophysiology, dielectrophoresis and Ringer's solutions were found to be similar in size to each other but significantly different to microspheres made with all other solvents. Microspheres made using external electrophysiology buffer were significantly larger than microspheres made using all other solvents (p < 0.005). Microspheres made with water as a solvent were significantly smaller than microspheres made with all other solvents except HBSS (p < 0.005). Microspheres made using HBSS were significantly smaller than microspheres made using all other solvents (p < 0.005). Barlett's test for variance was performed and microspheres made with external electrophysiology buffer and HBSS had significantly higher (p < 0.005) variance than microspheres generated with other solvents.
Figure 3.
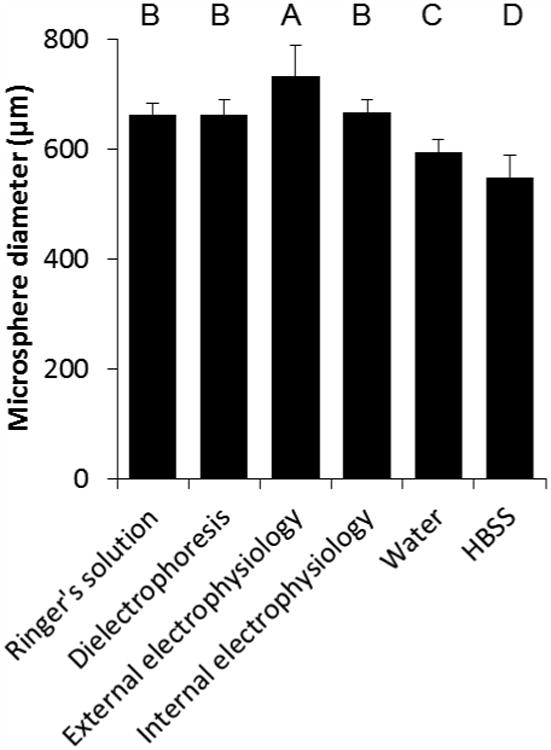
Effect of solvent on microsphere diameter. Alternative solvents were used to produce 1.5% Manugel DMB alginate solutions, which were electrosprayed using 0.4 mm i.d. nozzle, 10 ml hr-1 flow rate and ∼7 kV applied voltage. Values are mean of 30 microspheres measured and error bars represent standard deviation. Bars labelled with the same letter are not significantly different.
Microspheres were made using food-grade alginate solvated in HBSS over a wider range of voltages and flow rates (Figure 4). Microspheres made with alginate solvated in HBSS were significantly different in size to microspheres composed of alginate solvated in water (1092.2 ± 369 μm compared with 1118.8 ± 366 μm for water and HBSS respectively, p = 0.009; however, the difference between the mean diameters (26 μm) would not be considered substantial or scientifically significant). This result is in contrast to Al-Hajry et al. who observed a very significant difference between alginate microspheres produced using water as a solvent and an amino-acid rich plant growth medium as a solvent, where microspheres made using medium were observed to be much larger than microspheres made using water.[26] Interestingly, when HBSS was used to solvate alginate a significant effect (p = 0.033) of flow rate on microsphere diameter was observed for the lowest flow rate tested (2 ml hr-1). This observation is in contrast to using water to solvate alginate, where no effect of flow rate was observed. HBSS was chosen as the solvent to encapsulate cells as its effect on the size of microspheres produced is minimal and is optimal for cellular viability.
Figure 4.
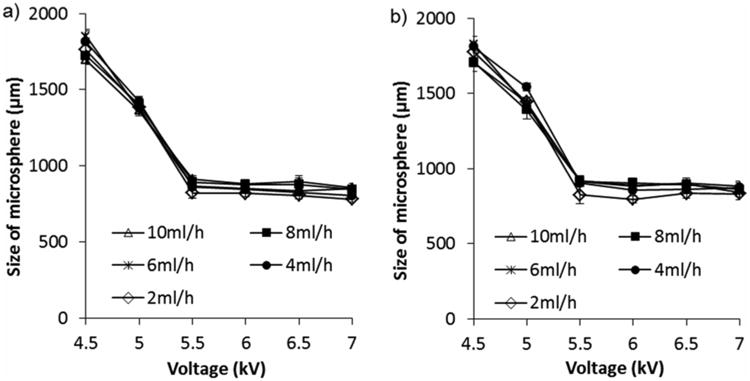
Use of HBSS compared with water does not affect the diameter of microspheres produced. Water a) or HBSS b) was used to produce 1.5% Manugel DMB alginate solutions, which were electrosprayed using 0.6 mm i.d. nozzle, various flow rates and applied voltages. Values are mean of 30 microspheres measured and error bars represent standard deviation.
2.3 Effect of alginate composition on microsphere size
Having characterised the optimal physical parameters for BES, we determined the effect of varying alginate type on microsphere formation. Commercially available alginates were chosen to investigate the effect of composition; one food-grade, one chemical-grade, and six ultrapure, well-characterised products (Table 1). The six ultrapure alginates were composed of different M/G ratios and viscosities; very low viscosity, high G content (VLVG), very low viscosity, high M content (VLVM), low viscosity, high G content (LVG), low viscosity, high M content (LVM), medium viscosity, high G content (MVG), and medium viscosity, high M content (MVM).
Table 1.
Specifications for each type of alginate used in this investigation. This information was provided by the manufacturers/suppliers of the alginate.
| Type of alginate | Supplier | M/G content | MW, kDa | Viscosity, mPas (1% solution) |
|---|---|---|---|---|
| VLVG | Novamatrix | 67% G | <75 | 5 |
| VLVM | Novamatrix | 56% M | <75 | 5.6 |
| LVG | Novamatrix | 68% G | 75-200 | 163 |
| LVM | Novamatrix | 59% M | 75-200 | 88 |
| MVG | Novamatrix | 68% G | >200 | 515 |
| MVM | Novamatrix | 54% M | >200 | 440 |
| Food-grade Manugel DBM | FMC Biopolymer | 60-70% G | 150 | 319 |
| Chemical-grade | Sigma | 61% M | 80 - 120 | ≥2,000 a) |
Measured for a 2% solution
All types of alginate were made into 1.5% solutions using HBSS as a solvent; VLVG and VLVM were also made into 3% solutions. Spherical microspheres did not form with 1.5% or 3% VLVG or VLVM alginate solutions. Shi et al. observed droplets that formed “tear” structures similar to those observed to form using VLVG and VLVM (data not shown).[24a] They attribute this “tear” formation to a lack of alginate molecules at the periphery of the droplet. Ouwerx et al. observed “flake” formation when alginate solutions with viscosity of less than ∼ 50 mPas were dripped into CaCl2 solution.[27] Indeed, as it has been observed that the viscosity of alginate solutions must be above ∼ 50 – 60 mPas to form spherical beads, it is not surprising that VLVG and VLVM alginate solutions did not form spheres.[24b] All other alginate solutions produced spherical microspheres using 0.4 mm i.d. nozzle, 10 ml h-1 alginate solution flow rate and ∼7 kV applied voltage (Figure 5). There was a significant effect of alginate type on size of microspheres produced (p < 0.005).
Figure 5.
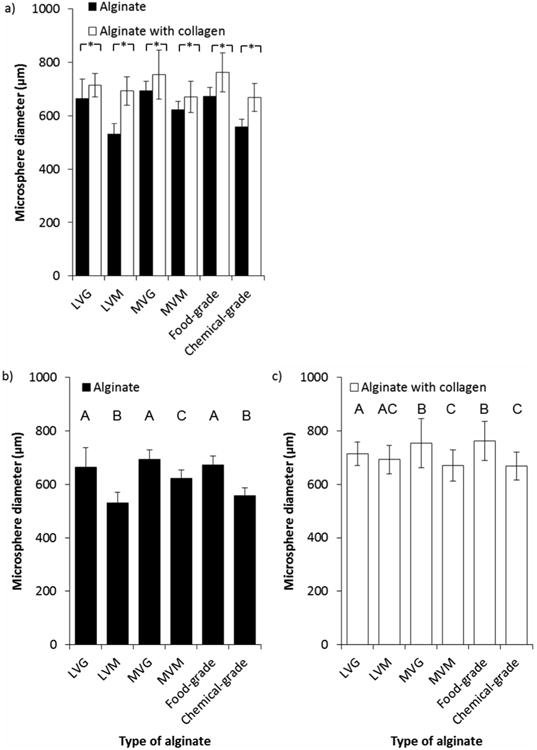
Effect of alginate type (black bars) and addition of collagen (white bars) on the diameter of produced microspheres. a) Addition of collagen produced microspheres of significantly larger diameter than alginate alone (*p < 0.005). Alginate type b) and addition of collagen c) affected size of microspheres produced. Alginate concentration was 1.5% Manugel DMB, final collagen concentration was 1 mg ml-1, BES conditions were 0.4 mm inner diameter nozzle, 10 ml h-1 alginate solution flow rate, ∼7 kV applied voltage. Values are from two replicate experiments where mean of 30 microspheres were measured for each replicate and error bars represent standard deviation. b and c: Bars labelled with the same letter are not significantly different.
Alginates with a high proportion of M residues are known to form smaller microspheres than alginates with a high proportion of G residues, due to increased shrinkage.[28] For microspheres formed of low viscosity alginate (LVG and LVM) this observation was true but did not hold for medium viscosity alginate (MVG and MVM). Chemical-grade alginate was also observed to form small microspheres, in keeping with an increased proportion of M residues. Food-grade alginate, composed of an increased proportion of G residues, formed large microspheres. Other studies have found LVM alginate to form smaller microspheres than LVG,[29] and this observation has also been reported for medium viscosity alginates.[16b, 28, 30]
As the predominant reason for developing a 3D cell culture model is to create a more physiological environment and to investigate the cellular biology in the context of extracellular matrix fibrils, the effect of adding collagen to each of the alginate types was observed. Again, spherical microspheres could not be produced using 1.5% or 3% VLVG or VLVM alginate solutions, but were successfully formed from all other alginate and collagen combinations (Figure 5). There was a significant effect of collagen addition on size of microspheres produced, with collagen increasing microsphere size in all conditions (p < 0.005).
The addition of collagen to alginate before generating microspheres appeared to ameliorate the effect of alginate type on size of microspheres. Microspheres containing collagen were all significantly larger than microspheres manufactured under the same conditions without collagen (mean difference of 86.7 μm, p < 0.005). There was a significant interaction effect between the addition of collagen and the alginate type (p < 0.005). The size of microspheres formed using LVM alginate was most strongly affected by the addition of collagen (529.91 ± 41 μm compared with 692.58 ± 53 μm, p < 0.005). Capone et al. report that alginate microspheres containing collagen produced using an air flow generator were 20 μm smaller than equivalently produced alginate microspheres.[31] Although Yao et al. produced collagen-containing alginate microspheres, microsphere diameter was not reported.[32] To the best of our knowledge, no work has been carried out comparing microspheres composed of different alginate types and collagen.
2.4 Applied voltage does not affect cell viability
Next, to determine whether high applied voltages affected cell viability, we passed THP-1 cells in sodium alginate solutions through the Encapsulator with and without applied voltage (∼7 kV) with subsequent encapsulation within MVG alginate microspheres. Microspheres formed without an applied voltage were large (3124.5 ± 77 μm, as seen in Figure 1); whereas microspheres formed with an applied voltage of ∼7 kV were much smaller (885.3 ± 30 μm, p < 0.0001). Microspheres formed with the addition of cells were not significantly different in size to microspheres formed without cells (885.3 ± 30 μm compared to 881 ± 37 μm respectively, p = 0.6229). Cells encapsulated using both methods proliferated initially (Figure 6a) and viability at 24 hours post-encapsulation was comparable to non-encapsulated control cells (figure 6b). Cells encapsulated using an applied voltage proliferated linearly over the time observed (Figure 6a), whereas cells encapsulated without using an applied voltage grew initially and then remained static. There was a significant effect due to voltage on the number of live cells in the microspheres (p = 0.014). Viability of cells encapsulated using both methods was observed to increase over time (Figure 6b). Voltage has no significant effect on the viability of the cells (p = 0.298).
Figure 6.
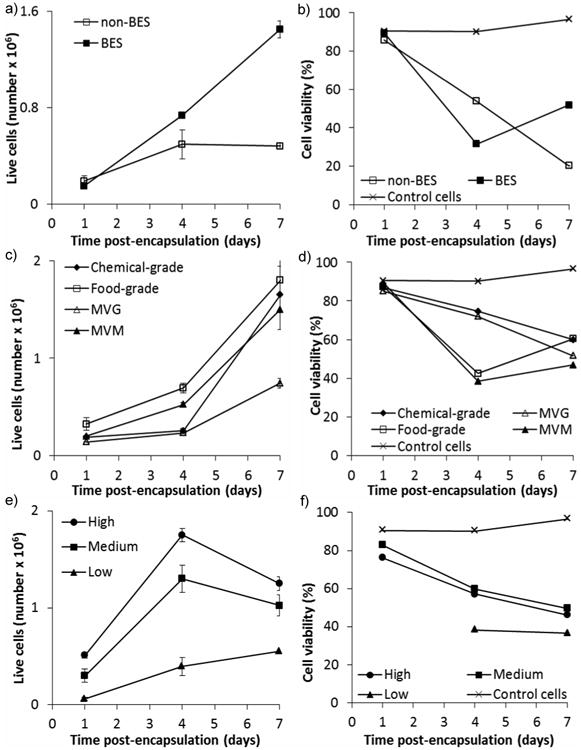
Effect of varying parameters on cell proliferation a), c), e) and cell viability b), d), f) of encapsulated THP-1 cells. Parameters varied were: a), b) voltage applied; no voltage (non-BES) or ∼7 kV applied voltage (BES), c), d) alginate type; food-grade, chemical-grade, MVG or MVM and e), f) initial cell concentration; 10 × 106 cells ml-1 (High), 5 × 106 cells ml-1 (Medium), and 1 × 106 cells ml-1 (Low). Alginate concentration was 1.5%, BES conditions were 0.4 mm inner diameter nozzle, 10 ml h-1 alginate solution flow rate, ∼7 kV applied voltage (unless otherwise stated). Cell counts were carried out using trypan blue. Viability was measured using PI staining and flow cytometry. Error bars represent standard deviation of two measurements.
As has previously been observed, use of high voltages in bio-electrospraying does not affect cell viability or ability of cells to grow, [14, 33] though these studies did not incorporate extracellular matrix. The lack of growth observed in cells encapsulated in microspheres formed without an applied voltage may be due to the large size of the microspheres. Cell necrosis has been observed in the centre of large capsules (>500 μm) after implantation into rats, due to ineffective diffusion of oxygen, nutrients and waste products.[34] Furthermore, modelling has shown that microspheres should be no more than 300 μm in diameter to ensure effective nutrient and waste diffusion and hence cell survival.[35]
2.5 Effect of alginate type on viability of THP-1 cells
THP-1 cells were encapsulated within microspheres composed of different types of alginate to investigate the effect of alginate composition on cell viability. Food-grade, chemical-grade, MVG and MVM alginate were investigated (Table 1). An increase in cell numbers was seen over the observed time for cells encapsulated in all types of alginate (Figure 6c). Representative images demonstrate cells encapsulated in food-grade alginate using fluorescent microscopy with calcein-AM staining live cells and ethidium homodimer staining dead cells (Figure 7). There was a significant effect of alginate type on the number of live cells contained within the microspheres; the number of live cells within the MVG alginate microspheres was significantly lower than the number of live cells encapsulated within the MVM or food-grade alginate microspheres (p < 0.005). In other words, the type of alginate used affected cell proliferation, however, there was no significant effect of alginate type on the viability of encapsulated cells (Figure 6d), with similar percentages of live cells in each microsphere type. Therefore, the growth of the cells in MVG alginate microspheres was slower than that seen for the other alginate types but this did not appear to be due to a higher rate of cell death. Other studies have shown that MVM and MVG do not significantly affect encapsulated cell viability.[36]
Figure 7.
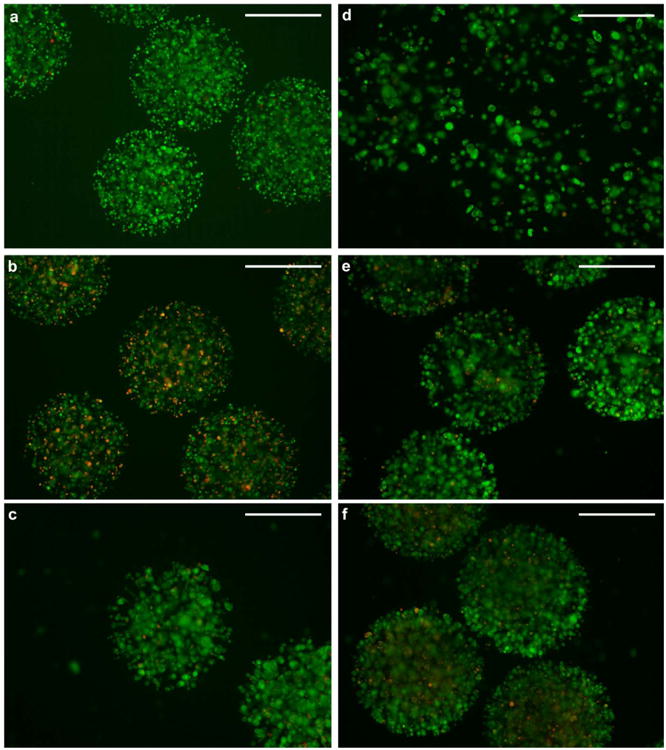
Fluorescent images showing encapsulated live (green) and dead (red) cells at different time points after BES. Cells encapsulated in 1.5% food-grade alginate are shown a) one day, b) 4 days, and c) 7 days post-encapsulation. Cells encapsulated at d) 1 × 106 cells ml-1 (Low), e) 5 × 106 cells ml-1 (Medium), and f) 10 × 106 cells ml-1 (High). Images were taken 7 days post-encapsulation. Green fluorescence is emitted from intracellular esterase-converted calcein in live cells, whereas red fluorescence is emitted from ethidium homodimer present in the nuclei of dead cells. BES conditions were 0.4 mm inner diameter nozzle, 10 ml h-1 alginate solution flow rate, ∼7 kV applied voltage. Scale bar 500 μm.
2.6 Initial cell concentration affects cell viability
We next hypothesised that cell viability would be modulated by the initial cell concentrations within the microspheres. To test this hypothesis, three different initial concentrations of cells were encapsulated; 10 × 106 cells ml-1 (high), 5 × 106 cells ml-1 (medium), and 1 × 106 cells ml-1 (low). All previous cell encapsulation experiments were carried out with cells at a concentration of 5 × 106 cells ml-1, corresponding to medium cell concentration in this experiment. Different numbers of cells were encapsulated within each set of MVG alginate microspheres corresponding to the initial cell concentrations encapsulated (Figure 6e). This difference in cell number was also observed for 7 days after encapsulation using fluorescent microscopy with calcein-AM (live cells) and ethidium homodimer (dead cells) staining (Figure 7d,e,f). All samples of microspheres showed increased cell numbers to 4 days post-encapsulation, after this time point cells encapsulated at high initial cell density decreased in number. Overall, a larger increase in cell number was observed in medium and low initial cell concentrations than high initial cell concentration. There was a significant effect of cell concentration on the number of live cells within the microspheres (p < 0.005). Cell viability was observed to decrease in all samples over time; however, cells encapsulated at low initial cell density increased in viability between 7 and 11 days post encapsulation (Figure 6f). There was no significant effect due to cell concentration observed on cell viability (p = 0.229); however, this is probably due to a lack of power in the experiment when attempting to find small differences.
Gasperini et al. encapsulated B50 neuroblastoma rat cells using a homemade electrospraying device at 5 × 106 cells ml-1 and 10 × 106 cells ml-1.[21b] They reported good viability as observed by confocal microscopy and live/dead staining, but did not report absolute values. We are not aware of any previously undertaken systematic evaluation of the effect of initial cell density on proliferation and cell survival within microspheres formed by electrospraying.
3. Conclusion
We have performed the first detailed and systematic evaluation of BES variables and investigated the effect on microsphere size, stability of microsphere generation and cellular survival. We identify critical variables and optimal parameters for generating microspheres containing both human cells and extracellular matrix components so that biological processes can be studied in a physiological 3D matrix.
We show that increasing voltage below the critical voltage leads to a decrease in microsphere size. We have also shown that decreasing nozzle size allows smaller alginate microspheres to be produced. The nozzle size and critical voltage are linearly related, which allows the critical voltage to be calculated for a given nozzle diameter, without the need for protracted empirical testing. The flow rate range investigated did not affect microsphere size. Low viscosity high-M alginate formed smaller microspheres than low viscosity high-G alginate, but this effect was not observed with medium viscosity alginate samples. The addition of collagen to alginate produced larger microspheres than alginate alone and LVM alginate was affected the most by collagen addition.
BES at the voltages studied did not adversely affect THP-1 cell viability. Of the alginate types tested, MVG provided the least optimal environment for cellular proliferation within the microspheres. However, none of the alginates tested adversely affected cell viability. Cell concentration was shown to have an effect on live encapsulated cell numbers, with an initial cell concentration of 5 × 106 cells ml-1 optimal for cell survival and proliferation.
In summary, we have shown that with careful optimisation BES can be used to produce 3D encapsulated models incorporating collagen. This model can be employed to increase researchers' understanding how 3D intercellular interactions and cell-matrix interactions regulate cell behaviour, which is of critical relevance to understanding basic biology and malignant, infectious, autoimmune and degenerative human diseases.
4. Experimental Section
Materials
Manugel DMB alginate supplied by FMC Biopolymer, Cork, Ireland. VLVG, VLVM, LVG, LVM, MVG and MVM alginate supplied by Novamatrix, Sandvika, Norway. LIVE/DEAD viability kit, HBSS, RPMI-1640, HEPES, and NaHCO3 was supplied by Life Technologies, Dorset, UK. VitroCol human Collagen solution, type I (3 mg ml-1) was supplied by Advanced BioMatrix, Inc. San Diego, CA. All other chemicals were supplied by Sigma-Aldrich, Dorset, UK.
Microsphere Production
Alginate solutions were made by dissolving alginate (type and concentration indicated in each experiment) in either distilled water or HBSS (without Ca2+ and Mg2+). During cell encapsulation cells were mixed with alginate solution to give a final cell concentration of 5 × 106 cells ml-1. Alginate solution was introduced into an electrostatic bead generator (Nisco Engineering AG, Zurich, Switzerland) using a programmable syringe driver (PHD 4400, Harvard Instruments, Kent, UK) connected via silicon tubing at various flow rates (indicated in each experiment). The effect of varying electrical potential and flow rate, as well as needle diameter, on microsphere size was investigated (as indicated in each experiment). The distance between the needle and the ring electrode was constant at 1 cm. The alginate droplets formed by the electrical potential fell into a magnetically stirred setting bath of 100 mm CaCl2 in either water or HBSS. Microspheres containing cells were washed three times in HBSS containing Ca2+ and Mg2+.
Alginate microspheres containing collagen were produced as follows; a solution containing 0.05 m NaOH in 0.2 m HEPES (9% final volume) was mixed with 7.5% NaHCO3 (18% final volume) and collagen (73% final volume) on ice. The collagen containing solution was mixed 50:50 with 3% alginate solution. Encapsulation settings were flow rate of 10 ml hr-1, electrostatic potential of ∼7 kV, and a needle with 0.7 mm o.d. and 0.4 mm i.d. A magnetically stirred setting bath of 100 mM CaCl2 in HBSS was used to solidify the droplets.
Microsphere Measurement
Microsphere diameter was measured using a Leica microscope and camera (Leica Microsystems (UK) Ltd, Milton Keynes, UK) in combination with Leica Application Software. The diameter of 30 individual microspheres was measured for each sample.
Alternative Solvents
Ringer's solution consisted of 100 mm NaCl, 2 mm KCl, 1 mm EGTA, 1 mm MgCl2, and 5 mm HEPES. The pH was adjusted to 7.4 before use. Dielectrophoresis buffer consisted of 9.5% ultrapure sucrose, 0.3% dextrose and 0.1% Pluronic F68. External electrophysiology buffer consisted of 140 mm NaCl, 2.8 mm KCl, 10 mm HEPES, 1 mm MgCl2·6H20, and 2 mm CaCl2·2H20. The pH was adjusted to 7.2 – 7.4 by addition of NaOH. Immediately before use 10 mm D-(+)-glucose was added. Internal electrophysiology buffer consisted of 10 mm NaCl, 145 mm KCl, 10 mm HEPES, and 1 mm MgCl2·6H20. The pH was adjusted to 7.2 – 7.4 by addition of KOH. Immediately before use 1 mm EGTA was added.
High Speed Photography
Bio-electrospraying was observed using a MotionBlitz high speed camera (Mikrotron GmbH, Unterschleißheim, Germany) capable of recording up to 523 frames per second.
Cell culture
THP-1 cells (American Type Culture Collection, TIB-202) and cell-containing beads were cultured in complete medium consisting of RPMI 1640 with 10% foetal calf serum, 2 mm glutamine, 0.1 mg ml-1 ampicillin and 1.25 μg ml-1 amphotericin B in an atmosphere of 5% CO2/95% air at 37 °C.
PI Staining and Flow Cytometry
A sample of microspheres was dissolved using 30 mm EDTA + 55 mm sodium citrate for 5 minutes at 37 ºC. A known number (500,000) of cells was stained with PI (1:50 dilution in HBSS) or LIVE/DEAD stain (HBSS containing 2 μm calcein stain and 4 μm of ethidium homodimer-1). Stained samples were detected using a CyAn flow cytometer (Beckman Coulter, High Wycombe, UK).
Statistics
Alginate microsphere properties are expressed as mean ± standard deviation. To determine significant differences between groups of data, analysis of variance (ANOVA) was performed with a Tukey–Kramer post-test. In all cases, P < 0.05 was considered statistically significant.
Acknowledgments
SNJ and PTE gratefully acknowledge the National Institutes of Health in the United States for funding this research program (RO21-AI102239) and for funding the post-doctoral research position for VLW. The authors wish to thank Mr Nicolai Suter, General Manger of Nisco Engineering AG for all the help and advice provided for these investigations.
Contributor Information
Dr Victoria L. Workman, BioPhysics Group, UCL Institute of Biomedical Engineering, UCL Centre for Stem Cells and Regenerative Medicine and Department of Mechanical Engineering, University College London, Torrington Place, London, WC1E 7JE, United Kingdom.
Dr Liku B. Tezera, Clinical and Experimental Sciences, Institute for Life Sciences, Faculty of Medicine, University of Southampton, Southampton, SO16 6YD, United Kingdom.
Dr Paul T. Elkington, Clinical and Experimental Sciences, Institute for Life Sciences, Faculty of Medicine, University of Southampton, Southampton, SO16 6YD, United Kingdom.
Dr Suwan N. Jayasinghe, Email: s.jayasinghe@ucl.ac.uk, BioPhysics Group, UCL Institute of Biomedical Engineering, UCL Centre for Stem Cells and Regenerative Medicine and Department of Mechanical Engineering, University College London, Torrington Place, London, WC1E 7JE, United Kingdom.
References
- 1.Streuli CH, Bailey N, Bissell MJ. J Cell Biol. 1991;115:1383. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pampaloni F, Reynaud EG, Stelzer EH. Nat Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 3.a) Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. Differentiation. 2002;70:537. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kleinman HK, Philp D, Hoffman MP. Curr Opin Biotechnol. 2003;14:526. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Poncelet D, de Vos P, Suter N, Jayasinghe SN. Adv Healthcare Mater. 2012;1:27. doi: 10.1002/adhm.201100001. [DOI] [PubMed] [Google Scholar]; b) Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WWL, Schricker SR, Shi S. J Biomed Mater Res A. 2013;101:3285. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 6.a) Purcell EK, Singh A, Kipke DR. Tissue Eng Part C, Methods. 2009;15:541. doi: 10.1089/ten.tec.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Weber LM, Hayda KN, Anseth KS. Tissue Eng Part A. 2008;14:1959. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wong HL, Wang MX, Cheung PT, Yao KM, Chan BP. Biomaterials. 2007;28:5369. doi: 10.1016/j.biomaterials.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Baer AE, Kraus VB, Setton LA. Spine. 2001;26:1747. doi: 10.1097/00007632-200108150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Poncelet D, Bugarski B, Amsden BG, Zhu J, Neufeld R, Goosen MFA. Appl Microbiol Biotechnol. 1994;42:251. [Google Scholar]
- 9.Tagalakis AD, Diakonov IA, Graham IR, Heald KA, Harris JD, Mulcahy JV, Dickson G, Owen JS. Biochim Biophys Acta. 2005;1686(3):190. doi: 10.1016/j.bbalip.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Seifert DB, Phillips JA. Biotechnol Prog. 1997;13:562. doi: 10.1021/bp970071a. [DOI] [PubMed] [Google Scholar]
- 11.a) Pruβe U, Fox B, Kirchhoff M, Bruske F, Breford J, Vorlop KD. Biotechnol Tech. 1998;12:105. [Google Scholar]; b) Zhou S, Fan J, Datta SS, Guo M, Guo X, Weitz DA. Adv Funct Mater. doi: 10.1002/adfm.201301030. [DOI] [Google Scholar]
- 12.Poncelet D, Neufeld RJ, Goosen MFA, Burgarski B, Babak V. AIChE J. 1999;45:2018. [Google Scholar]
- 13.Chakraborty S, Liao IC, Adler A, Leong KW. Adv Drug Delivery Rev. 2009;61:1043. doi: 10.1016/j.addr.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasinghe SN, Qureshi AN, Eagles PAM. Small. 2006;2:216. doi: 10.1002/smll.200500291. [DOI] [PubMed] [Google Scholar]
- 15.Mongkoldhumrongkul N, Swain SC, Jayasinghe SN, Sturzenbaum S. J R Soc, Interface. 2010;7:595. doi: 10.1098/rsif.2009.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Bugarski B, Li Q, Goosen MFA, Poncelet D, Neufeld RJ, Vunjak G. AIChE J. 1994;40:1026. [Google Scholar]; b) Klokk TI, Melvik JE. J Microencapsulation. 2002;19:415. doi: 10.1080/02652040210144234. [DOI] [PubMed] [Google Scholar]; c) Koch S, Schwinger C, Kressler J, Heinzen C, Rainov NG. J Microencapsulation. 2003;20:303. doi: 10.1080/0265204021000058438. [DOI] [PubMed] [Google Scholar]; d) Goosen MFA, Al-Ghafri AS, Mardi OE, Al-Belushi MIJ, Al-Hajri HA, Mahmoud ESE, Consolacion EC. Biotechnol Prog. 1997;13:497. [Google Scholar]
- 17.Smidsrod O. Faraday Discuss Chem Soc. 1974;57:263. [Google Scholar]
- 18.Gaserod O, Smidsrod O, Skjak-Braek G. Biomaterials. 1998;19:1815. doi: 10.1016/s0142-9612(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 19.Martinsen A, Skjak-Braek G, Smidsrod O. Biotechnol Bioeng. 1989;33:79. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Int J Cancer. 1980;26:171. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 21.a) Strand BL, Gaserod O, Kulseng B, Espevik T, Skjak-Baek G. J Microencapsulation. 2002;19:615. doi: 10.1080/02652040210144243. [DOI] [PubMed] [Google Scholar]; b) Gasperini L, Maniglio D, Migliaresi C. J Bioact Compat Polym. 2013;28:413. [Google Scholar]
- 22.Lewinska D, Rosinski S, Werynski A. Artif Cells, Blood Substitutes, Immobilization Biotechnol. 2004;32:41. doi: 10.1081/bio-120028667. [DOI] [PubMed] [Google Scholar]
- 23.De Castro M, Orive G, Hernandez RM, Gascon AR, Pedraz JL. J Microencapsulation. 2005;22:303. doi: 10.1080/026520405000099893. [DOI] [PubMed] [Google Scholar]
- 24.a) Shi P, He P, Teh TKH, Morsi YS, Goh JCH. Powder Technol. 2011;210:60. [Google Scholar]; b) Chan ES, Lee BB, Ravindra P, Poncelet D. J Colloid Interface Sci. 2009;338:63. doi: 10.1016/j.jcis.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Meng F, Jiang Y, Sun Z, Yin Y, Li Y. J Appl Polym Sci. 2009;113:526. [Google Scholar]
- 26.Al-Hajry HA, Al-Maskry SA, Al-Kharousi LM, El-Mardi O, Shayya WH, Goosen MFA. Biotechnol Prog. 1999;15:768. doi: 10.1021/bp990069e. [DOI] [PubMed] [Google Scholar]
- 27.Ouwerx C, Velings N, Mestdagh MM, Axelos MAV. Polym Gels Networks. 1998;6:393. [Google Scholar]
- 28.Darrabie MD, Kendall WF, Opara EC. J Microencapsulation. 2006;23:29. doi: 10.1080/02652040500286144. [DOI] [PubMed] [Google Scholar]
- 29.a) Moya ML, Morley M, Khanna O, Opara EC, Brey EM. J Mater Sci Mater Med. 2012;23:903. doi: 10.1007/s10856-012-4575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wandrey C, Espinosa D, Rehor A, Hunkeler D. J Microencapsulation. 2003;20:597. doi: 10.1080/0265204031000148022. [DOI] [PubMed] [Google Scholar]
- 30.Kendall WF, Jr, Darrabie MD, El-Shewy HM, Opara EC. J Microencapsulation. 2004;21:821. doi: 10.1080/02652040400015452. [DOI] [PubMed] [Google Scholar]
- 31.Capone SH, Dufresne M, Rechel M, Fleury MJ, Salsac AV, Paullier P, Daujat-Chavanieu M, Legallais C. PLoS One. 2013;8:e62032. doi: 10.1371/journal.pone.0062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao R, Zhang R, Lin F, Luan J. Biotechnol Bioeng. 2013;110:1430. doi: 10.1002/bit.24784. [DOI] [PubMed] [Google Scholar]
- 33.a) Braghirolli DI, Zamboni F, Chagastelles PC, Moura DJ, Saffi J, Henriques JAP, Pilger DA, Pranke P. Biomicrofluidics. 2013;7:044130. doi: 10.1063/1.4819747. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moyer HR, Kinney RC, Singh KA, Williams JK, Schwartz Z, Boyan BD. Ann Plast Surg. 2010;65:497. doi: 10.1097/SAP.0b013e3181d37713. [DOI] [PubMed] [Google Scholar]
- 34.De Vos P, Van Straaten JF, Nieuwenhuizen AG, de Groot M, Ploeg RJ, De Haan BJ, Van Schilfgaarde R. Diabetes. 1999;48:1381. doi: 10.2337/diabetes.48.7.1381. [DOI] [PubMed] [Google Scholar]
- 35.a) Dulong JL, Legallais C. Biotechnol Bioeng. 2007;96:990. doi: 10.1002/bit.21140. [DOI] [PubMed] [Google Scholar]; b) Gross JD, Constantinidis I, Sambanis A. J Theor Biol. 2007;244:500. doi: 10.1016/j.jtbi.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.a) Ceccaldi C, Fullana SG, Alfarano C, Lairez O, Calise D, Cussac D, Parini A, Sallerin B. Cell Transplant. 2012;21:1969. doi: 10.3727/096368912X647252. [DOI] [PubMed] [Google Scholar]; b) Tran NM, Dufresne M, Duverlie G, Castelain S, Defarge C, Paullier P, Legallais C. Tissue Eng Part A. 2013;19:103. doi: 10.1089/ten.TEA.2012.0139. [DOI] [PubMed] [Google Scholar]


