Abstract
The widely used immunosuppressant cyclosporin A (CsA), a potent calcineurin inhibitor, significantly increases the incidence of cancer in organ transplant patients. Calcineurin signaling is an important mediator of VEGF signaling in endothelial cells. Negative regulation of calcineurin by its endogenous inhibitor, Down Syndrome Candidate Region-1 (DSCR1), suppresses tumor growth and angiogenesis, in contrast to the effect observed after long-term CsA treatment. Despite the significance of calcineurin signaling in endothelial cells, the consequences of CsA on tumor angiogenesis has not been investigated. Using an in vivo model of skin carcinogenesis, prolonged treatment with CsA promoted tumor growth and angiogenesis. The addition of CsA to endothelial cells in vitro increased proliferation and migration in a calcineurin-independent manner and is associated with increased mitochondrial reactive oxygen species (ROS). Co-treatment with antioxidants significantly abrogated CsA-induced endothelial cell activation. Furthermore, mice treated with antioxidants were protected against CsA-mediated tumor progression. Taken together, these findings suggest that CsA affects endothelial cells in a calcineurin-independent manner to potentiate tumor growth by promoting tumor angiogenesis through increasing mitochondrial ROS production. This work identifies a previously undescribed mechanism underlying a significantly adverse off-target effect of CsA and suggests that co-treatment with antioxidants would inhibit the tumor promoting effects of CsA.
Keywords: calcineurin-independent, cyclosporin A, angiogenesis, mitochondria
Introduction
Organ transplantation has revolutionized treatment for end stage organ failure. Cyclosporin A (CsA) is a commonly used immunosuppressant for organ transplant patients (1). CsA binds to intracellular cyclophilins, complexing specifically with cyclophilin A to inhibit calcineurin, a calcium responsive ser/thr phosphatase that is best known for its dephosphorylation and activation of the nuclear factor of activated T-cells (NFAT) family of transcription factors. Activation of the T-cell receptor increases intracellular calcium and results in NFAT-induced transcription of key cytokines such as IL-2, IFNγ and IL-4, critical for the expansion and function of effector T cells. Inhibition of calcineurin activity with CsA results in potent suppression of the adaptive immune response (2). However, an adverse side effect of patients who remain on chronic CsA treatment is an elevated risk of malignancies. The incidence of skin cancers is significantly increased, with a ≥65 fold increase in risk when compared to the rest of the population (1,3). The progression of skin cancers in CsA-treated patients is significantly more aggressive, with an increased incidence of metastasis and a poor prognosis (3). With advances in post-transplant care, patients receiving allografts are living longer and post-transplant malignancies are one of the major causes of morbidity and mortality in this population (4).
The increased incidence in cancer observed in transplant recipients has been attributed to CsA’s function as an immunosuppressant leading to the loss of tumor immunosurveillance. This is supported by an increased incidence of human papillomavirus observed in transplant recipients with skin cancers as compared to skin cancers in patients not on immunosuppressive therapy (5). In contrast, patients on FK506 (tacrolimus) for immunosuppression have a significantly lower incidence of cancer post-transplant as compared to patients on CsA (1) suggesting that while important, immunosuppression alone is not sufficient to promote tumorigenesis. CsA treatment has also been shown to potentiate tumor growth and metastasis of xenograft tumors in SCID mice (6). These studies demonstrate that while CsA mediated immunosuppression contributes to tumorigenesis, CsA also has pro-tumor effects independent of a functional adaptive immune system.
A few studies have examined the immune-independent mechanisms of CsA-induced tumorigenesis. CsA has been shown to suppress both apoptosis and the expression of DNA repair proteins after UV exposure (7,8), permitting the persistence of sporadic mutations while evading cell death. In another study, oncogenic Ras-induced senescence is circumvented by CsA inhibition of calcineurin-NFAT signaling in keratinocytes leading to ATF3-dependent suppression of p53 (9). CsA has also been shown to increase TGF-β production by tumor cells, leading to enhanced tumor invasion and metastasis in SCID mice (6).
The role of the calcineurin pathway in tumor progression is complicated by the identification of the endogenous calcineurin inhibitor DSCR1, (also known as RCAN1). Increased expression of DSCR1 has been shown by us and others, to inhibit tumor growth by blocking VEGF-calcineurin-NFAT signaling in endothelial cells attenuating tumor angiogenesis (10–15). The physiological relevance of this pathway was illustrated in Down Syndrome mouse models whereby chromosome 21 encoded inhibitors of the calcineurin-NFAT pathway, including Dscr1, are overexpressed blocking tumor angiogenesis and tumor progression (10). Despite the importance of calcineurin signaling in endothelial cells, the effect of CsA on endothelial cells in a tumorigenic context has not been extensively examined.
CsA is an 11 amino acid fungal-derived peptide and although it binds to cyclophilin A to inhibit calcineurin, it also binds to other cyclophilin family members. Cyclophilins are a family of 16 conserved members with intracellular peptidyl-prolyl cis-trans isomerase activity functioning mostly as chaperone proteins (16). Cyclophilins have been associated with breast and lung cancers, but their role in tumorigenesis is not understood (17). Cyclophilin D is located in the mitochondrial inner membrane as a component of the mitochondrial permeability transition pore (MPTP). Binding of CsA to cyclophilin D has been shown to be cytoprotective against several forms of mitochondrial mediated cell death (18,19) and alters the flux of mitochondrial contents through the MPTP. Changes in mitochondrial physiology can have a range of effects on endothelial cells since the endothelium is particularly sensitive to changes in oxidative state, activating signaling pathways that affect vascular tone, permeability, and angiogenesis (20,21). An elevation in reactive oxygen species (ROS) levels due to altered mitochondrial metabolism has been shown to promote tumorigenesis by increasing mitogen-activated protein kinase (MAPK) signaling to a proliferative level, directly promoting the cell division of cancer cells (22), or through stabilization of hypoxia inducible factor and increased tumor angiogenesis (23). The effect of CsA binding to cyclophilin D on the MPTP in endothelial cells has not yet been examined in a non-apoptotic context.
Here we show that CsA promotes tumorigenesis by increasing tumor angiogenesis in a calcineurin-independent manner. Using an in vivo skin carcinogenesis model, we demonstrate that CsA potentiates tumor growth by upregulating tumor angiogenesis as evidenced by increased microvessel density. Our data demonstrates that CsA treatment stimulates a proliferative and migratory phenotype in endothelial cells and is associated with elevated mitochondrial ROS. Pharmacological quenching of CsA-induced ROS with antioxidants is sufficient to abolish CsA-induced endothelial cell proliferation and migration in vitro and tumor growth in vivo. Our findings suggest that prophylaxis treatment with an anti-oxidant to target ROS may decrease transplant-associated malignancies without affecting CsA’s immunosuppressive capabilities.
Materials and Methods
Reagents
NIM811 was a gift from Novartis. CsA, FK506, N-acetyl cysteine (NAC) and Angiotensin II were from Sigma-Aldrich. Mn(III)tetrakis(4-benzoic acid)porphyrin Chloride was from Millipore.
Animals and Tumor Studies
WT C57Bl/6 mice were from The Jackson Laboratory. Dscr1 targeted transgenic mice (10) and Calcineurin Bf/f mice were previously described (24). Mice were orally gavaged daily with 10mg/kg of CsA oral solution USP modified or 0.15mg/kg FK506 (Hospital of University of Pennsylvania Pharmacy) diluted in peanut oil or simple syrup, respectively, starting 7–14 days prior to tumor initiation, or supplemented with 40mM NAC in the drinking water starting 14 days prior to CsA treatment. Chemically induced papillomas (25) and B16-F10 melanoma xenograft tumors (10) were generated as previously described. B16-F10 melanoma cells, originally from ATCC, were authenticated to be of C57Bl/6 murine origin using microsatellite markers (RADIL) in 2011. Mice were euthanized if mice became moribund or tumors became ulcerated prior to experimental endpoint. Mice were 8–12 weeks old. All animal experiments were performed according to protocols approved by the University of Pennsylvania IACUC.
Immunofluorescence and CD31 quantification
Tumors were harvested from mice and frozen in OCT freezing medium (Tissue-Tek), then sectioned for staining as previously described (26). Primary and secondary antibody was rat anti-mouse CD31 antibody (1:50, BD Biosciences) and Alexa 594 goat anti-rat (1:2000, Invitrogen), respectively. Five random 10X magnification pictures were taken of each slide and the area of CD31+ structures, visible lumens, total vessels, and vessels ≥100µm were counted. Images were taken with a 10× or 20× magnification objective lens and with a digital camera AxioCAM HRc (Zeiss, Thornwood, CT) mounted on Zeiss Imager M1 Axio using Zeiss AxioVision Acquisition software (version 4.5).
Primary endothelial cell isolation
Primary murine lung endothelial cells (LuEC) were isolated from 3–4 week old mice as previously described (27).
In vitro proliferation and TUNEL apoptosis assays
LuEC were plated in triplicate at 5 × 103 cells per well in 0.1% gelatin coated 24-well tissue culture plates. Cells were counted by a coulter counter (Beckman). For 5-bromo-2-deoxyuridine (BrdU) labeling, 2.5 × 103 LuEC were plated onto 0.1% gelatin-coated glass coverslips and then serum starved for 24 hours, then incubated with drug treatment and then pulsed with 10µM BrdU (BD Biosciences) for 1.5 hours. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.1% TBST, and denatured with 2N HCL. Endothelial cells were stained with anti-BrdU mouse antibody (1:50, Dako). Secondary antibody was Alexa 594 goat anti-rat (1:2000, Invitrogen) and nuclei were identified with Hoechst33342 (1:1000, Invitrogen). Seven random 10X magnification pictures were taken of each slide using 10× or 20× magnification objective lens with a digital camera AxioCAM HRc (Zeiss, Thornwood, CT), BrdU positive cells and total cell number were counted. In situ terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) labeling was performed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) according to manufacturer's instructions. Flow cytometry of TUNEL stained cells was performed on a FACS Canto flow cytometer (BD Biosciences) and analyzed with FlowJo Software (TreeStar, Ashland, OR).
Migration Assay
Basal endothelial media (27) with 0.5% FBS and lacking ECGs was placed in the lower chamber of a modified Boyden chamber (Corning) separated by a 8µm pore filter. LuEC were serum starved overnight, treated with experimental reagents for 2 hours and 2 × 104 cells were plated in the upper chamber and allowed to migrate for 2–4 hours at 37°C. Filters were stained with Diff-Quick solution (Baxter, Miami, FL) and the cells that migrated across the filter were counted in five random images taken at 10× or 20× magnification objective lens and with a digital camera AxioCAM HRc (Zeiss, Thornwood, CT).
AdCre infection
LuEC from CnBf/f mice were infected with Cre recombinase adenovirus (Ad5CMVCre; University of Iowa, Gene Transfer Vector Core) at 500 MOI overnight, followed by PBS wash and media change.
Mitosox and TMRE Staining
Mitosox (Invitrogen) and TMRE (Life Technologies) staining on LuEC were performed following manufacturer’s instructions. Mitosox fluorescence was detected by flow cytometry using a FACS Canto flow cytometry machine reading at 610 or 613 nm and TMRE fluorescence was detected reading at 575 nm. Data were analyzed with FlowJo software.
Western blotting
Whole LuEC lysates were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with the following antibodies: p-ERK, total ERK, p-AKT, total AKT (Cell Signaling Technologies), Calcineurin A (Santa Cruz Biotechnology), β-actin (Sigma). Band densities of phospho-proteins were quantified with densitometry analysis using ImageJ software (NIH) and then normalized the total protein.
Statistical analysis
Data is represented as either the mean ± standard deviation (SD), or the mean ± standard error of the mean (SEM) and indicated in figure legends. P values between two groups are calculated using Student’s t-test. Differences were considered significant when p<0.05.
Results
CsA treatment in vivo promotes skin tumorigenesis and angiogenesis
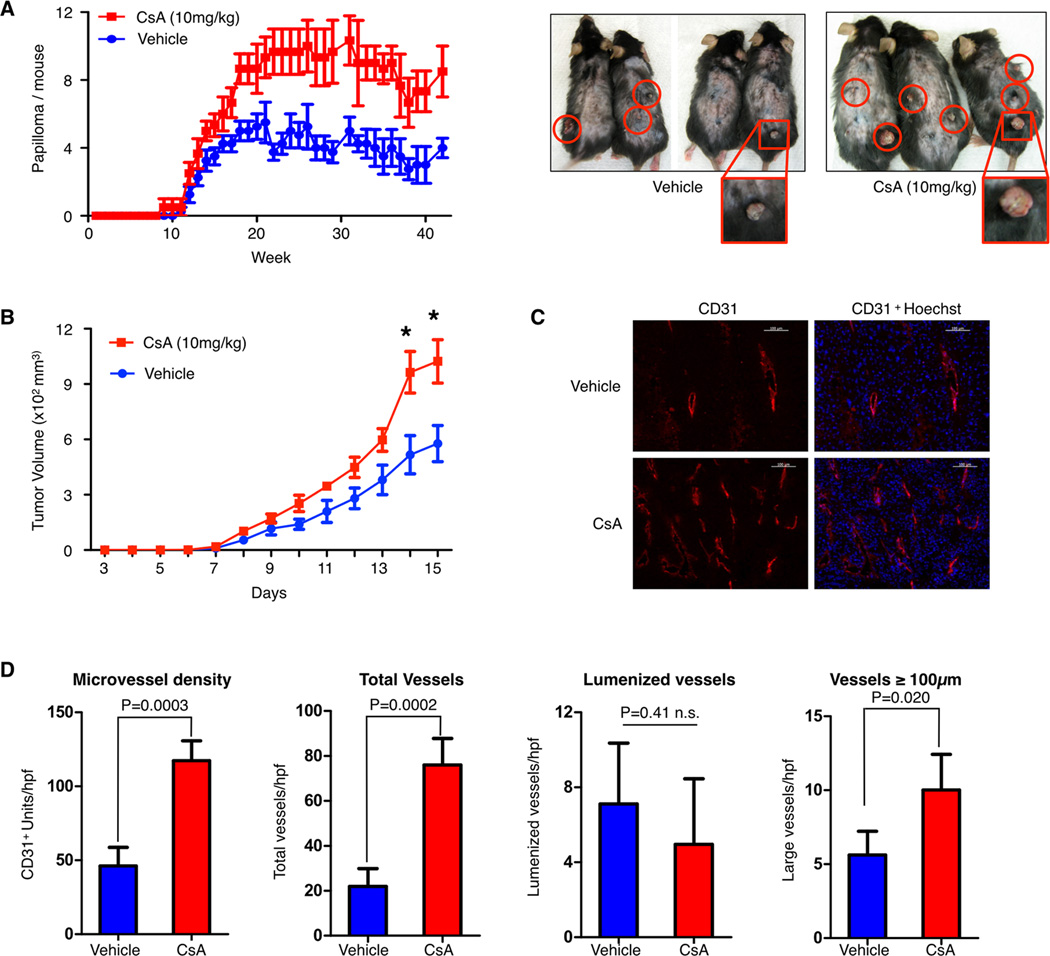
A well characterized carcinogen-induced model of skin carcinogenesis uses a single application on the skin of wild-type mice with 7,12-dimethylbenz[α]anthracene (DMBA) followed by twice weekly application of 12-O-tetradecanoylphorbol-13-acetate (TPA), leading to dermal papillomas and eventual carcinomas (25). CsA treatment of mice during DMBA and TPA application promotes tumor growth. Consistent with a previous study examining the applicative value of the DMBA-TPA carcinogenesis model in immunosuppressive contexts (28), clinically relevant dosing of CsA significantly increases the number of dermal papillomas on wild-type mice (Fig. 1A). Similarly, CsA treatment of wild-type mice after flank injection of syngeneic B16-F10 melanoma cells causes a significant increase in tumor growth when compared to mice treated with vehicle alone (Fig. 1B). To examine the effect of CsA on tumor angiogenesis, we immunostained tumors with anti-CD31 identifying a pronounced increase in the microvessel density of CsA treated tumors (Fig. 1C). The total number of vessels was higher in CsA treated tumors with numerous short CD31-positive vessels as compared to untreated controls. However, the number of mature lumenized vessel structures did not change and vessels larger than 100um were only moderately increased in CsA treated tumors (Fig. 1D). This characterization of tumor angiogenesis suggests that CsA treatment may promote the early stages of vessel formation.
Figure 1. Cyclosporin A promotes tumor angiogenesis and tumor growth.
A, quantification of chemically-induced papillomas in mice treated with either cyclosporin A (CsA) or vehicle. Representative photos are shown on the right with papillomas highlighted in red. N=5 mice per group, results shown are representative of two independent experiments. B, tumor growth over time of subcutaneous B16-F10 melanoma tumors in mice treated with CsA or vehicle. Data are shown as the mean ± SEM, with n=3–5 mice per group. Representative results of two independent experiments are shown. C, immunofluorescent images of B16-F10 tumor sections stained for the endothelial cell marker CD31 (red) and the DNA stain Hoechst (blue). Scale bar, 100µm. D, quantification of microvessel density, total vessels, lumenized vessels, and large (≥100µm) vessels in anti-CD31-stained tumor sections. Data are shown as mean ± SD, statistical analysis was performed by a student t-test, * P<0.05.
CsA promotes endothelial cell proliferation and migration in vitro
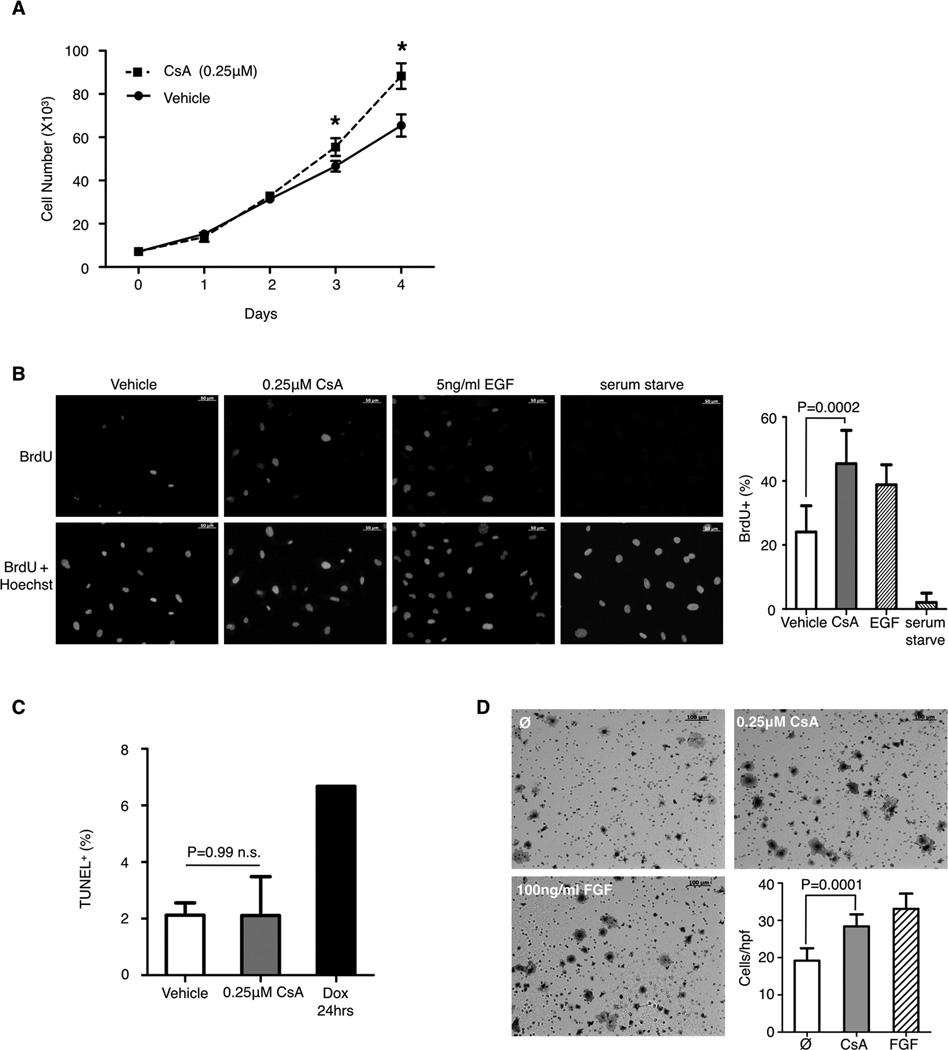
To investigate the effect of CsA on endothelial cell activation, primary lung microvascular endothelial cells (LuEC) were isolated from wild-type mice and treated with CsA or vehicle alone. CsA treatment increases LuEC number and BrdU incorporation as compared to vehicle alone (Fig. 2A and 2B). Since CsA has been shown to decrease apoptosis under UV or calcium overload stress (8) we assayed CsA treated LuEC for apoptosis. After treatment with either CsA or vehicle for 72 hours in normal culture conditions and in absence of any apoptotic inducers, we found no differences in TUNEL staining (Fig. 2C). Pre-treatment of LuEC with CsA also shows increased migration through a transwell filter towards CsA in comparison to vehicle treated cells towards basal media (Fig. 2D). Taken together, this data indicates that CsA promotes endothelial cell proliferation and migration in vitro.
Figure 2. Cyclosporin A promotes endothelial cell proliferation and migration in vitro.
A, proliferation of primary lung endothelial cells (LuEC) with CsA or vehicle, n=3 per group. B, representative images of BrdU uptake by LuEC treated with 0.25µM CsA, 5ng/mL EGF or vehicle for 48 hours. Quantification of BrdU+ cells is shown on the right. C, quantification of TUNEL+ LuEC after treatment with vehicle or CsA for 72 hours or doxorubicin (Dox) for 24 hours, n=5 per group. D, representative images of LuEC migration through a transwell filter in response to vehicle or 0.25µM CsA treatment. 100ng/mL FGF is used as a positive control. Scale bar, 100µm. Quantification of cells per high-powered field (hpf) from 8–10 randomly taken images is shown on the bottom. Representative results of three independent experiments is shown. Data is presented as mean ± SD, statistical analysis was performed by a student t-test, * P<0.05.
Calcineurin is not required for CsA-induced proliferation and migration
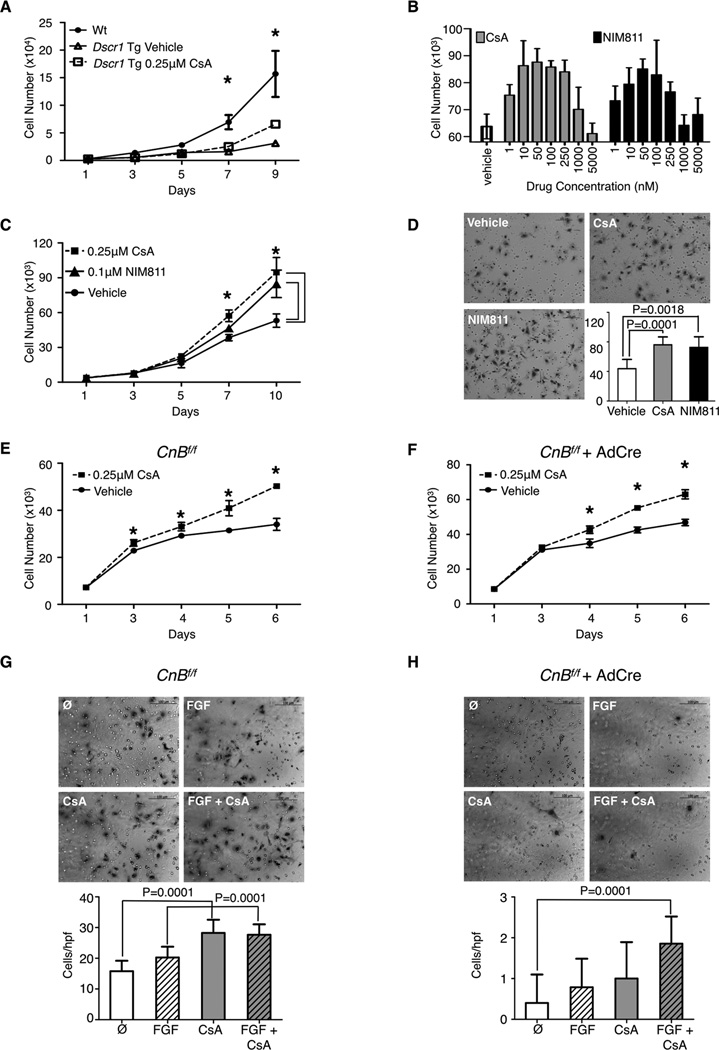
Since calcineurin is downstream of VEGF signaling in endothelial cells, inhibition of the calcineurin pathway is expected to decrease tumor angiogenesis, as demonstrated by overexpression of the endogenous calcineurin inhibitor Dscr1 (10). Despite being a potent calcineurin inhibitor, CsA treatment instead positively regulates tumor angiogenesis and endothelial cell activation. Therefore, we set out to investigate the calcineurin dependence of the endothelial phenotypes induced by CsA. CsA treatment of LuEC overexpressing DSCR1 still increases endothelial cell growth, despite the fact that DSCR1 overexpression alone decreases endothelial cell proliferation compared to wild-type (Fig. 3A). Furthermore, treatment of B16-F10 tumor bearing mice with FK506, an independent calcineurin inhibitor, showed no increase tumor growth compared to mice treated with vehicle alone (Supplementary Fig. S1A). FK506 treatment of endothelial cells in vitro decreases endothelial cell growth (Supplemental Fig. S1B) and VEGF-induced migration (Supplemental Fig. S1C) as compared to CsA treatment or vehicle alone. These data suggest a differential and non-overlapping mechanism by which these calcineurin inhibitors regulate endothelial cell activity.
Figure 3. Calcineurin is not required for cyclosporin A-induced endothelial cell proliferation and migration.
A, proliferation of wild-type (Wt) alone or Dscr1 transgenic (Tg) LuEC after treatment with CsA or vehicle. B, proliferation of wild-type LuEC for 6 days in response to vehicle alone or increasing concentrations of CsA or NIM811, n=4. C, proliferation of wild-type LuEC treated with CsA, NIM811, or vehicle. D, representative images of wild-type LuEC migration through a transwell filter in response to 0.25µM CsA, 0.1µM NIM811 or vehicle alone. Scale bar, 100µm. Quantification of cells per hpf is shown on the lower right. Representative results are shown from two independent experiments. E & F, proliferation of uninfected (E) or adenovirus-Cre infected (F) Calcineurin Bf/f (CnBf/f) LuEC in the presence of CsA or vehicle alone. G & H, representative images of uninfected (G) or adenovirus-Cre infected (H) CnBf/f LuEC migration through a transwell filter after treatment with 0.25µM CsA, 100ng/mL FGF, CsA+ FGF or vehicle alone. Scale bar, 100µm. Quantification of cells per hpf is shown on the bottom. A-G, n=3–4 per group, representative result of two independent experiments is shown. Data are shown as mean ± SD, statistical analysis was performed by a student t-test, * P<0.05.
To dissect out the immunosuppressive effects of CsA from its regulation of the mitochondrial permeability transition pore, we utilized the non-immunosuppressive cyclosporin analog NIM811, which binds to cyclophilins but does not interact with calcineurin (29). We find that treatment with either NIM811 or CsA results in a similar dose-dependent response in endothelial cell numbers (Fig. 3B). Both compounds also induce comparable levels of LuEC proliferation and migration (Fig. 3C and D) suggesting a calcineurin-independent mechanism by which CsA and NIM811 affects endothelial cell activation.
To further examine the requirement for calcineurin in CsA-mediated endothelial cell activation, we deleted the calcineurin B subunit in Calcineurin Bf/f LuEC through adenovirus-cre infection. Upon genetic deletion of Calcineurin B and the subsequent degradation of calcineurin A (Supplementary Fig. S2) leading to loss of calcineurin function (24), treatment with CsA still increases endothelial cell proliferation (Fig. 3E and 3F). Our studies show that deletion of Calcineurin B in LuEC results in a near-complete loss of cell migration, however CsA treatment in combination with the growth factor FGF causes a slight but meaningful increase in migration when compared to untreated cells (Fig. 3G and H). Taken together, these data indicate that CsA promotes endothelial cell activation through a calcineurin-independent mechanism.
CsA induces ROS release from the mitochondria
In addition to forming an intracellular complex with cyclophilin A and inhibiting calcineurin function, CsA also binds to another cyclophilin family member, cyclophilin D (30), at a similar affinity as cyclophilin A (31). Since our data shows comparable effects between CsA and NIM811, a non-immunosuppressive cyclosporin analog, we hypothesize that the mechanism by which CsA and NIM811 affects endothelial cell activation is similar. NIM811 binds to other intracellular cyclophilins and in particular cyclophilin D, a regulatory subunit of the mitochondrial permeability transition pore (MPTP) (19). Thus we examined whether CsA-mediated endothelial effects occurs through the mitochondrial-localized cyclophilin D.
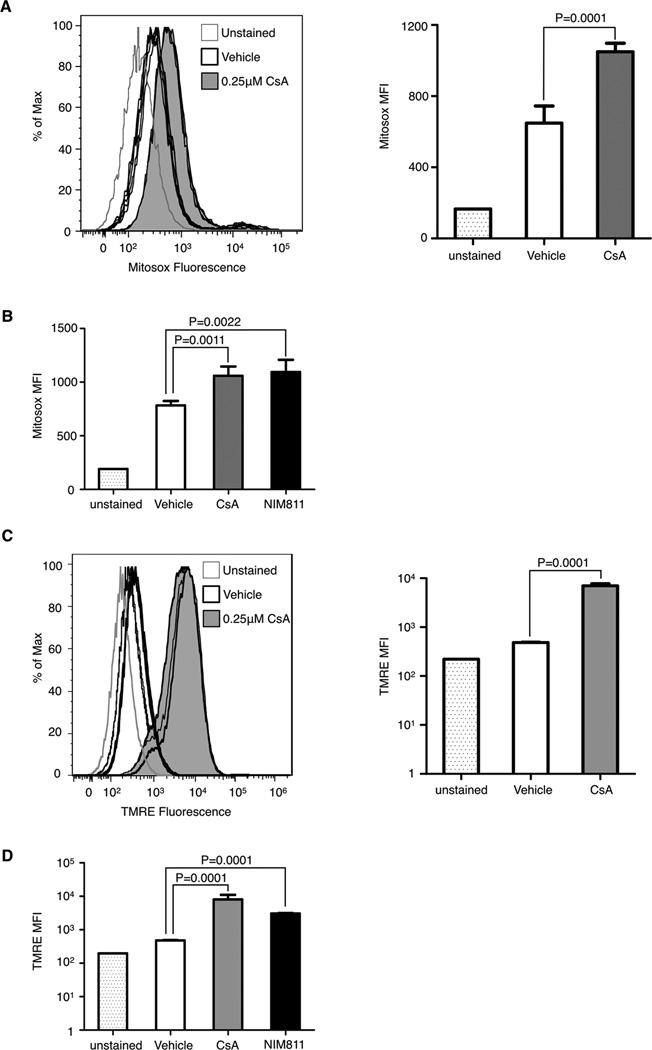
Binding of CsA to cyclophilin D has been shown to affect MPTP activity, including the release of ROS from the mitochondria in the cytosol, either directly through pore opening (31,32) or indirectly through changes in membrane potential (33). Release of mitochondrial ROS can stimulate mitogenic pathways in tumor and stromal cells (22,34). Staining of CsA-treated LuEC with the mitochondrial superoxide marker Mitosox (8,20) shows increased fluorescence compared to vehicle treated cells, (Fig. 4A) indicating CsA treatment increases mitochondrial ROS. Similarly, treatment with NIM811 results in increased Mitosox fluorescence (Fig. 4B), consistent with the notion that CsA increases is acting on the mitochondria in a calcineurin-independent manner to increase mitochondrial ROS production in endothelial cells.
Figure 4. Cyclosporin A targets the mitochondria to increase ROs in vitro.
A & B, Mitosox fluorescence in LuEC either unstained or treated with vehicle, (A) 0.25µM CsA, or (B) 0.1µM NIM811 for 24 hours. Mitosox fluorescence was quantified by flow cytometry with mean fluorescence intensity (MFI) shown on the right (A). C & D, tetramethylrhodamine, ethyl ester (TMRE) fluorescence in LuEC either unstained or treated with vehicle, (C) 0.25µM CsA or (D) 0.1µM NIM811 for 24 hours. TMRE fluorescence was quantified by flow cytometry with MFI show on the right (C). N=4–5 per group, data are shown as mean ± SD, statistical analysis was performed by a student t-test.
Binding of CsA to cyclophilin D has been shown to desensitize MPTP opening by increasing the stimulus threshold required to open the pore, thus decreasing MPTP-mediated cell death (8,30). In the absence of apoptotic stimuli, the MPTP has been described to transiently open, regulating mitochondrial membrane potential and affecting ROS production, among other things (35). This transient MPTP opening is sensitive to CsA treatment (35). Thus it is possible that CsA may increase mitochondrial ROS production by altering MPTP-regulated mitochondrial membrane potential. Tetramethylrhodamine, ethyl ester (TMRE) is a fluorescent lipophilic cation that accumulates in the mitochondria in direct proportion to the mitochondrial membrane potential. High levels of TMRE fluorescence would indicate a high mitochondrial membrane potential. We examined TMRE staining of LuEC after CsA treatment and found a dramatic increase in TMRE fluorescence (Fig. 4C) indicating a change in the mitochondrial membrane potential. Treatment with LuEC with the non-immunosuppressive cyclosporin analog NIM811 also increases TMRE staining similar to CsA treatment (Fig. 4D). Our studies with Mitosox and TMRE lend support to our hypothesis whereby CsA can activate endothelial cells through its interaction with mitochondrial cyclophilin D and in a calcineurin-independent manner.
Antioxidant treatment abolishes CsA-induced endothelial cell activation
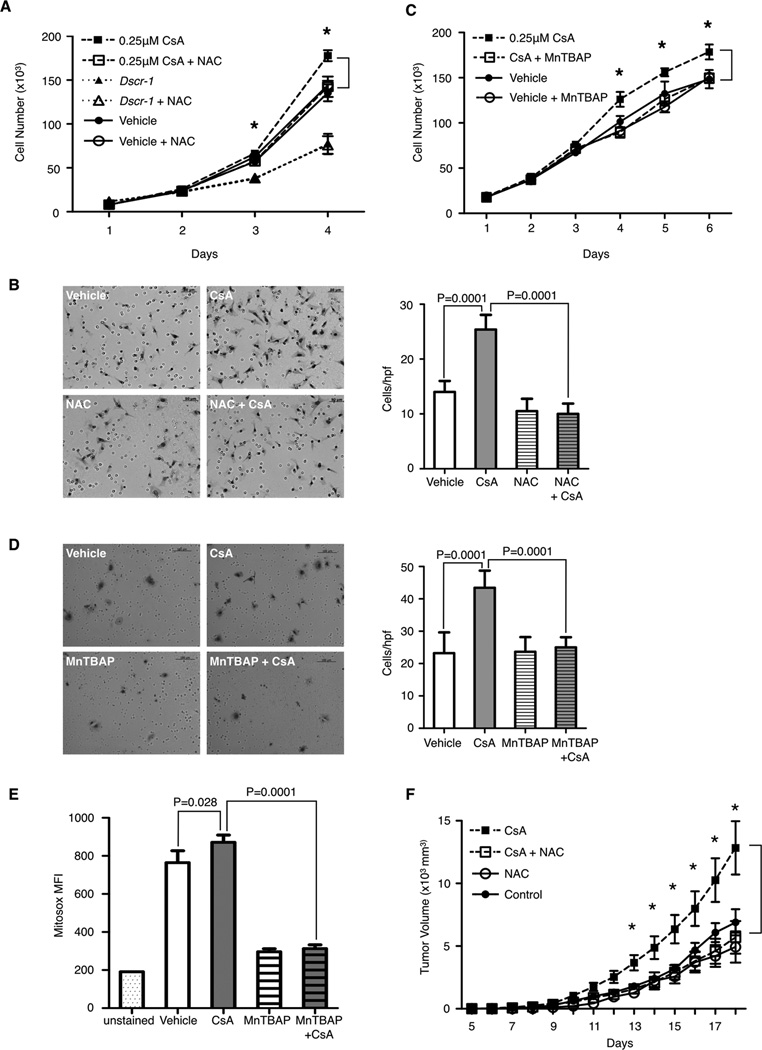
One method to inhibit cellular or mitochondrial-derived ROS is to treat cells with antioxidants, a class of drugs that quench intracellular ROS by either boosting endogenous antioxidant systems or scavenging ROS directly. Co-treatment of CsA with the cellular antioxidant N-acetyl-cysteine (NAC) decreases CsA-induced LuEC proliferation in vitro (Fig. 5A). NAC treatment alone had no effect on either wild type or Dscr1 transgenic LuEC. Similarly, NAC treatment also blocks CsA-induced LuEC migration (Fig. 5B). Co-treatment with the mitochondrially permeant superoxide dismutase mimetic and peroxynitrate scavenger, manganese(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP) (36) also attenuates CsA-induced proliferation and migration (Fig. 5C and 5D). These data support the notion of CsA treatment triggering an increase in mitochondrial ROS. Mitosox staining confirms that MnTBAP co-treatment with CsA reduces mitochondrial generated ROS (Fig. 5E). Both pre-treatment and simultaneous co-treatment of mice with the clinically utilized antioxidant NAC decreases B16-F10 tumor growth in animals on CsA therapy (Fig. 5F), demonstrating that quenching ROS levels protects against CsA-induced tumorigenesis in vivo.
Figure 5. Antioxidant treatment abolishes cyclosporin A induced endothelial cell activation and tumorigenesis.
A & C, proliferation of LuEC after treatment with CsA plus (A) 5mM N-acetyl cysteine (NAC), or (C) 10µM Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP). N=3 per group, with representative results of two independent experiments shown. B & D, representative images of LuEC migration through a transwell filter in response to 0.25µM CsA, (B) 5mM NAC, (D) 10µM MnTBAP, or vehicle. Scale bar, (B) 50µm, (C) 100µm. Quantification of cells per hpf is shown on the right. Representative results of two independent experiments is shown. E, quantification of Mitosox fluorescence (MFI) in LuEC treated with 0.25µM CsA or vehicle for 24 hours, with or without 10µM MnTBAP co-treatment. Representative results from two independent experiments is shown, with n=4 per group. F, B16-F10 tumor growth in WT mice ± CsA and ± NAC. Representative results of two independent experiment are shown, with n=5–9 mice per group. A-E, data are shown as mean ± SD, (F) data are shown as mean ± SEM, statistical analysis was performed by a student t-test, * P<0.05.
CsA-induced ROS activates ERK signaling
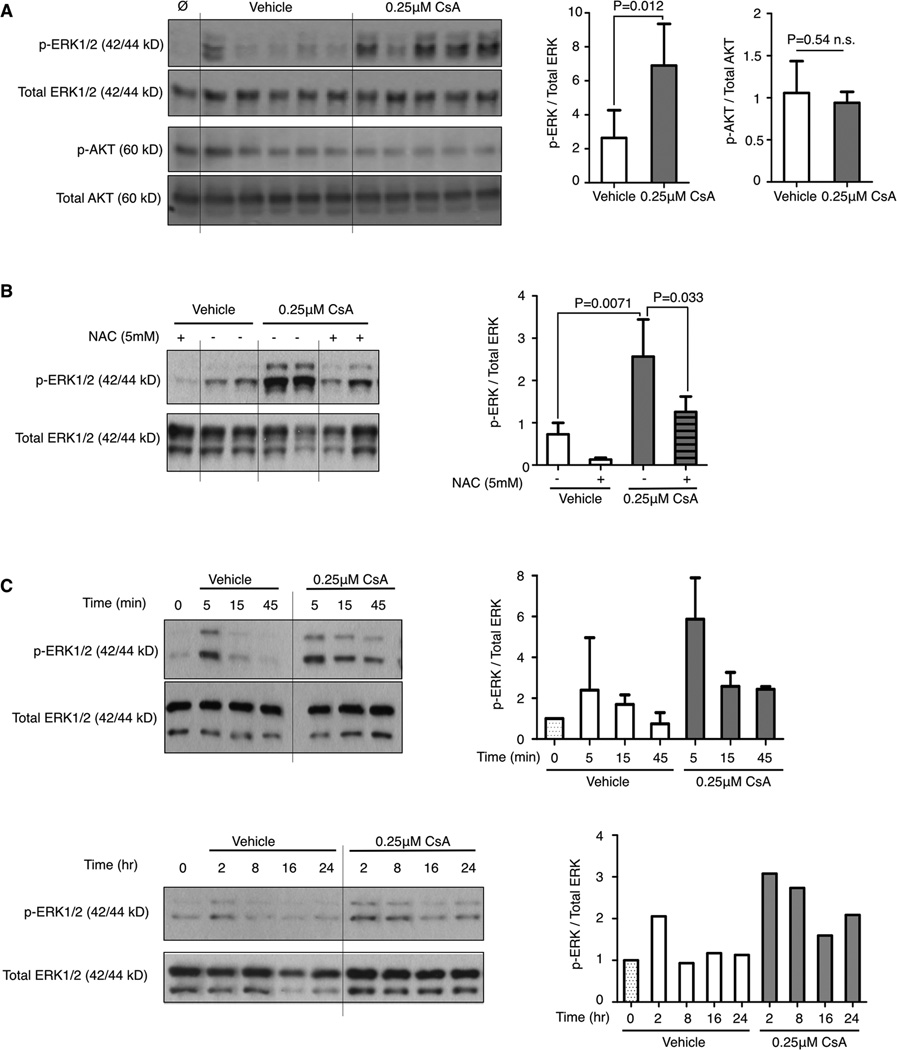
Moderate increases in cellular ROS levels have been shown to activate mitogenic signals (22,37,38). Many triggers of ROS have been identified including oncogenic Ras signaling which has been shown to elevate mitochondrial ROS, an important contributor for anchorage-independent growth (22). Conversely, various components of the Ras pathway have been demonstrated to be redox-sensitive. Phosphatases that regulate MAPK signaling, such as the ERK1/2 directed phosphatases, can be inactivated by elevated intracellular ROS, thereby prolonging ERK1/2 phosphorylation (38). CsA treatment of LuEC specifically increases ERK1/2 phosphorylation with no effect on AKT phosphorylation (Fig. 6A). Antioxidant pre-treatment with NAC blocks CsA-induced ERK phosphorylation (Fig. 6B). While ERK activation during normal growth is transient, CsA treatment shows sustained ERK1/2 phosphorylation as compared to vehicle alone (Fig. 6C). These data suggest that one mechanism by which CsA-mediated increased ROS levels promote endothelial activation is through increased MAPK signaling.
Figure 6. Cyclosporin A induced reactive oxygen species upregulates MAPK signaling.
A, western blot of phospho- and total ERK and AKT in LuEC after treatment with CsA or vehicle for 5 min. Each lane is an individual sample. B & C, western blot of phospho- and total ERK in LuEC after treatment with CsA for 5 min with pre-treatment with NAC for 30 min followed by CsA or vehicle for 5 min (B), or with CsA or vehicle for the indicated time (C). A-C, phosphorylated proteins are normalized to their respective total protein levels with relative expression levels quantified on the right. Results are representative of one experiment (C, 2 hr–24 hr) or pooled from 3–5 experiments (A, B, C, 5 min–45 min). Data are shown as mean ± SD, statistical analysis was performed by a student t-test, * P<0.05.
Discussion
The use of cyclosporin A (CsA) in immunosuppressive therapy for transplant recipients has long been associated with an increased cancer incidence (1). Here we identify a previously undescribed mechanism whereby CsA promotes tumor growth through its activation of tumor angiogenesis in a calcineurin-independent manner. Our data indicates that CsA increases endothelial cell proliferation and migration by increasing mitochondrial ROS levels. Antioxidant treatment mitigated CsA-induced tumorigenesis in mouse models, identifying a potential therapeutic intervention for CsA-associated tumorigenesis.
Previous studies investigating the mechanism underlying the increased cancer incidence with CsA treatment focused on the effect of CsA on tumor cells. In response to UV irradiation, CsA suppresses DNA repair genes and apoptosis in keratinocytes (7,8), allowing UV-induced DNA damage to go unrepaired while escaping cell death. Another study examined oncogenic Ras-driven skin lesions and found that CsA treatment induced ATF3 expression, blocking p53-dependent senescence evading a crucial tumor suppressive mechanism in a cancer cell autonomous manner (9). These data offer an explanation for the high incidence of skin cancers observed in patients on CsA regimens. Furthermore, in the presence of CsA, renal carcinoma cells were found to secrete TGF-β, inducing an invasive cancer phenotype with pronounced metastasis (6). CsA-associated cancers, in addition to their high metastatic potential, grow rapidly despite the presumed tumor initiating events, such as chronic sun exposure, occurring years or decades prior to treatment onset (3). These data suggest that CsA may also play a role in tumor promotion.
Systemic CsA treatment has also been proposed to affect tumor vasculature. CsA treatment has been shown to induce VEGF (39) and heme oxygenase-1 (40,41) production in tumor cells, which signals to the surrounding tumor microenvironment to promote angiogenesis. Furthermore, treatment of endothelial cells in vitro with low levels of CsA can be cytoprotective against nutrient deprivation (42). The consequences of CsA treatment directly on endothelial cells in a tumorigenic context, however, have not been extensively investigated.
In this work, we utilized a well-characterized and clinically relevant skin tumor model with long-term CsA treatment. Our data demonstrates a significant increase in tumor angiogenesis with CsA treatment resulting in an abundance of small, short vessels in tumors and preservation of large lumenized vessels, suggesting CsA promotes the formation of immature vessels. The early stages of angiogenesis are characterized by degradation of the extracellular space, followed by migration and proliferation of endothelial cells to generate new vessels. New angiogenic structures are pruned and stabilized through pericyte coverage, resulting in mature vessels (43). This concerted process is an infrequent event in adults and is tightly regulated by angiogenic factors to ensure proper neovascularization. Tumor vessels are often disorganized, abnormal, and inefficient due to imbalances in angiogenic regulators (44). An increase in tumor microvessel density, even when comprised mostly of small or immature vessels, can aid in nutrient and oxygen delivery. In vitro studies confirmed a pro-proliferative and pro-migratory effect of CsA treatment on primary endothelial cell cultures. Variations in batches of primary endothelial cell cultures are due in part to isolation technique and passage number among other factors and can translate to differences in raw data for endothelial cell activation assays. This was notable in our migration assays thus data was analyzed as fold change relative to day 1 as well as by differences in raw numbers with conclusions drawn from consistent changes observed when data was analyzed by multiple methods.
Studies from our lab and others have shown that inhibition of calcineurin by overexpression of its endogenous inhibitor DSCR1, or by deletion of calcineurin, negatively regulates endothelial cell activation (10,14,45). The calcineurin pathway in endothelial cells lies downstream of VEGFR2 activation and transmits angiogenic signals through one of its effectors, the NFAT family of transcription factors (10,11,27). We have previously shown that calcineurin inhibition in endothelial cells by DSCR1 blocks tumor angiogenesis (10,14). Given CsA is a potent calcineurin inhibitor, we would predict that CsA treatment would also block tumor angiogenesis. In contrast, our data shows a significant increase in tumor angiogenesis in CsA-treated tumor bearing mice, implicating the possibility that the pro-angiogenic effect of CsA may be calcineurin independent. To prove this concept we compared the effect of CsA on endothelial cells with a non-immunosuppressive cyclosporin analog NIM811 that does not bind calcineurin. Despite its inability to inhibit calcineurin, NIM811 acts similarly to CsA to increase endothelial cell proliferation and migration. To further confirm the calcineurin-independent effects of CsA on endothelial cell activation, we examined the proliferation and migration of calcineurin-null endothelial cells after CsA treatment. We found that endothelial activation after CsA treatment was similar in wild-type and calcineurin-null endothelial cells. Despite being a downstream mediator of VEGF signaling, proliferation in vitro was preserved in calcineurin null endothelial cells, possibly due to signaling through calcineurin-independent VEGF pathways as well as other pro-angiogenic pathways. Calcineurin signaling has also been implicated in tumor cell migration (46), and its deletion results in the near complete loss of migration in vitro, an outcome likely made more pronounced by the serum and growth factor depletion conditions used in the migration assay.
While CsA preferentially binds to cyclophilin A forming a calcineurin inhibitory complex, it also binds to other intracellular cyclophilins, a family of peptidyl-prolyl isomerases with diverse yet poorly understood functions (16,17). Binding of CsA to other cyclophilin family members can result in additional cellular effects that are calcineurin-independent. CsA is known to bind to cyclophilin D, a mitochondrial localized cyclophilin and part of the mitochondria permeability transition pore (MPTP). During calcium and oxidative stress, CsA limits superoxide release by inhibiting high-conductive irreversible MPTP opening (30), but has also been shown in other situations to increase mitochondrial ROS levels (8,33,34). Under basal, or non-apoptotic conditions, the MPTP has also been found to open briefly in transient “superoxide flashes” (35,47), or ‘low-conductive’ openings which do not result in mitochondrial swelling or massive release of mitochondrial contents. This “low-conductive” MPTP opening has possible roles in mitochondrial-cytosol crosstalk or cellular metabolism, and its opening temporarily lowers the membrane potential, serving as an overflow mechanism to maintain mitochondrial membrane potential (47,48). Therefore, regulation of the MPTP by CsA can have very different outcomes depending on pore state. Published studies show that CsA increases the apoptotic threshold in response to calcium or oxidative stress by inhibiting irreversible MPTP opening (8), and prevents the release of damaging levels of mitochondrial content, including ROS, into the cytosol. In the absence of apoptotic stimuli, CsA increases the mitochondrial membrane potential (33), possibly through the inhibition of ‘low conductance’ MPTP opening, which is associated with increased mitochondrial ROS generation (49). Under non-apoptotic conditions, our data shows CsA treatment of endothelial cells increases the mitochondrial membrane potential and mitochondrial superoxide levels. At higher concentrations (>5.0 µM), CsA has also been shown to inactivate a major mitochondrial superoxide detoxifier, resulting in damaging levels of mitochondrial ROS and consequent vascular damage (33). While the dose of CsA used in our study is within a clinically relevant range and the effects are pro-angiogenic, it remains to be determined whether the increased mitochondrial ROS is due to increased membrane potential or inactivation of an antioxidant enzyme. Future studies examining the cellular consequences of CsA binding to other intracellular cyclophilins will be important to determine towards understanding the full range of CsA effects on endothelial cells.
While high levels of ROS are associated with lipid peroxidation, DNA damage, and ultimately cell death, low levels of ROS can act as physiological signaling molecules in a variety of pathways (50). In particular, mitogenic pathways have been directly linked to ROS (37), a process that has been co-opted by cancer cells. In oncogenic Kras driven tumors, mitochondrial-derived ROS is necessary to maintain anchorage-independent growth and modulate ERK phosphorylation to a proliferative level (22). The vascular system, in particular, is highly regulated by changes in oxidative and nitration states (20). A number of angiogenic pathways are redox-sensitive, including the MAPK pathway and endothelial cell migratory pathways. We show that CsA treatment in endothelial cells increases ERK1/2 phosphorylation in a ROS-dependent manner. Treatment with antioxidants abolished CsA-induced endothelial ERK activation, proliferation and migration. Antioxidant treatment of tumor bearing mice also decreased CsA-induced tumor growth. Collectively, our data suggest that neutralization of ROS with anti-oxidants during CsA therapy may be an effective prophylactic treatment to decrease CsA-associated malignancies.
Our study highlights a previously undescribed mechanism for increased tumorigenesis after long-term CsA treatment. We show that CsA acts in a calcineurin-independent manner to induce endothelial cell activation and tumor angiogenesis by increasing mitochondrial ROS release. Importantly our work demonstrates that CsA-induced tumor angiogenesis is calcineurin-independent, thus preserving calcineurin inhibition as a feasible target for anti-angiogenic therapy. Furthermore, our data suggests that manipulating mitochondrial ROS may be useful in not only CsA-associated malignancies, but also for the development of new anti-angiogenic therapies for all solid tumors.
Supplementary Material
Implications.
Targeting the pro-angiogenic effects of cyclosporin A may be useful in the management of transplant-associated cancers.
Acknowledgement
We thank all the members of the Ryeom laboratory for their input, especially Keri Schadler, Dong Ha Bhang, and Diana Blidarescu. We also thank David Feldser, Alan Diehl, Doug Wallace, Todd Ridky, Meenhard Herlyn and Mitchell Weiss for scientific discussion and input. The authors would like to thank Novartis Pharmaceuticals for providing NIM811.
Grant Support
Funding was provided by NIH grant R01 CA118374 (SWR), Garrett B. Smith Foundation (SWR), and the TedDriven Foundation (SWR).
Footnotes
The authors declare no conflict of interest
Author Contributions
AYZ designed and performed experiments, interpreted the data, and wrote the manuscript. SWR designed the experiments, interpreted the data, and wrote and edited the manuscript.
References
- 1.Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transpl Int. 2006;19:607–620. doi: 10.1111/j.1432-2277.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 2.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 3.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 4.London NJ, Farmery SM, Will EJ, Davison AM, Lodge JP. Risk of neoplasia in renal transplant patients. Lancet. 1995;346:403–406. doi: 10.1016/s0140-6736(95)92780-8. [DOI] [PubMed] [Google Scholar]
- 5.Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby C. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–297. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 7.Kuschal C, Thoms K-M, Boeckmann L, Laspe P, Apel A, Schön MP, et al. Cyclosporin A inhibits nucleotide excision repair via downregulation of the xeroderma pigmentosum group A and G proteins, which is mediated by calcineurin inhibition. Exp Dermatol. 2011;20:795–799. doi: 10.1111/j.1600-0625.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 8.Norman KG, Canter Ja, Shi M, Milne GL, Morrow JD, Sligh JE. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion. 2010;10:94–101. doi: 10.1016/j.mito.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Nguyen B-C, Dziunycz P, Chang S, Brooks Y, Lefort K, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek K-H, Zaslavsky A, Lynch RC, Britt C, Okada Y, Siarey RJ, et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesser BA, Liang XH, Camenisch G, Yang S, Lewin DA, Scheller R, et al. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood. 2004;104:149–158. doi: 10.1182/blood-2004-01-0273. [DOI] [PubMed] [Google Scholar]
- 12.Iizuka M, Abe M, Shiiba K, Sasaki I, Sato Y. Down syndrome candidate region 1,a downstream target of VEGF, participates in endothelial cell migration and angiogenesis. J Vasc Res. 2004;41:334–344. doi: 10.1159/000079832. [DOI] [PubMed] [Google Scholar]
- 13.Minami T, Yano K, Miura M, Kobayashi M, Suehiro J, Reid PC, et al. The Down syndrome critical region gene 1 short variant promoters direct vascular bed – specific gene expression during inflammation in mice. J Clin Invest. 2009;119:2250–2270. doi: 10.1172/JCI35738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin J, Lee J, Baek K-H. A single extra copy of Dscr1 improves survival of mice developing spontaneous lung tumors through suppression of tumor angiogenesis. Cancer Lett. 2013;1:1–12. doi: 10.1016/j.canlet.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Yao Y-G, Duh EJ. VEGF selectively induces Down syndrome critical region 1 gene expression in endothelial cells: a mechanism for feedback regulation of angiogenesis? Biochem Biophys Res Commun. 2004;321:648–656. doi: 10.1016/j.bbrc.2004.06.176. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Kim SS. Current implications of cyclophilins in human cancers. J Exp Clin Cancer Res. 2010;29:97. doi: 10.1186/1756-9966-29-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Shimizu S, Watanabe T. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 19.Waldmeier PC, Feldtrauer J-J, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:2023–2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 21.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res. 2006;71:236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–2566. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 25.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, et al. Endothelial HIF-2 α regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy K, Zhou Z, Schadler K, Jia S-F, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol Cancer Res. 2008;6:929–936. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryeom S, Baek K-H, Rioth MJ, Lynch RC, Zaslavsky A, Birsner A, et al. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–431. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Yajima Y, Sueki H, Oguro T, Yoshida T, Iijima M. Effects of oral administration of ciclosporin A on skin carcinogenesis: a study using the two-stage carcinogenesis protocol in mice. Clin Exp Dermatol. 2008;33:478–483. doi: 10.1111/j.1365-2230.2008.02763.x. [DOI] [PubMed] [Google Scholar]
- 29.Rosenwirth BE, Billich A, Datema R, Donatsch P, Hammerschmid F, Harrison R, et al. Inhibition of Human Immunodeficiency Virus Type 1 Replication by SDZ NIM 811 , a Nonimmunosuppressive Cyclosporine Analog. Antimicrob Agents Chemother. 1994;38:1763–1772. doi: 10.1128/aac.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, et al. Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta. 2010;1797:1113–1118. doi: 10.1016/j.bbabio.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malouitre S, Dube H, Selwood D, Crompton M. Mitochondrial targeting of cyclosporin A enables selective inhibition of cyclophilin-D and enhanced cytoprotection after glucose and oxygen deprivation. Biochem J. 2010;425:137–148. doi: 10.1042/BJ20090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 33.Redondo-Horcajo M, Romero N, Martínez-Acedo P, Martínez-Ruiz A, Quijano C, Lourenço CF, et al. Cyclosporine A-induced nitration of tyrosine 34 MnSOD in endothelial cells: role of mitochondrial superoxide. Cardiovasc Res. 2010;87:356–365. doi: 10.1093/cvr/cvq028. [DOI] [PubMed] [Google Scholar]
- 34.Akool E-S, Gauer S, Osman B, Doller A, Schulz S, Geiger H, et al. Cyclosporin A and tacrolimus induce renal Erk1/2 pathway via ROS-induced and metalloproteinase-dependent EGF-receptor signaling. Biochem Pharmacol. 2012;83:286–295. doi: 10.1016/j.bcp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Jian C, Zhang X, Huang Z, Xu J, Hou T, et al. Superoxide flashes: elemental events of mitochondrial ROS signaling in the heart. J Mol Cell Cardiol. 2012;52:940–948. doi: 10.1016/j.yjmcc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Ferro E, Goitre L, Retta SF, Trabalzini L. The Interplay between ROS and Ras GTPases: Physiological and Pathological Implications. J Signal Transduct. 2012;2012:3657–3669. doi: 10.1155/2012/365769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng T-C, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 39.Basu A, Contreras AG, Datta D, Flynn E, Zeng L, et al. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008;68:5689–5698. doi: 10.1158/0008-5472.CAN-07-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee P, Basu A, Wegiel B, Otterbein LE, Mizumura K, et al. Heme oxygenase-1 promotes survivial of renal cancer cells through modulation of apoptosis- and autophagy-regulating molecules. J Biol Chem. 2012;287:32113–32123. doi: 10.1074/jbc.M112.393140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee P, Basu A, Arbiser JL, Pal S. The natural product honokiol inhibits calcineurin-inhibitor-induced and Ras-mediated tumor promoting pathways. Cancer Letters. 2013;338:292–299. doi: 10.1016/j.canlet.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez-Arroyo MV, Yague S, Wenger RM, Pereira DS, Jimenez S, Caramelo C, et al. Cyclophilin-mediated pathways in the effect of cyclosporin A on endothelial cells: role of vascular endothelial growth factor. Circulation Res. 2002;91:202–209. doi: 10.1161/01.res.0000027562.91075.56. [DOI] [PubMed] [Google Scholar]
- 43.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 44.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 45.Gollogly LK, Ryeom SW, Yoon SS. Down syndrome candidate region 1-like 1 (DSCR1-L1) mimics the inhibitory effects of DSCR1 on calcineurin signaling in endothelial cells and inhibit angiogenesis. J Surg Res. 2007;142:129–136. doi: 10.1016/j.jss.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen AHT, Béland M, Gaitan Y, Bouchard M. Calcineurin a-binding protein, a novel modulator of the calcineurin-nuclear factor of activated T-cell signaling pathway, is overexpressed in wilms’ tumors and promotes cell migration. Mol Cancer Res. 2009;7:821–831. doi: 10.1158/1541-7786.MCR-08-0402. [DOI] [PubMed] [Google Scholar]
- 47.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 48.Huser J, Blatter L. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem Soc. 1999;317:311–317. [PMC free article] [PubMed] [Google Scholar]
- 49.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 50.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.