Abstract
Purpose
This phase I study was conducted to determine the dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) for the combination of bortezomib and alvocidib in patients with B-cell malignancies (multiple myeloma [MM], indolent lymphoma, Waldenstrom's macroglobulinemia, and mantle cell lymphoma).
Experimental Design
Patients received bortezomib (intravenous push), followed by alvocidib (1-hour infusion), on days 1, 4, 8, and 11 of a 21-day treatment cycle. Patients experiencing responses or stable disease continued on treatment at the investigator's discretion. A standard 3+3 dose-escalation design was used to identify the MTD based on DLTs, and pharmacokinetic and pharmacodynamic studies were conducted.
Results
A total of 44 patients were enrolled, with 39 patients assessed for response. The MTD was established as 1.3 mg/m2 for bortezomib and 40 mg/m2 for alvocidib. The most common hematologic toxicities included leukopenia, lymphopenia, neutropenia, and thrombocytopenia. The most common non-hematologic toxicities included diarrhea, fatigue, and sensory neuropathy. Three complete remissions (8%) and 10 partial remissions (26%) were observed for a total response rate of 33%. Pharmacokinetic findings with the current dosing regimen were consistent with the comparable literature and the hybrid dosing regimen. Pharmacodynamic study results did not correlate with clinical responses.
Conclusions
The combination of bortezomib and alvocidib is tolerable and an MTD has been established for this schedule. The regimen appears to be efficacious in patients with relapsed/refractory MM or indolent non-Hodgkin's lymphoma. As the non-hybrid regimen is less cumbersome than the previous hybrid dosing schedule regimen, the current schedule is recommended for successor studies.
Keywords: Alvocidib, bortezomib, B-cell neoplasm, clinical trial
Introduction
Despite numerous therapeutic options (e.g., cytotoxic chemotherapy, monoclonal antibodies, radioimmunotherapy, and combinations thereof) for patients with indolent B-cell non-Hodgkin lymphoma (NHL), cures largely remain elusive. Allogeneic stem cell transplantation (SCT) using reduced-intensity conditioning (RIC) regimens, which carry a lower treatment-related mortality (TRM) risk than myeloablative regimens, can theoretically produce cures via a graft-versus-lymphoma effect, but definitive evidence of efficacy of this strategy is lacking (1). Similarly, while proteasome inhibitors and immunomodulatory drugs have dramatically altered the therapeutic landscape in multiple myeloma (MM), the disease remains incurable aside from allogeneic SCT. Although RIC regimens have lowered the high TRM-associated with myeloablative conditioning, convincing evidence is lacking that allogeneic SCT improves survival compared with autologous SCT, and the former is only recommended in the context of clinical trials (2). Novel therapeutic approaches are, therefore, clearly needed for patients with these diseases.
Bortezomib, the first-in-class proteasome inhibitor, received regulatory approval in the United States in 2003 after large phase II and III trials demonstrated promising single-agent activity in patients with relapsed or refractory MM (3, 4). Its mechanism of action (MOA) is partly mediated through nuclear factor-kappa B (NF-κB) inhibition that results in apoptosis, decreased angiogenic cytokine expression, and interference with the tumor microenvironment. Additional MOAs include c-Jun N-terminal kinase activation and growth factor expression modulation (5). Subsequent combination phase III studies established the drug for frontline MM therapy (6). Based on a 33% response rate in a large multicenter phase II trial (7), bortezomib was also approved for patients with relapsed or refractory mantle cell lymphoma (MCL). More recently, regimens combining bortezomib with chemoimmunotherapy have yielded high response rates (83-88%) in patients with relapsed/refractory indolent B-cell NHL and MCL (8, 9).
As perturbations of the cell cycle are almost universal in human malignancies, the cyclin-dependent kinases (CDKs) have become attractive targets for cancer therapy (10, 11). Alvocidib (flavopiridol), the first CDK inhibitor to enter the clinic, globally represses transcription by non-ATP-competitive inhibition of positive transcription elongation factor b (P-TEFb, CDK9/cyclin T1) (12), inducing down-regulation of the short-lived anti-apoptotic protein myeloid cell leukemia-1 (Mcl-1), which may represent the drug's principal MOA in malignant hematopoietic cells (13). Indeed, in MM cells, for which Mcl-1 is an essential survival protein (14), alvocidib induces apoptosis through Mcl-1 transcriptional repression and down-regulation (15).
Alvocidib has been administered via a variety of schedules (10, 11). While the single-agent activity in patients with MCL or MM using 1-hour intravenous bolus administration was modest to nonexistent (16, 17), marked clinical efficacy was noted in studies in patients with genetically high-risk chronic lymphocytic leukemia (CLL) using a pharmacologically-derived “hybrid” administration schedule (18, 19). However, hybrid schedule results were disappointing in a phase I trial in patients with relapsed/refractory acute leukemias (20). Similarly, hybrid dosing did not improve results compared to a 1-hour bolus infusion in a phase I trial of alvocidib, fludarabine, and rituximab that reported an overall response rate of 82% in patients with MCL, B-NHL, or CLL (21). Finally, a recent randomized phase II comparison of bolus and hybrid dosing of alvocidib (followed sequentially by cytarabine and mitoxantrone) in patients with newly diagnosed poor-risk acute myeloid leukemia (AML) showed comparably encouraging results for both schedules, prompting selection of a bolus administration for successor studies (22).
Malignant cells are highly susceptible to a strategy in which survival signaling and cell cycle regulatory pathways are simultaneously disrupted (23). Furthermore, Mcl-1 accumulation is an undesirable molecular consequence of proteasome inhibitor exposure, attenuating proapoptotic effects, and arguing that targeting Mcl-1 may increase the effectiveness of these agents (24). In support of these concepts, bortezomib and alvocidib interact synergistically to induce apoptosis in various leukemic cells (25, 26). In this context, alvocidib also inhibits IκB kinase, and by extension, NF-κB (27), and in this way may cooperate with bortezomib in triggering malignant hematopoietic cell death (26).
Given these observations, a multicenter phase I trial of this novel drug combination was initiated in patients with recurrent or refractory B-cell neoplasms. Patients received bortezomib by intravenous push followed by a 1-hour infusion of alvocidib on days 1, 4, 8, and 11. Based on encouraging results in the CLL trial (18, 19), a hybrid schedule of alvocidib as a 30-minute bolus infusion followed by a 4-hour infusion on days 1 and 8 was explored and MTD determined (28). During this time the original non-hybrid schedule of this trial was put on hold without reaching the MTD and accrual was resumed after completion of the hybrid schedule (28). The results of the original non-hybrid schedule are described in this manuscript.
Patients and Methods
Drug supply
Bortezomib (PS-341; NSC 681239; Millennium Pharmaceuticals, Inc., Whitehouse Station, NJ) and alvocidib (flavopiridol; NSC 649890; Sanofi-Aventis Pharmaceuticals, Inc., Bridgewater, NJ) were obtained through the Cancer Therapy Evaluation Program (CTEP) Pharmaceutical Management Branch, NCI.
Patient eligibility
Patients must have had a diagnosis of relapsed or refractory follicle center lymphoma (follicular or diffuse), mantle cell lymphoma (MCL), marginal zone B-cell lymphoma (splenic, nodal or extranodal), lymphoplasmacytoid lymphoma/immunocytoma, plasma cell myeloma, plasmacytoma, plasma cell leukemia, or Waldenstrom's macroglobulinemia with no potential curative therapeutic options . Patients with a history of a central nervous system involvement were ineligible. Prior autologous SCT, but not prior allogeneic SCT, was allowed. Prior bortezomib was also allowed.
Additional eligibility criteria included being at least 18 years of age, an Eastern Cooperative Oncology Group performance status (ECOG) score of 1 or less, no grade 2 or greater neuropathy, preserved kidney and liver function, hemoglobin levels of 8 g/dL or higher, an absolute neutrophil count of 1.5 × 109/L or greater, and platelet counts of 100 × 109/L or greater.
Treatment plan
This study was designed as a phase I, nonrandomized, dose-escalation study to determine the MTD for the bortezomib and alvocidib combination, where alvocidib is administered as a 1-hour infusion. The dose of alvocidib ranged from 15 mg/m2 at dose level 1 to 90 mg/m2 at dose level 9. The dose of bortezomib for dose level 1 was 1.0 mg/m2 and for dose levels 2 through 9 was 1.3 mg/m2. On days 1, 4, 8, and 11 of a 3-week cycle, bortezomib was administered via intravenous push over 3 to 5 seconds, after which alvocidib was administered as an intravenous infusion over 1 hour. Treatment was repeated every 3 weeks.
Disease status was assessed after the first 6 weeks of treatment and every 6-8 weeks thereafter. Patients experiencing a response or stable disease were allowed to continue treatment indefinitely at the investigator's discretion. Patients received full supportive care including herpes zoster prophylaxis. No specific precautions for TLS or cytokine release syndrome (CRS) were used prior to August 2007, but upon resumption of accrual to the non-hybrid regimen in May 2010, specific measures were applied. Pretreatment with allopurinol and overnight in-hospital observation for CRS and TLS monitoring were strongly encouraged for all patients. Clinical discretion was used to determine if more stringent tumor lysis precautions were indicated based on tumor burden and risk. For any signs of CRS, patients were treated with dexamethasone and prophylaxis was encouraged for those with circulating tumor cells documented by routine blood count.
Study design, definition of dose-limiting toxicity, and identification of the MTD
The standard 3+3 dose-escalation design was used, with a dose level expansion of up to 6 evaluable patients if a dose-limiting toxicity (DLT) was noted. The MTD was defined as the highest dose level at which fewer than 2 of 6 patients experienced a DLT.
A DLT was initially defined as any of the following occurring during the first cycle of treatment and determined to be possibly, probably, or definitely related to study treatment: (1) grade 4 anemia; (2) grade 4 absolute neutrophil count or platelet toxicity of greater than one week duration; (3) grade 3 or greater febrile neutropenia, and (4) any grade 3 or greater nonhematologic adverse events except grade 3 or greater nausea and vomiting without prophylactic and/or symptomatic treatment, grade 3 or greater diarrhea without maximum opioid and/or octreotide treatment, and grade 3 or greater infections, opportunistic infection with grade 2 or greater lymphopenia (specifically herpes zoster or herpes simplex infection). A protocol amendment (October 2008) modified the DLT definition to include instances in which both drugs are omitted due to dose modifications on at least two days of the scheduled drug administration during the first cycle.
Toxicity evaluation
NCI Common Terminology Criteria for Adverse Events (CTCAE) v3.0 was used for reporting adverse events until April 1, 2011, when mandatory conversion to CTCAE v4.0 supervened.
Response evaluation
Response criteria used were based on the nature of the disease as follows: (a) patients with lymphomas were evaluated using the NCI-sponsored Working Group Lymphoma Response Criteria (29); (b) patients with plasma cell myeloma or plasmacytoma were evaluated according to European Group for Blood and Bone Marrow Transplant (EBMT) criteria (30); (c) patients with plasma cell leukemia were evaluated according to the criteria of Vela-Ojeda et al (31); and (d) patients with Waldenstrom's macroglobulinemia were evaluated according to the Second International Workshop on Waldenstrom's macroglobulinemia criteria (32).
Alvocidib pharmacokinetic studies
Samples from the first 29 enrolled patients were obtained for alvocidib pharmacokinetic analysis. The samples were obtained prior to and following treatment on Cycle 1 Day 1 and Cycle 3 Day 8 according to the following schedule: pre-infusion, immediately post-infusion, and 1, 2, 4, 8, 12, and 24 hours post-infusion. Alvocidib concentrations were analyzed as previously described (28).
Enrichment of CD138+ MM cells from bone marrow
Paired bone marrow aspirates were obtained from MM patients only: (1) prior to initial study treatment, and (2) on Day 2 of Cycle 1, approximately 24 hours following initial treatment. CD138+ MM cells were enriched from bone marrow as previously described (28).
Protein extraction and Western blot analysis
Protein extraction and Western blot analyses with primary antibodies to pJNK, Mcl-1, XIAP, and GAPDH were done as previously described (28). A PARP-specific antibody (BD Pharmingen) was also included. An Odyssey Imager (LI-COR Biosciences) was used to quantify binding of IRDye 680LT-conjugated secondary antibodies (LI-COR Biosciences).
Localization and quantitative immunofluorescence of p65/RelA
RelA belongs to a family of transcription factors of NF-kappa-B complex. Cytospin preparations of enriched CD138+ plasma cells were prepared from study patients before and during treatment. The cells were fixed, stained, and visualized by fluorescence microscopy as previously described (28). Analysis of NF-kB within the nucleus was performed using the Definiens® Developer v1.5 software suite (Definiens, Munich, Germany). The nuclear region was determined with automatic threshold segmentation on the DAPI and Alexa 488 stains; further refined for plasma cells by the size and shape of the nuclei. Mean pixel intensity of the Alexa 488 signal was determined from the nuclei of the plasma cells.
Statistical analysis
Basic demographics, adverse events, DLTs, dose levels, clinical responses were summarized by basic descriptive statistics such as frequency, proportion, mean, median, and range. Pharmacokinetic and pharmacodynamic analyses including all biomarkers and p65/ReIA were limited to descriptive statistics.
Human investigation studies
Studies were performed after Institutional Review Board approval and in accordance with an assurance filed with and approved by the Department of Health and Human Services. Informed consent was obtained from each patient. ClinicalTrials.gov trial registration ID: NCT00082784.
Results
Patient characteristics
A total of 44 patients, 12 female and 32 male, were enrolled on study between March 2004 and July 2012 (Table 1). The study participants included 35 Caucasians (including 4 Hispanics) and 9 African-Americans (including 1 Hispanic). Median age of patients was 64.5 years (range: 40-79). Twenty-four patients were diagnosed with MM, 2 were diagnosed with Waldenstrom's macroglobulinemia (WM), and 18 were diagnosed with NHL (5 of whom had MCL, an NHL subset). The median number of prior treatments was 3, with a range of 1 to 10. Ten patients had received prior autologous SCT and 10 patients had prior bortezomib therapy. Patients received a median of 5 cycles of study treatment, with a range of 1-16 cycles.
Table 1.
Patient enrollment and characteristics
| Gender | No. of patients |
| Female | 12 |
| Male | 32 |
| Total | 44 |
| Race | No. of patients |
| Black or African American | 9 |
| White | 35 |
| Ethnicity | No. of patients |
| Hispanic or Latino | 5 |
| Non-Hispanic | 39 |
| Age Range | Years |
| Median | 64.5 |
| Range | 40-79 |
| Performance Status | No. of patients |
| 0 | 21 |
| 1 | 23 |
| Diagnosis | No. of patients |
| Multiple Myeloma | 24 |
| Waldenstrom's macroglobulinemia | 2 |
| NHL (subset of NHL-Mantle Cell Lymphoma) | 18 (5) |
| Prior Treatment | No. of regimens |
| Mean | 3.4 |
| Median | 3 |
| Range | 1-10 |
| Prior Treatment | No. of patients |
| Autologous stem cell transplantation | 10 |
| Bortezomib | 10 |
| Study Treatment | No. of cycles initiated |
| Mean | 4.5 |
| Median | 5 |
| Range | 1-16 |
Safety and tolerability
Myelosuppression was a common hematologic toxicity and typically manifested itself as grade 3 leukopenia (30%), lymphopenia (25%), neutropenia (34%), or thrombocytopenia (27%) (Table 2). Grade 4 neutropenia (23%) and thrombocytopenia (14%) were also commonly observed. The most common non-hematologic toxicities included grade 3 diarrhea (20%) and fatigue (16%). Grade 3 pain and grade 3 sensory neuropathy were slightly less commonly experienced. One patient experienced grade 4 sensory neuropathy. A patient with follicular lymphoma developed grade 3 oral mucositis/esophagitis (which lasted more than 7 days) on Day 2 of Cycle 1, associated with both paraneoplastic pemphigus and a grade 4 CD4 lymphocyte count without HIV infection. The patient's prior treatment regimen was rituximab and bendamustine. The most common grade 2 hematologic and non-hematologic toxicities, occurring in at least 20% of patients are listed in Table 2. Prior to initiation of prophylactic antiviral treatment with acyclovir or comparable agents, 2 patients experienced grade 3 herpes zoster.
Table 2.
Hematologic and nonhematologic toxicities occurring during any treatment cyclea
| Events/Patients |
|||
|---|---|---|---|
| Hematologic toxicities | Grade 2b | Grade 3 | Grade 4 |
| Anemia | 24/13 | 1/1 | 0/0 |
| Leukopenia | 46/20 | 29/13 | 6/3 |
| Lymphopenia | 43/12 | 18/11 | 1/1 |
| Neutropenia | 33/20 | 33/15 | 15/10 |
| Thrombocytopenia | 50/24 | 19/12 | 9/6 |
| Events/Patients |
|||
|---|---|---|---|
| Nonhematologic toxicities | Grade 2b | Grade 3 | Grade 4 |
| Anorexia | 16/12 | ||
| CD4 lymphocytes decreased | 1/1 | 0/0 | |
| Dehydration | 3/3 | 0/0 | |
| Diarrhea | 33/20 | 9/9 | 0/0 |
| Esophagitis | 1/1 | 0/0 | |
| Fatigue | 53/27 | 8/7 | 0/0 |
| Febrile neutropenia | 2/2 | 0/0 | |
| Hypoglycemia | 2/1 | 0/0 | |
| Hypotension | 1/1 | 0/0 | |
| Infection: blood (normal ANC) | 0/0 | 1/1 | |
| Infection: peripheral nerve (zoster)c | 2/2 | 0/0 | |
| Mucosal Infection | 1/1 | 0/0 | |
| Mucositis oral/pharyngeal | 1/1 | 0/0 | |
| Muscle weakness LE | 1/1 | 0/0 | |
| Myalgia | 1/1 | 0/0 | |
| Nausea | 10/9 | 1/1 | 0/0 |
| Neuropathy: sensory | 11/9 | 5/5 | 1/1 |
| Neuropathy: motor | 1/1 | 0/0 | |
| Oral dysesthesia | 1/1 | 0/0 | |
| Pain: Abdomen | 1/1 | 0/0 | |
| Pain: Back | 1/1 | 0/0 | |
| Pain: Bone | 1/1 | 0/0 | |
| Pain: Extremity | 3/3 | 0/0 | |
| Pain: Kidney | 1/1 | 0/0 | |
| Pain-neuralgia/peripheral nerve | 3/2 | 0/0 | |
| Pneumonia | 1/1 | 0/0 | |
| Syncope | 1/1 | 0/0 | |
| Tumor Lysis Syndrome | 1/1 | 0/0 | |
| Weight loss | 1/1 | 0/0 | |
Only those toxicities deemed possibly, probably, or definitely related to the treatment are included in the table.
Only grade 2 toxicities that occurred in at least 20% of the patients are reported.
Cases appeared prior to a protocol amendment dated Jan 2005, which included use of prophylactic antiviral treatment for herpes zoster.
DLT and MTD
In the initial enrollment phase to the non-hybrid schema, 1 DLT was seen at dose level 5, necessitating expansion to 6 DLT-evaluable patients (Table 3). No other DLTs were noted in that expansion cohort. Patient enrollment continued through 3 DLT-evaluable patients at dose level 7 before the trial was interrupted and switched to the evaluation of the hybrid schema. Upon return to the non-hybrid schema, the 3+3 dose-escalation design of the study allowed progression through dose level 9. DLT accumulation at subsequent higher dose levels required a de-escalation to lower dose levels until the MTD was reached at dose level 5. Grade 3 DLTs, experienced by 1 patient each, included back pain, fatigue, peripheral neuropathy, febrile neutropenia, TLS, diarrhea, and esophagitis/oral mucositis. TLS was manifested by short-lived laboratory perturbations (elevated phosphate and uric acid) resolving within 12 hours without sequelae or recurrence. One patient (dose level 9) experienced neutropenia that prompted dose omission of 2 scheduled doses of both agents (due to grade 4 ANC on day 4 and grade 3 ANC on day 8). Although grade 4 neutropenia lasting less than 7 days did not meet DLT criteria, omission of 2 scheduled doses of both investigational agents for this adverse event did meet DLT criteria. The MTD for a schedule of drug administration on days 1, 4, 8 and 11 of a 21-day cycle was determined to be 1.3 mg/m2 bortezomib (iv push) and 40 mg/m2 alvocidib (1-hour infusion).
Table 3.
Dose Levels and DLTs
| Dose Level | Bortezomib (mg/m2) | Alvocidib (mg/m2) | Patients treated/# DLTs | DLT |
|---|---|---|---|---|
| 1 | 1.0 | 15 | 3/0 | |
| 2 | 1.3 | 15 | 5/0 | |
| 3 | 1.3 | 22 | 3/0 | |
| 4 | 1.3 | 30 | 3/0 | |
| 5a | 1.3 | 40 | 7/1 | Grade 3 back pain |
| 6b | 1.3 | 50 | 6/2 | Grade 3 fatigue Grade 3 peripheral neuropathy |
| 7b | 1.3 | 60 | 8/2 | Grade 3 febrile neutropenia Grade 3 tumor lysis syndrome |
|
The protocol underwent a mandatory amendment at dose level 7 (prior to reaching the MTD of the pre-hybrid schedule) to pursue a hybrid dosing schedule (28). After determining the MTD of the hybrid-dosing schedule, accrual resumed to the pre-hybrid dosing schedule at dose level 8. Patients were enrolled through dose level 9, when DLTs required retrograde expansion of dose levels 8 through 6 in order to find the MTD of the pre-hybrid schedule. | ||||
| 8b | 1.3 | 75 | 6/2 | Grade 3 diarrhea Grade 3 esophagitis/oral mucositis |
| 9b | 1.3 | 90 | 3/2 | Grade 3 febrile neutropenia Omission of 2 scheduled treatments due to neutropeniac |
Maximum tolerated dose (MTD)
Exceeded MTD
Cycle 1 Day 4 treatment omitted for grade 4 ANC; Cycle 1 Day 8 treatment omitted for grade 3 ANC. Because the grade 4 ANC lasted less than 7 d, it was not by itself a DLT by the criteria in the protocol; however, it met DLT criteria by prompting 2 dose omissions in Cycle 1.
Disease response
Although this study was not powered to assess response, 3 complete responses (CRs) and 10 partial responses (PRs) were observed among the 39 patients evaluable for response (overall response rate = 33%; Table 4). The overall response rate was similar for both NHL patients (33%) and MM patients (32%), with a 50% response rate in a limited sample of patients with WM. CRs were experienced by 2 (one of whom had mantle cell lymphoma) of the 15 NHL patients (13%) and 1 of the 24 MM patients (4%). The MM patient with the CR had previously been treated with bortezomib. PRs were achieved in 26% of the patients—3 with NHL (20%), 6 with MM (27%), and 1 with WM (50%). A total of 20 patients had stable disease (SD) as best response and 6 patients had progressive disease (PD). Among the patients not evaluable for response, 3 patients came off treatment for adverse events prior to the disease assessment interval, 1 patient came off treatment in Cycle 1 due to a second cancer (renal) and did not have a response assessment, and 1 patient came off treatment in Cycle 1 for convenience (moved far from the treatment center) and did not have response assessment
Table 4.
Treatment response by diagnosisa
| Diagnosis | NHL | MM | WM | Total |
|---|---|---|---|---|
| (n=15) | (n=22) | (n=2) | (n=39) | |
| Complete Response (CR) | 2b,c | 1d | 0 | 3 |
| Partial Response (PR) | 3 | 6 | 1 | 10 |
| Total CR + PR (%) | 5 (33%) | 7 (32%) | 1 (50%) | 13 (33%) |
| Stable Disease (SD) | 7 | 12 | 1 | 20 |
| Progressive Disease (PD) | 3e | 3 | 0 | 6 |
Excluded from the totals are 5 patients who were not evaluable for response: 3 patients came off treatment for adverse events prior to the disease assessment time point, 1 patient came off treatment in Cycle 1 due to a second cancer (renal mass) and did not have a response assessment, and 1 patient came off treatment in Cycle 1 for convenience (moved a far distance from the treatment center) and did not have a response assessment.
Includes 1 patient with prior autologous SCT
Includes 1 patient with mantle cell lymphoma
Includes 1 patient with prior bortezomib
Includes 3 patients with mantle cell lymphoma
Pharmacokinetic studies
Samples were collected from the first 29 patients enrolled to the non-hybrid schedule. Alvocidib pharmacokinetics were evaluated using a non-compartmental (model-independent) approach. A sample immediately at the end of the 1-hr alvocidib infusion was only collected in 11 of the 29 patients. Consequently, a CMAX could be reported for these 11 patients. Of these 11 patients, only 9 had sufficient data to calculate a terminal elimination rate for the determination of half-life, clearance, and AUCINF. No lag in peak alvocidib concentration (CMAX) was observed. Maximum alvocidib concentration was observed immediately at the end of the infusion (Supplemental Fig. 1). Cycle 1 Day 1 alvocidib pharmacokinetic parameters per dose level are shown in Supplemental Table 1.
Pharmacodynamics
To determine the feasibility of measuring pharmacodynamic markers as a predictor of response, pre- and post-treatment samples (24 hours after the first doses of drugs) were obtained from the MM patients for quantitative analysis of p65/RelA nuclear localization by immunofluorescence staining (Fig. 1) and for quantitative analysis of pJNK, Mcl-1, PARP, and XIAP by Western blotting (Fig. 2). Changes in expression of these proteins were previously observed in cells co-exposed to alvocidib and bortezomib in vitro (25).
Figure 1.
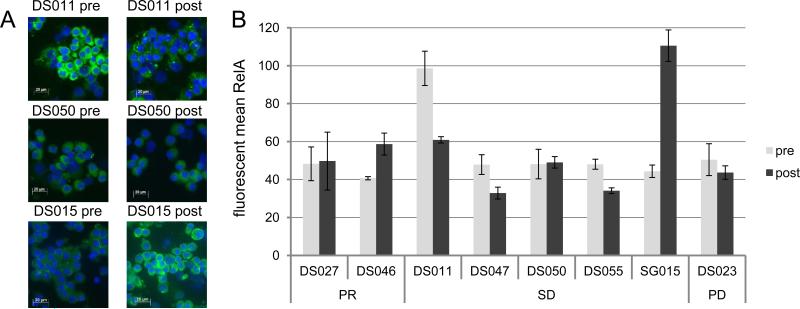
Expression of p65/RelA in plasma cells obtained from MM patients. A. p65/RelA fluorescent microscopy of plasma cells isolated from 3 patients with stable disease. Bone marrow aspirates were collected prior to treatment and 24 hours following treatment, and plasma cells were stained for RelA (green) and DAPI (blue). Relative to the pre-treatment samples, sample DS011 displays a decrease in expression, sample DS050 displays no change in expression, and sample SG015 displays an increased in expression. B. Expression of nuclear p65/RelA was analyzed by immunofluorescence as in Panel A. The mean pixel intensity for RelA is shown (mean ± SD). PR: partial response; SD: stable disease; PD: progression disease.
Figure 2.
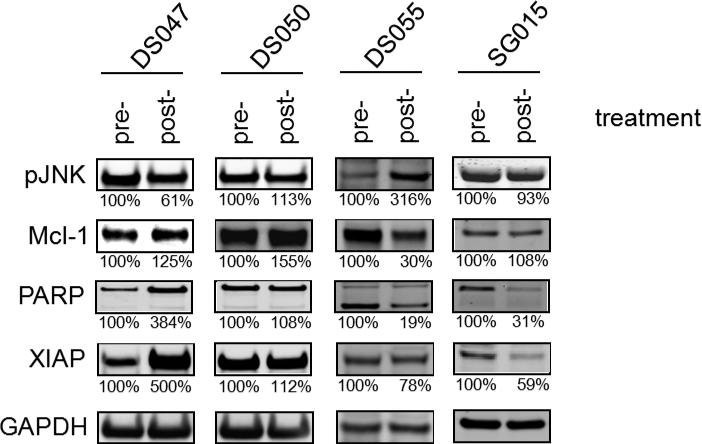
Pharmacodynamic analysis of pre-treatment and post-treatment bone marrow samples. CD138+ cells were isolated at baseline (pre-treatment) and 24 hours after the first dose of alvocidib and bortezomib treatment (post-treatment). Whole cell lysates were prepared, electrophoresed on a 4% to 12% NuPAGE gel, and Western blotted with primary antibodies to pJNK, Mcl-1, PARP, XIAP, and GAPDH. Following development with an IRDye 680LT-conjugated (LI-COR) secondary antibody, the fluorescent signals were quantitated on an Odyssey Imager. Values below the figures represent the level of assayed proteins determined by densitometric analysis normalized to GAPDH levels, where the normalized level of the pre-treatment sample is defined as 100%.
Cytospin preparations of enriched CD138+ plasma cells were prepared from the pre- and post-treatment bone samples from 8 MM patients including 2 patients from the PR group (DS027 and DS046), 5 patients from the SD group (DS011, DS047, DS050, DS055, and SG015), and 1 patient from the PD group (DS023). NF-κB activation was measured by monitoring changes in nuclear p65/RelA localization, as a function of mean pixel intensity, in pre- and post-treatment samples as shown in Fig.1A. Minimal changes in nuclear RelA localization were detected in 3 patient samples (DS027, DS050 and DS023). Down-regulation of nuclear p65/RelA after treatment was observed in 3 patient post-treatment samples (DS011 with 38% lower expression, DS047 with 31% lower expression, and DS055 with 29% lower expression). Up-regulation of nuclear RelA was observed in 2 patients (DS046 with 44% higher expression, and SG015 with 149% higher expression).
Expression levels of Mcl-1, pJNK, PARP, and XIAP in the pre- and post-treatment CD138+ cells from 4 patients (DS047, DS050, DS055, and SG015) in the SD group were determined by Western blot analysis (Fig. 2). Although some samples exhibited high magnitude changes in the post-treatment sample in comparison to the pre-treatment sample (e.g., an 81% decrease of PARP for patient DS055; a 500% increase in XIAP for patient DS047), there was no correlation of the changes with clinical activity as consistent increases or decreases in expression were not observed for any of the proteins across all of the patients.
Discussion
In the original study of the alvocidib/bortezomib regimen initiated in March 2004, 29 patients were first enrolled to the non-hybrid schedule at dose levels 1-7 without reaching the MTD. In 2007, the protocol underwent a CTEP-recommended amendment to proceed to a hybrid schedule based on promising data in CLL (19). Results from the hybrid schedule trial have been published (28). A total of 16 patients were treated, consisting of 11 male and 5 female patients. Nine patients had NHL (6 patients had mantle cell lymphoma subtype), 6 had multiple myeloma, and 1 had an extramedullary plasmacytoma. Treatment was on a 21-day cycle: bortezomib by intravenous push on days 1, 4, 8, and 11; and alvocidib on days 1 and 8 by 30-minute bolus infusion followed by a 4-hour continuous infusion. The MTD was established as 1.3 mg/m2 for bortezomib and 30 mg/m2 for alvocidib (both the 30-minute bolus and 4-hour infusions). There were 2 CR (12%) and 5 PR (31%) observed (overall response rate = 44%). After determining the MTD for the hybrid schedule (28), data from the pre-hybrid schedule were re-evaluated and determined to demonstrate a similar response and toxicity profile. With the agreement of CTEP, the non-hybrid schema trial was re-opened to patient enrollment in May of 2010.
The results of this study indicate that this schedule of the combination of bortezomib and alvocidib can be administered safely to patients with recurrent or refractory indolent B-cell neoplasms (including NHL and MM). The MTD was determined to be 1.3 mg/m2 of bortezomib (intravenous push) followed by 40 mg/m2 of alvocidib (1-hour infusion) on days 1, 4, 8, and 11 on a 21-day cycle (Table 3). In a previous hybrid study of these agents in the same patient population, the MTD was found to be 1.3 mg/m2 of bortezomib (intravenous push) on days 1, 4, 8, 11 followed by a hybrid schedule of 30 mg/m2 of alvocidib (30-minute infusion) and then 30 mg/m2 of alvocidib (4-hour infusion) on days 1 and 8 of a 21 day cycle (28).
The most common hematologic toxicities for both the current and previous study involved myelosuppression (including grade 3 leukopenia and lymphopenia) and grades 3 and 4 neutropenia and thrombocytopenia [Table 2 and reference (28)]. Likewise, the non-hematologic toxicity profile for both studies was very similar with grade 3 diarrhea, fatigue, and sensory neuropathy most frequently reported. One of the DLTs in the current regimen was grade 3 laboratory TLS in 1 patient with Waldenstrom's Macroglobulinemia at dose level 7 (Table 2). The event was managed with IV fluids and laboratory abnormalities normalized within 12 hours. This patient received a total of 6 cycles of the treatment and achieved a PR. No TLS was observed with the hybrid schedule (28). DLTs for both studies included grade 3 fatigue and febrile neutropenia, while the previous study also reported a single case of grade 3 aspartate aminotransferase elevation. Two patients on the present study developed herpes zoster (Table 2), but no additional episodes were reported following prophylactic antiviral therapy. Similarly, no episodes of herpes zoster were reported in the hybrid study, which included prophylactic antiviral therapy. (28). Patients received a median of 5 cycles of treatment on the current study (Table 1) compared to a median of 4 cycles of treatment on the previous study (28). Thus, both study regimens displayed a similar safety profile.
Although the primary objective of this phase I study was not to determine the efficacy of the treatment regimen, 3 CRs (NHL = 2, MM = 1) and 10 PRs (NHL = 3, MM = 6, WM = 1) were observed for an overall CR+PR response rate of 13 patients out of 39 evaluable patients (33%) (Table 4). This compares to an overall CR+PR response rate of 7 patients out of 16 evaluable patients (total = 44%, NHL = 33%, MM = 57%) in the previous study of the hybrid regimen (28). As the MM patient in the present study who achieved a CR had previously progressed on bortezomib, this raises the possibility that either alvocidib exhibited single agent activity, or, as previously observed in in vitro studies(25), it may have cooperated with bortezomib to lower the cell death threshold. Previously reported response rates for bortezomib as a single agent in relapsed/refractory disease are approximately 33% for MCL (7), 35% MM (3), and 13.3% for indolent NHL including follicular lymphoma, marginal zone lymphoma, and small lymphocytic lymphoma (33). Because this study was not powered to characterize efficacy, firm conclusions about the activity of the combination of bortezomib and alvocidib in comparison to bortezomib as a single agent cannot be drawn. Nevertheless, the response rates in this phase I trial, which involved multiply-treated relapsed/refractory patients, are encouraging and warrant further study.
This non-hybrid schedule produced comparable pharmacokinetic parameter values as the hybrid schedule (28). Furthermore, the pharmacokinetics of the non-hybrid schedule were consistent with published results using 1-hr infusion schedule (34). Alvocidib pharmacokinetics in the absence of bortezomib were not evaluated in this clinical trial. Consequently, direct comparison cannot be made. However PK parameters are in very close agreement with a previous study (34, 35) for dose-normalized CMAX, dose-normalized AUC, half-life, and clearance. Together, these results suggest that the administration of bortezomib immediately prior to the alvocidib infusion does not affect its disposition.
Based on previous findings demonstrating that bortezomib and alvocidib interact synergistically to induce apoptosis in human leukemia cells (25, 26), it was plausible to postulate that co-administration of these agents in vivo to patients with B-cell malignancies might induce analogous perturbations in apoptotic-regulatory pathways in tumor cells as those previously observed in vitro. To assess the feasibility of acquiring the samples, and to begin evaluating pharmacodynamic markers of biological effects of treatment, CD138+ plasma cells were obtained from the bone marrow of the MM patients, both prior to and 24 hours following treatment with bortezomib and alvocidib. Because bortezomib can inactivate NF-κB, which may activate the JNK pathway (26) as well as down-regulate XIAP (36), translocation of the p65/RelA subunit of NF-κB to the nucleus and the accumulation of pJNK and XIAP were selected as candidate markers. Similarly, because alvocidib can attenuate Mcl-1 cytoprotective effects by preventing proteasome inhibitor-mediated Mcl-1 accumulation (25), total Mcl-1 protein levels were monitored. Finally, PARP cleavage was used as an indicator of apoptosis.
Of 24 MM patients on the study, immunofluorescence staining for p65/RelA was conducted on 8 paired plasma cell samples and Western blot analysis for pJNK, Mcl-1, PARP, and XIAP was conducted on 4 paired plasma cell samples. Among the samples assayed for nuclear p65/RelA expression, there was no clear pattern of expression associated with disease status. Although a decrease in expression was observed in 3 samples (all PRs), an increase in expression was observed in 2 samples (1 PR and 1 SD) (Fig. 1). Similarly, among the 4 paired samples (all SD) analyzed by Western blot, and despite the fact that single-agent alvocidib has been shown to diminish Mcl-1 expression (37), only one sample showed a decrease in expression. While this result was unanticipated, it is possible that in this instance, diminished Mcl-1 proteasomal degradation may have predominated. Although a major mode of action of the bortezomib/alvocidib regimen is presumed to be apoptosis induction, only sample SG015 (from a patient with SD) displayed enhanced post-treatment PARP cleavage. Whether the inconclusive results seen here reflect the small sample size, differing drug doses, or the possibility that these molecules may not be robust markers of therapeutic activity cannot be determined in the context of this phase I trial. While bone marrow sampling was mandatory for patients with myeloma, it proved logistically cumbersome, thereby limiting the number of samples obtained. These questions could possibly be answered in more a more highly powered phase II trial with larger patient numbers and more uniform drug dosing.
In conclusion, this phase I study has determined the MTD for the combination of bortezomib and alvocidib administered as a 1-hour infusion in patients with recurrent or refractory indolent B-cell cancers. Both the observed hematologic and non-hematologic toxicities were similar to those observed with bortezomib as a single agent combined with a hybrid dose schedule of alvocidib (28). The dosing regimen employed here resulted in 3 CRs and 10 PRs in a heavily pretreated patient population. The frequency of these CRs and PRs was encouraging and similar to that observed in a previous trial of these agents that employed a more cumbersome hybrid dosing schedule (28). Because the simpler regimen used in the current trial demonstrated comparable activity to the hybrid dosing scheme, and because the nature and frequency of the toxicities associated with the regimens did not differ substantively, the results reported here argue for selection of the non-hybrid regimen for successor combination trials of alvocidib. As the toxicities of the current regimen were not inconsequential, plans are under development for a new phase I trial of alvocidib and a next-generation orally-active proteasome inhibitor, MLN9708, administered in conjunction with the non-hybrid schedule of alvocidib in MM patients who have progressed following any proteasome inhibitor treatment.
Supplementary Material
Statement of Translational Relevance.
Preclinical studies with alvocidib (a cyclin-dependent kinase inhibitor) and bortezomib (a proteasome inhibitor) have shown that these agents interact synergistically to induce apoptosis in malignant hematopoietic cells. The clinical relevance of this finding for patients with B-cell neoplasms was previously tested in a phase I trial in which the dose-limiting toxicities (DLTs) and maximum tolerated dose of the combination of these agents was determined, with alvocidib administered by a “hybrid”, pharmacologically-derived infusional schedule. The phase I trial described here extends this study by administering alvocidib according to a standard and considerably more easily managed bolus schedule. Here, we report that the two regimens were associated with similar toxicity and response profiles, including responses in patients who had previously progressed following bortezomib treatment. Consequently, based on the results of the present trial, the non-hybrid dosing schedule is recommended for subsequent phase II evaluation.
Acknowledgments
The authors thank Sarah Kolla (VCU) for assistance with the pharmacodynamic studies, Erin Gardner (NCI) for her assistance with the pharmacokinetic studies, and Joe Johnson (Moffitt) for his valuable assistance in the Analytical Microscopy Core Facility supported by NCI P30 CA76292.
Grant Support: The study was supported by grants from the National Institutes of Health (NCI R01 CA93738, NCI R01 CA100866, NCI R21 CA110953, NCI R01 CA167708, NCI P50 CA130805 [Lymphoma SPORE], NCI P30 CA016059 [Cancer Center Support Grant to Massey Cancer Center], NCI P30 CA76292 [Cancer Center Support Grant to H. Lee Moffitt Cancer Center & Research Institute], NCI N01 Contract HHSN261201100100C for the Southeast Phase 2 Consortium, NCRR M01 RR000065 [GCRC], and National Center for Advancing Translational Sciences KL2TR000057 [CTSA]) and an award from the Multiple Myeloma Research Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest: G. David Roodman is a consultant for Amgen and receives research funding from Eli Lilly; John D. Roberts has a research contract with Selexys, Inc.
References
- 1.Chakraverty R, Mackinnon S. Allogeneic transplantation for lymphoma. J Clin Oncol. 2011;29:1855–63. doi: 10.1200/JCO.2010.32.8419. [DOI] [PubMed] [Google Scholar]
- 2.Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, Perez-Simon JA, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–30. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 7.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 8.Fowler N, Kahl BS, Lee P, Matous JV, Cashen AF, Jacobs SA, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study. J Clin Oncol. 2011;29:3389–95. doi: 10.1200/JCO.2010.32.1844. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg JW, Vose JM, Kelly JL, Young F, Bernstein SH, Peterson D, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–12. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 12.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–9. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 13.Grant S, Dent P. Gene profiling and the cyclin-dependent kinase inhibitor flavopiridol: what's in a name? Mol Cancer Ther. 2004;3:873–5. [PubMed] [Google Scholar]
- 14.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–9. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 15.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–38. [PubMed] [Google Scholar]
- 16.Kouroukis CT, Belch A, Crump M, Eisenhauer E, Gascoyne RD, Meyer R, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–5. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Gertz MA, Lacy MQ, Geyer SM, Fitch TR, Fenton RG, et al. Flavopiridol in patients with relapsed or refractory multiple myeloma: a phase 2 trial with clinical and pharmacodynamic end-points. Haematologica. 2006;91:390–3. [PubMed] [Google Scholar]
- 18.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni W, Albanese KA, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95:1098–105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TS, Blum KA, Fischer DB, Mitchell SM, Ruppert AS, Porcu P, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–23. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp JE, Garrett-Mayer E, Estey EH, Rudek MA, Smith BD, Greer JM, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012;97:1736–42. doi: 10.3324/haematol.2012.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant S, Dent P. Simultaneous interruption of signal transduction and cell cycle regulatory pathways: implications for new approaches to the treatment of childhood leukemias. Curr Drug Targets. 2007;8:751–9. doi: 10.2174/138945007780830764. [DOI] [PubMed] [Google Scholar]
- 24.Nencioni A, Hua F, Dillon CP, Yokoo R, Scheiermann C, Cardone MH, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005;105:3255–62. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene. 2003;22:7108–22. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 26.Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–18. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 27.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem. 2004;279:4750–9. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 28.Holkova B, Perkins EB, Ramakrishnan V, Tombes MB, Shrader E, Talreja N, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin Cancer Res. 2011;17:3388–97. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 30.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 31.Vela-Ojeda J, Garcia-Ruiz Esparza MA, Rosas-Cabral A, Padilla-Gonzalez Y, Garcia-Chavez J, Tripp-Villanueva F, et al. Intermediate doses of melphalan and dexamethasone are better than vincristine, adriamycin, and dexamethasone (VAD) and polychemotherapy for the treatment of primary plasma cell leukemia. Ann Hematol. 2002;81:362–7. doi: 10.1007/s00277-002-0480-5. [DOI] [PubMed] [Google Scholar]
- 32.Weber D, Treon SP, Emmanouilides C, Branagan AR, Byrd JC, Blade J, et al. Uniform response criteria in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:127–31. doi: 10.1053/sonc.2003.50037. [DOI] [PubMed] [Google Scholar]
- 33.Di Bella N, Taetle R, Kolibaba K, Boyd T, Raju R, Barrera D, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–80. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 34.Tan AR, Headlee D, Messmann R, Sausville EA, Arbuck SG, Murgo AJ, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol. 2002;20:4074–82. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Tan AR, Yang X, Berman A, Zhai S, Sparreboom A, Parr AL, et al. Phase I trial of the cyclin-dependent kinase inhibitor flavopiridol in combination with docetaxel in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:5038–47. doi: 10.1158/1078-0432.CCR-04-0025. [DOI] [PubMed] [Google Scholar]
- 36.Dai Y, Rahmani M, Dent P, Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol. 2005;25:5429–44. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.