Abstract
Although we have known for decades that B cells contribute to immune responses by secreting antibody, it is now clear that B cells are more than simply factories for immunoglobulin production and that B cells also play key roles as modulators of T cell-dependent immunity. Indeed, the evidence showing that antigen-presenting and cytokine-producing B cells can alter the magnitude and quality of CD4 T cell responses continues to grow. In this article we review the data showing that B cells, working in partnership with dendritic cells, regulate the development of Th2 cells and the subsequent allergic response.
Introduction
In 1986 Mosmann and Coffman identified two CD4 T cell subsets, referred to as T helper 1 (Th1) and T helper 2 (Th2) cells, which express unique patterns of cytokines following restimulation (1). Subsequent work from many laboratories demonstrated that the development of these two CD4 T cell populations is reliant on different transcriptional programs and that the CD4 effectors play distinct roles during immune responses (2). For example, the IFNγ-producing Th1 cells are thought to be critical for elimination of intracellular pathogens while the IL-4-producing Th2 cells are believed to regulate immune responses to multicellular organisms like nematodes. Collectively, these findings established the backbone of the helper T cell differentiation hypothesis (3) and paved the way for the subsequent identification of additional T helper subsets including the IL-17 producing Th17 cells, the IL-10 producing regulatory T cells (Treg) and the IL-21 producing T follicular helper (TFH) cells (4). Each of these T cell subsets exhibits different functional properties and the development of each lineage is programmed by a distinct transcription factor (4). Although we know much about the molecular cues that initiate development of Th1, Treg and Th17 cells (4), our understanding of the signals that initiate the Th2 developmental pathway are less clear, despite almost three decades of intense study. In this review we discuss how dendritic cells (DCs) and B cells, working in concert, can initiate and sustain Th2 development.
Th2 development is regulated by multiple different cell types, including DCs and B cells
Effective priming of naïve CD4 T cells is dependent on “professional” antigen-presenting cells (APCs) that express co-stimulatory molecules and present antigen (Ag)-derived peptides complexed with Major Histocompatibility Complex Class II (MHCII) (5). DCs are thought to be the key professional APCs and are critical for T cell priming as transient depletion of DCs in vivo impairs naive CD4 T cell priming in most experimental settings (6). Not surprisingly, given the important role of DCs in CD4 T cell priming, DCs are also thought to provide signals that are critical for expression of the transcriptional factors that control the differentiation of the primed CD4 T cells into the different effector populations (7). For example, IL-12 producing mature DCs induce expression of the Th1 lineage specifying transcription factor, T-bet, in the primed CD4 T cells and this DC-dependent signal is required to induce full Th1 development (8). Likewise, it is reported that DCs are necessary to induce Th2 development (9, 10) and are also sufficient for Th2 differentiation as adoptive transfer of DCs, isolated from the lymph nodes (LNs) of animals exposed to house dust mite (HDM) allergen, into the lungs of naive mice is sufficient to induce a Th2 response in the mice following aerosol challenge with HDM (11). However, the paradigm that DCs are the only APC involved in Th2 development has been challenged with recent data showing that Ag presentation solely by DCs may not induce optimal Th2 development. For example, basophils, which express MHCII molecules and can produce the Th2 lineage inducing cytokine, IL-4, are reported to be sufficient to induce Th2 development (12). Although these findings are controversial, additional studies looking at mice in which DCs are the only cell type able to present peptide-MHC II complexes to T cells in vivo show that Th2 development is impaired following exposure to pathogens like Trichuris muris (13) or allergens like papain (14). Thus, the data suggest that additional APCs likely provide signals that facilitate the generation, expansion or maintenance of Th2 cells.
B cells, just like DCs, express MHCII and, when appropriately activated by Ag, cytokines and/or pathogen-derived TLR ligands, also upregulate co-stimulatory molecules and can present Ag to naive CD4 T cells (15). Although initial studies looking at allergic responses in the genetically B cell deficient μMT mouse strain suggest that B cells play no role in the development of the Th2 response (16, 17), later studies using additional pathogens and allergens reveal that B cells can, in some settings, modulate Th2 development. For example, Th2 development in response to infection with the helminth Heligmosomoides polygyrus is impaired in B cell deficient μMT mice and in transiently B cell depleted mice (18-20). Similarly, Th2 cytokines in the lung airways and tissue are significantly lower in μMT mice exposed to HDM and the HDM-exposed B cell deficient mice exhibit decreased airway hyperreactivity after methacholine challenge (21). Following an erythrocytic infection with Plasmodium chabaudi chabaudi, C57BL/6 mice develop a Th1 response that switches to a Th2-dominant response later in the primary response (22). By contrast, B cell deficient μMT mice continue to retain a Th1-like response to the malarial Ags throughout the primary infection, resulting in a chronic relapsing parasitemia (22). In other experimental settings, B cell deficiency is associated with immune deviation away from a Th2 polarized response toward a Th1-dominant response. For example, following exposure to Schistosoma mansoni eggs (23) or infection with T. muris (24), T cells from B cell deficient mice make an IFNγ dominant response. Likewise, following infection with Leishmania major LV39, CD4 T cells from normal BALB/c mice make IL-4 and IFNγ following restimulation while CD4 T cells from B cell deficient BALB/c mice produce only IFNγ, rendering this normally susceptible strain of mice now resistant to L. major (25). Taken together, the data demonstrate that B cells can influence the development and/or maintenance of the Th2 response.
B cells regulate Th2 development by presenting Ag and providing co-stimulation
Given the ability of B cells to specifically capture Ag bound to the B cell receptor (BCR), one obvious way in which B cells might modulate CD4 T cell responses is by presenting Ag and providing co-stimulation to CD4 T cells (15). Although studies examining mice in which only the B cells are competent to present Ag indicate that B cells have only a limited ability to initiate CD4 T cell responses following intravenous or subcutaneous vaccination (26), it is clear that the development and maintenance of the TFH lineage is highly dependent not only on B cells but on Ag-presenting, ICOS-L-expressing B cells that present Ag and provide co-stimulation (27, 28). Given the recent reports suggesting that Th2 cells and an IL-4 competent TFH subset may arise from a common precursor (29), some of the earlier studies showing alterations in the development of IL-4 producing T cells in B cell deficient mice may be attributable, at least in part, to an inability of these mice to sustain the IL-4 producing TFH cells (TFH2). However, recent studies from our lab (18) using transient B cell depletion in the H. polygyrus infection model to study the IL-4 producing TFH2 and the IL-4 + IL-13 producing Th2 cells in the LN and peripheral sites clearly demonstrate that B cells regulate the maintenance or expansion of both IL-4 producing cell lineages. It is tempting to speculate that B cells, in concert with DCs, regulate the development of the putative IL-4 competent TFH/Th2 precursor and that sustained interactions between B cells and this precursor reinforce the TFH developmental program, while transient and non-sustained interactions between B cells and the TFH/Th2 precursor favor commitment to the Th2 lineage. Nevertheless, it is clear that any reported roles for B cells in sustaining or expanding different CD4 effector populations – be they Th1, Th2, Th17 or Treg – will need to be carefully assessed to ensure that the changes observed are not due to the loss of B cell-dependent TFH cells that retain the ability to produce effector cytokines like IFNγ, IL-17 and IL-4.
Given the data from our lab (18) and others showing that Ag specific B cells regulate the development of the TFH and Th2 response in the LN and the magnitude of the Th2 response at effector sites, suggest that B cells are likely presenting antigen to the T cells. In support of this hypothesis, multiple publications report that CD4 Th2 effector responses are diminished in mice that are selectively unable to express MHCII in B lineage cells (B-MHCII−/− mice). For example, CD4 T cell expansion is reduced and IL-4 production by the restimulated T cells is greatly decreased in B-MHCII−/− mice following immunization with protein Ags in alum (30) and after repeated exposures to cockroach Ags (21). Likewise, Th2 primary and memory responses are significantly impaired in H. polygyrus-infected B-MHCII−/− animals (20). Interestingly, although Ag presentation by B cells is required for the development of both primary and memory Th2 responses following vaccination or infection, Th1 differentiation is reportedly normal in B-MHCII−/−mice (30), suggesting that B cells provide unique co-stimulatory signals that specifically promote or sustain Th2 development.
Several studies support the concept that particular co-stimulatory pathways enhance Th2 development. Indeed, experiments using CD28 deficient mice reveal that Th2 responses to some pathogens and allergens are impaired in the absence of CD28 signaling (31). Interestingly, these mice are competent to initiate normal Th1 responses to other Ags (31), suggesting that Th2 development is more dependent than Th1 development on engagement of CD28 by co-stimulatory CD80/CD86 ligands. Likewise, signaling through OX40 seems to be critical for Th2 differentiation in various experimental models (32-34) and studies with ICOS deficient mice and ICOS/ICOS-L blockade reveal a role for this T cell co-stimulatory pathway in Th2 differentiation (35). It is reported that OX40L expression by B cells is required for the development of a primary effector and memory Th2 response following vaccination with protein Ags in alum (36). Likewise, expression of both CD80 and CD86 by the B cells is required for the development of the primary Th2 response to H. polygyrus (19). Although the experiment to assess the role of ICOS-L expressing B cells in Th2 development has not been performed, prior studies show that ICOS-L expression by B cells is critical for TFH development and maintenance (28). Given the role for B cell-derived co-stimulation during the development of both TFH and Th2 cells, it is again tempting to speculate that B cells may provide co-stimulatory signals that promote the development of a common TFH2/Th2 precursor. Regardless, the collective data suggest a role for B cell-mediated co-stimulation during the development of Th2 responses.
Th2 development is not only influenced by the type of co-stimulation provided to the naive T cells by the APC but also by the strength of the TCR signal received by the T cell. Indeed, the combination of peptide/Ag dose and co-stimulation provided by the APC affects the balance between Th1 and Th2 development with low TCR signal strength interactions promoting Th2 development (37). For example, in vitro priming of naïve CD4+ T cells with low concentrations of MHCII-peptide complexes (38, 39) or low-affinity MHCII-peptide complexes (40) preferentially induces Th2 differentiation. Likewise, low affinity or avidity TCR stimulation promotes IL-4 independent expression of the Th2 master regulator transcription factor, GATA-3 (41). By contrast, stimulation with high concentrations of peptide abrogates early GATA-3 expression and prevents Th2 development (41). In agreement with the “low TCR-strength model”, the T2 ribonuclease/omega-1, a glycoprotein derived from Schistosoma mansoni egg with Th2 adjuvant-like properties, can condition DCs to drive T helper 2 (Th2) polarization by altering the ability of the DC to form stable conjugates with the T cell (42). This Ag-directed weak conjugate formation is presumed to decrease the TCR signal that the naïve CD4+ T cell can receive and thereby facilitate Th2 development. Likewise, it is thought that low expression of Ag-MHCII complexes on the surface of an APC may also decrease the stability of the DC:T cell conjugates (43), resulting in weaker TCR stimulation and favoring Th2 development. Supporting this concept, basophils, which express low levels of MHCII molecules, can act as APCs for CD4+ T cells, however these cells preferentially prime Th2 responses when tested in vitro and in vivo (12).
B cells, like basophils, may preferentially induce weak TCR stimulation. However, in the case of B cells, this is not due to constitutively low MHCII expression but rather to the unique ability of B cells to present Ag late in the immune response when Ag levels are declining (44, 45). B cells, unlike DCs or basophils, collect Ag in a specific fashion through binding of the Ag to the BCR (44). As the levels of soluble Ag decline following pathogen clearance, B cells become more efficient than DCs at capturing and presenting Ag to CD4 T cells (46). It is proposed that T cell priming occurs through sequential stable interactions between the responding T cells and different APCs (47). In a sequential model of T cell priming, DCs may initiate T cell priming and clonal expansion when Ag is present in excess whereas B cells may play a more important role in sustaining the CD4 T cell differentiation program when Ag becomes limiting. This model appears to describe TFH development, which is known to be dependent initially on Ag-presenting DCs for the first priming and lineage commitment steps and later on Ag-presenting B cells to complete the developmental process and sustain the lineage (27, 28). Given that B cells may be the predominant APC in LNs for new cohorts of T cells late in the primary response when Ag is limiting, it is likely that the number of MCHII-peptide complexes presented by the B cells to the T cells will be decreased relative to earlier in the response and that these new cohorts of Ag-specific T cells will receive a weaker TCR signal from the B cells. Strikingly, some pathogens, like malaria, induce an initial Th1 response, which spontaneously switches to a Th2 response later during the primary response (22). This switch from the Th1 dominant response to a Th2-driven response fails to occur in B cell deficient mice (22). However, this switch from Th1 to Th2 development clearly doesn't occur in all infection models, even as antigen becomes limiting, so there must be other signals beyond antigen availability that dictate Th2 development in response to malaria infection. Regardless, the data suggest that establishment of late cognate interactions between the B cells and CD4 T cells may facilitate the full commitment of the T cells to the Th2 program, both through the unique co-stimulatory signals provided by the B cells and through the selective low TCR signal strength interactions that take place late in the response.
Tuning the Th2 priming capacity of DCs by B cells
According to the current paradigm (7), Pathogen-Associated Molecular Pattern molecules (PAMPs), expressed by the pathogens, can activate Pathogen-Associated molecular pattern Recognition Receptors (PRRs), expressed by DCs. This interaction causes the up-regulation of MHCII and co-stimulatory molecules on the DCs and induces the DCs to secrete the Th1 polarizing cytokine, IL-12. Naive CD4 T cells, activated in the presence of IL-12 or IFNγ, upregulate T-bet and T-bet directs the responding T cells to differentiate into Th1 lineage cells and secrete Th1 cytokines like IFNγ (8). IL-12 can induce the differentiation of TFH cells with the ability to produce IFNγ (TFH1 cells, (48-51)). Interestingly, B cells can also produce IL-12 and IFNγ and, under some conditions, B cells are important for the expansion or maintenance of the Th1 response (52, 53). Given the important role for both DCs and B cells in the development and maintenance of TFH cells, it is possible that B cells and DCs cooperate to induce optimal TFH1 and Th1 responses.
A similar cytokine-dependent model (37) was originally proposed for Th2 development whereby the lineage-driving cytokine, IL-4, produced by the APC in response to pathogen-directed signals will induce upregulation of the Th2 lineage-specifying transcription factor GATA-3 in the T cell. However, the in vivo evidence to support the model that DCs are conditioned by pathogen signals to differentiate into a DC that produces Th2 polarizing cytokines (DC-2 cell) is limited (54). First, no specific PRRs, PAMPs or Damage-associated molecular pattern molecules (DAMPs) are known to initiate IL-4 production by DCs (54). Second, there is no compelling evidence to support the supposition that DCs must be the initial source of IL-4 for the T cells (37, 54) and it is now proposed that other cell types, including the newly identified type 2 innate lymphoid cells (ILC2) (55), basophils (12) or the conventional T cells themselves (37) may serve as the initial source of IL-4. Finally, IL-4R or STAT6 deficient CD4 T cells can develop into Th2 cells in some settings (37), indicating that IL-4 signals are not obligate for Th2 commitment. Indeed, these in vivo results are in agreement with the in vitro studies showing that weak TCR signals in the absence of IL-4 are sufficient to initiate Th2 development (39, 41). Thus, while IL-4 may be critical for expansion and maintenance of the Th2 response, early Th2 development to some pathogens and Ags may, in fact, occur independently of IL-4 signals provided by the APC.
If DCs are not instructed by the pathogen to make IL-4, how do DCs initiate Th2 induction and how might B cells provide Th2 conditioning signals to the DCs? Several studies suggest that Th2 development may occur as a default pathway when Th1 polarizing cytokines or PAMPs/DAMPs that can activate PRRs expressed by the DCs are lacking (56). Based on this model, Th2 differentiation will occur whenever DCs are activated in the absence of IL-12 inducing pathogen-derived adjuvants. Conversely, the model predicts that the presence of IL-12 at the site of contact between the T cells and Ag-presenting DCs will inhibit Th2 development even when the pathogen or Ag would normally promote the development of a Th2 response. B cells can secrete many cytokines (53), including IL-12 and IFNγ (52), both of which promote Tbet expression in T cells and Th1 differentiation (8), as well as IL-10 (57), which can suppress IL-12 production by DCs (58). Multiple studies suggest that B cells may be able to regulate the DC-mediated priming of Th2 responses by modulating the cytokine microenvironment. As one example, B cell deficient mice are reported to make IFNγ and not IL-4 following infection with the nematode T. muris (24). However, treatment of the infected μMT mice with blocking anti-IL-12 antibodies is sufficient to restore the normal Th2 response in the B cell deficient animals (24), suggesting that B cells may tune the Th2 priming capacity of the DCs by creating a Th2 permissive microenvironment. In support of this hypothesis, another study shows that adoptively transferred KLH-pulsed DCs from B cell deficient μMT mice are unable to induce the development of IL-4 producing CD4 effectors (59). This study further shows that DCs obtained from B cell deficient μMT mice secrete more IL-12p40 when compared to DCs obtained from control animals (59). The increased production of IL-12 by the DCs from the B deficient mice appears to be due to the loss of IL-10 producing B cells as treatment of the μMT DCs with IL-10 restores the IL-4 priming capacity of the DCs (59). Interestingly, mice containing B cells that are selectively deficient in IL-10 (60) or IL-2 (20) also show impaired Th2 responses. Whether cytokines produced by B cells control T cell polarization through direct signaling in the T cells, as might by the case for IL-2 (61), or by preventing the DCs from making IL-12, as might be the case for IL-10 (59, 62), is still unknown. However, it is clear that the loss of Th2 immunity observed in B cell deficient mice is not always associated with immune deviation toward a Th1 response. In fact, although B cell deficient mice show impaired Th2 responses after infection with H. polygyrus (18-20), exposure to allergens (21) or vaccination with Ags adsorbed in alum (30, 36), Th1 responses are not enhanced in these settings. These data suggest that B cells may be able to influence the capacity of DCs to prime Th2 responses by mechanisms other than simply preventing the production of Th1 polarizing cytokines by DCs.
B cell-dependent remodeling of LN architecture facilitates DC-dependent Th2 development
Encounters between naïve CD4 T cells and Ag-bearing APCs are regulated by a finely-tuned balance between expression of homeostatic chemokines by stromal cells in the LN and chemokine receptors expressed by the T cells and APCs (63). According to the accepted model (64), CCR7-expressing naïve T cells enter the T cell area of the LN through high endothelial venules (HEV) and are retained within this region of the LN by the ligands for CCR7, CCL19 and CCL21, which are produced by the stromal-derived fibroblastic reticular cells. DCs upregulate CCR7 in response to PRR activation and migrate in a CCR7-dependent fashion through the lymphatics to the LN (65). Upon entering the LN through the subcapsular sinus, the CCR7+ DCs migrate through the cortical sinuses past the B cell follicles to the CCL19/CCL21 expressing T cell zone of the LN where the DCs encounter T cells, present Ag and initiate T cell priming and differentiation (65). CXCR5-expressing B cells localize to the B cell follicles in close proximity to the follicular dendritic cells (FDCs) that produce the CXCR5 ligand, CXCL13 (66). Thus, the B cells are most likely to encounter T cells during the initial migration of the B cells from HEVs to the B cell follicles, at the borders between the T cell zone and B cell follicle (T/B border) or between B cell follicles in the interfollicular region (66). As might be expected, disrupting T cell/DC colocalization within the LN can affect T cell priming. In fact, primary T cell responses to several experimental Ags and pathogens is impaired in CCR7 deficient mice (63). However, plt/plt mice, which lack the CCR7 ligands, CCL19 and CCL21a, can make normal or even exacerbated Th2 responses (18, 67), suggesting that Th2 differentiation may not need to take place within the T cell area of the LN and/or that the APC that initiates a Th2 response may not need to express CCR7 at the time of priming. Interestingly, multiple studies show that Th2 cells preferentially accumulate at the interface of the T/B border (19, 68), whereas Th1 cells are found primarily within the T cell area (68, 69). The preferential localization of Th2 cells in close proximity to B cells suggests that unique signals or cells that facilitate the development of Th2 responses might be present in this specialized interfollicular microenvironment. This view is supported by the observation that initial priming of Th2 responses following Nippostrongylus brasiliensis infection occurs at the border of the B cell follicle and T cell zone, and not in the T cell area (19). Recent work from our lab using the H. polygyrus infection system further supports the model that Th2 differentiation requires the close interactions between CD4 T cells and B cells. In fact, we find that CXCR5 is rapidly upregulated on pathogen-responsive CD4 T cells following H. polygyrus infection and that these T cells accumulate in the interfollicular areas and T/B border (18). The altered localization profile of the CD4 T cells is controlled by Ag-activated B cells (18) that produce Lymphotoxin-α (LT) – a cytokine that induces expression of the CXCR5 ligand, CXCL13, by the FDCs via a positive feedback loop (70). Thus, in the absence of Ag-specific B cells or B cell-derived LT, activated CD4 T cells fail to localize to the interfollicular region or T/B border of the LN and Th2 development is significantly impaired.
Interestingly, the CD4 T cells are not the only cell type to reposition near the B cell follicles. In fact, following H. polygyrus infection, the intestinal-derived migratory DCs upregulate expression of CXCR5 and gradually lose expression of CCR7 (18). These DCs are preferentially retained in the subcapsular sinus and interfollicular areas of the LN and appear unable to be recruited to or retained in the T cell zone (18). Furthermore, similar to what we observe for the CD4 T cells, the positioning of the DCs near the B cell follicle is controlled by the LT-expressing B cells, which regulate expression of CXCL13 in the reactive LN (18). More importantly, eliminating CXCR5 expression on either the DCs or the CD4 T cells is sufficient to prevent optimal TFH and Th2 development following H. polygyrus infection (18). Although one can argue that deleting CXCR5 expression in either the DCs or T cells simply prevents the co-localization of these cells, we find that blocking CXCR5 signaling in both cell types results in co-localization of the DCs and T cells in the T cell zone – yet Th2 development is still impaired (18). Collectively, these data indicate that following H. polygyrus infection, DCs and CD4 T cells are both instructed, potentially by pathogen-derived Ags, to upregulate CXCR5 and co-localize in close proximity to the CXCL13-expressing stromal cells and B cells. This early encounter between naïve CD4 T cells with DCs cells near the T/B border, which is controlled by the LT-producing B cells, appears to be an important determinant for Th2 commitment.
One remaining question is why the co-localization of DCs and T cells near the B cell follicles preferentially favors Th2 development. One possibility is that the CXCR5+ DCs do not receive critical signals in the T cell zone that condition the DCs to induce Th1 differentiation and that these DCs induce Th2 differentiation via the “default pathway” described earlier (56). In support of this possibility, engagement of CCR7 on DCs by CCL19 induces upregulation of costimulatory molecules and secretion of IL-12 by the DCs (71) – all of which favor the preferential priming of Th1 responses. Thus, the CXCR5+ DCs may not receive sustained CCR7-dependent activation signals and are simply unable to produce sufficient IL-12 to drive a Th1 response.
Alternatively, we can speculate that a positive signal delivered by CXCL13 alters the activation/maturation program of the DCs in a way that facilitates the Th2 priming capacity of the DCs. Finally, it is possible that the specialized microenvironment in the interfollicular areas and T/B border may specifically condition the DCs or T cells in a way that favors Th2 development. We can envision a scenario whereby serial cognate interactions between T cells and the DCs and the ICOS-L- and OX40-expressing B cells at the T/B border might specifically favor Th2 development. Alternatively, perhaps interactions between DCs and B cells or between DCs with other cells located in the interfollicular area including mast cells (37) and marginal reticular cells (72), might condition the Ag-bearing DCs to initiate Th2 development. Although we still do not understand how this specialized microenvironment promotes Th2 induction, the data indicate that one of the major, and completely unexpected, ways in which B cells can regulate Th2 development is by producing a cytokine that is required to orchestrate the CXCL13-dependent encounters between the Ag-bearing DCs and T cells in close proximity to the Ag-activated and Ag-presenting B cells.
Conclusions
Until recently, the role(s) for B cells in regulating T cell-mediated immunity have not been well appreciated. However, over the last decade it has become increasingly clear that B lineage cells can, in defined infection and autoimmune settings, regulate the development, maintenance and/or expansion of multiple effector and memory CD4 T cell subsets including Th1, TFH, Treg (reviewed in (53)), Th17 (73) and, as discussed here, Th2 cells. In the case of Th2 development, the data suggest that B cells can play at least three separate roles (Fig. 1). First, B cells through their ability to present antigen and provide specific co-stimulatory signals to T cells, can cooperate with antigen-presenting IL-12 non-producing DCs to initiate and sustain Th2 development. Second, B cells can produce cytokines, like IL-2 and IL-10, which act on the T cells or the DCs to promote Th2 development and prevent alternate fate decisions. Finally, B cells can ramp up the LT:CXCL13 positive feedback loop in the LN to facilitate the co-localization of DCs, CD4 T cells and B cells within the interfollicular region of the LN – a microenvironment that appears to favor Th2 development. Although the experiments supporting a role for B cells in Th2 development have come from animal models, the use of B cell depletion therapy to treat patients suffering from a number of chronic, supposedly T cell-mediated, inflammatory diseases (74) reveals that human CD4 T cell responses are also regulated by B cells. Although B cell depletion therapies have not been tested in humans suffering from Th2-mediated inflammatory diseases, patients with x-linked agammaglobulimia (XLA), who have decreased numbers of mature B cells, are reported to exhibit a skewed Th1 profile with reduced Th2 activity (75). These data and the data from B cell depleted and B cell deficient mice strongly suggest that B cells do play an important role in modulating Th2 responses. In the future, a better understanding of the mechanism(s) by which B cells influence the development and maintenance of Th2 responses may provide insights into potential B cell-, LT- or CXCL13-directed therapeutic approaches to treat Th2 mediated inflammatory diseases.
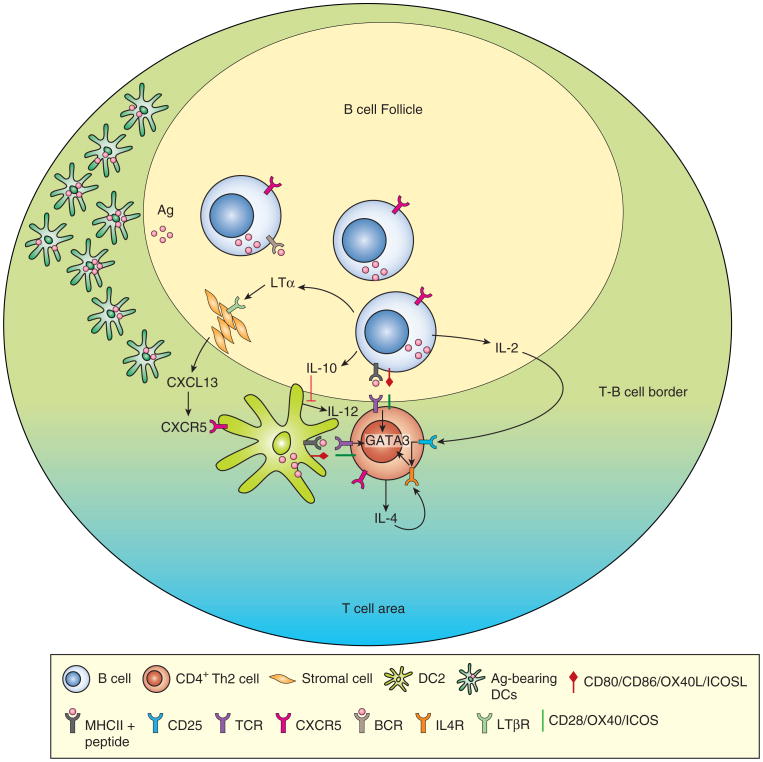
Figure 1. Cooperation between B cells and DCs can facilitate Th2 development in response to nematode infection.
The accumulating data suggest that DCs are necessary, but not always sufficient, to initiate the priming of naive CD4 T cells and the initial commitment of the primed T cells to the Th2 developmental pathway. Antigen-specific activated B cells can play an integral and cooperative role in this DC-dependent process by producing LT, which induces increased CXCL13 production by the LN stromal cells. Nematode infection induced activation of the LT:CXCL13 positive feedback loop facilitates the recruitment and retention of antigen-bearing, CXCR5-expressing DCs and CXCR5-expressing CD4 T cells near the B cell follicles. Localization of the DCs, T cells and B cells in this LN microenvironment allows for exchange of cytokine and co-stimulatory signals between these three cell types and with other cells, such as the CXCL13-producing stromal cells that are present in this specialized niche. These location-dependent signals favor development of IL-4 producing TFH and Th2 cells and prevent the initiation of alternate effector fates.
Acknowledgments
Funding: This work was supported by National Institutes of Health grant NIAID R01 AI104725 to F.E. Lund and B. León.
Abbreviations used in this article
- DAMPs
Damage Associated Molecular Patterns
- DC
Dendritic Cell
- FDC
Follicular Dendritic Cell
- HDM
House Dust Mite
- HEV
High Endothelial Venule
- ILC2
Type 2 Innate Lymphoid Cell
- LN
Lymph Node
- LT
Lymphotoxin alpha
- PAMPs
Pathogen Associated Molecular Patterns
- PRR
Pattern Recognition Receptor
- TFH
T follicular helper
- Th1
T helper 1
- Th2
T helper 2
Footnotes
Disclosures: The authors have no financial conflicts of interest to disclose
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Liew FY. T(H)1 and T(H)2 cells: a historical perspective. Nat Rev Immunol. 2002;2:55–60. doi: 10.1038/nri705. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hivroz C, Chemin K, Tourret M, Bohineust A. Crosstalk between T lymphocytes and dendritic cells. Crit Rev Immunol. 2012;32:139–155. doi: 10.1615/critrevimmunol.v32.i2.30. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 8.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–801. doi: 10.1016/j.jaci.2013.07.046. quiz 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leon B, Ballesteros-Tato A, Misra RS, Wojciechowski W, Lund FE. Unraveling effector functions of B cells during infection: the hidden world beyond antibody production. Infect Disord Drug Targets. 2012;12:213–221. doi: 10.2174/187152612800564437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 17.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–387. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]
- 18.Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Liu Z, Rozo CT, Hamed HA, Alem F, Urban JF, Jr, Gause WC. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindell DM, Berlin AA, Schaller MA, Lukacs NW. B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. PLoS One. 2008;3:e3129. doi: 10.1371/journal.pone.0003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci U S A. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez HJ, Wang Y, Stadecker MJ. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J Immunol. 1997;158:4832–4837. [PubMed] [Google Scholar]
- 24.Blackwell NM, Else KJ. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infect Immun. 2001;69:3860–3868. doi: 10.1128/IAI.69.6.3860-3868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronet C, Voigt H, Himmelrich H, Doucey MA, Hauyon-La Torre Y, Revaz-Breton M, Tacchini-Cottier F, Bron C, Louis J, Launois P. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J Immunol. 2008;180:4825–4835. doi: 10.4049/jimmunol.180.7.4825. [DOI] [PubMed] [Google Scholar]
- 26.Archambault AS, Carrero JA, Barnett LG, McGee NG, Sim J, Wright JO, Raabe T, Chen P, Ding H, Allenspach EJ, Dragatsis I, Laufer TM, Wu GF. Cutting edge: Conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J Immunol. 2013;191:545–550. doi: 10.4049/jimmunol.1201598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballesteros-Tato A, Randall TD. Priming of T follicular helper cells by dendritic cells. Immunol Cell Biol. 2014;92:22–27. doi: 10.1038/icb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 29.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 30.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 31.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 32.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 33.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coyle AJ, Gutierrez-Ramos JC. The role of ICOS and other costimulatory molecules in allergy and asthma. Springer Semin Immunopathol. 2004;25:349–359. doi: 10.1007/s00281-003-0154-y. [DOI] [PubMed] [Google Scholar]
- 36.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- 41.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 44.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 45.Malynn BA, Romeo DT, Wortis HH. Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation. J Immunol. 1985;135:980–988. [PubMed] [Google Scholar]
- 46.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13:1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 47.Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–191. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 48.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 50.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, Schwartzberg PL, Hager GL, O'Shea JJ. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 53.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 55.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72 Suppl 2:ii80–84. doi: 10.1136/annrheumdis-2012-202253. [DOI] [PubMed] [Google Scholar]
- 58.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 61.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 64.Cyster JG. Leukocyte migration: scent of the T zone. Curr Biol. 2000;10:R30–33. doi: 10.1016/s0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 65.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 66.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grinnan D, Sung SS, Dougherty JA, Knowles AR, Allen MB, Rose CE, 3rd, Nakano H, Gunn MD, Fu SM, Rose CE., Jr Enhanced allergen-induced airway inflammation in paucity of lymph node T cell (plt) mutant mice. J Allergy Clin Immunol. 2006;118:1234–1241. doi: 10.1016/j.jaci.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 68.Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286:2159–2162. doi: 10.1126/science.286.5447.2159. [DOI] [PubMed] [Google Scholar]
- 69.Kalies K, Blessenohl M, Nietsch J, Westermann J. T cell zones of lymphoid organs constitutively express Th1 cytokine mRNA: specific changes during the early phase of an immune response. J Immunol. 2006;176:741–749. doi: 10.4049/jimmunol.176.2.741. [DOI] [PubMed] [Google Scholar]
- 70.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 71.Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, Nakano H, Nembrini C, Saudan P, Kopf M, Bachmann MF. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 73.Bermejo DA, Jackson SW, Gorosito-Serran M, Acosta-Rodriguez EV, Amezcua-Vesely MC, Sather BD, Singh AK, Khim S, Mucci J, Liggitt D, Campetella O, Oukka M, Gruppi A, Rawlings DJ. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORgammat and Ahr that leads to IL-17 production by activated B cells. Nat Immunol. 2013;14:514–522. doi: 10.1038/ni.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 75.Amedei A, Romagnani C, Benagiano M, Azzurri A, Fomia F, Torrente F, Plebani A, D'Elios MM, Del Prete G. Preferential Th1 profile of T helper cell responses in X-linked (Bruton's) agammaglobulinemia. Eur J Immunol. 2001;31:1927–1934. doi: 10.1002/1521-4141(200106)31:6<1927::aid-immu1927>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]