Abstract
Purpose
The first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib is a standard therapy for patients with ALK-rearranged NSCLC. Several next-generation ALK-TKIs have entered the clinic and have shown promising activity in crizotinib-resistant patients. As patients still relapse even on these next-generation ALK-TKIs, we examined mechanisms of resistance to the next-generation ALK-TKI alectinib and potential strategies to overcome this resistance.
Experimental Design
We established a cell line model of alectinib resistance, and analyzed a resistant tumor specimen from a patient who had relapsed on alectinib. We developed Ba/F3 models harboring alectinib-resistant ALK mutations and evaluated the potency of other next-generation ALK-TKIs in these models. We tested the antitumor activity of the next-generation ALK-TKI ceritinib in the patient with acquired resistance to alectinib. To elucidate structure-activity-relationships of ALK mutations, we performed computational thermodynamic simulation with MP-CAFEE.
Results
We identified a novel V1180L gatekeeper mutation from the cell line model and a second novel I1171T mutation from the patient who developed resistance to alectinib. Both ALK mutations conferred resistance to alectinib as well as to crizotinib, but were sensitive to ceritinib and other next-generation ALK-TKIs. Treatment of the patient with ceritinib led to a marked response. Thermodynamics simulation suggests that both mutations lead to distinct structural alterations that decrease the binding affinity with alectinib.
Conclusions
We have identified two novel ALK mutations arising after alectinib exposure which are sensitive to other next generation ALK-TKIs. The ability of ceritinib to overcome alectinib-resistance mutations suggests a potential role for sequential therapy with multiple next-generation ALK-TKIs.
Keywords: ALK-rearranged NSCLC, gatekeeper mutation, resistance, crizotinib, alectinib (RO5424802/CH5424802), ceritinib (LDK378)
Introduction
The EML4-ALK fusion oncogene was first reported in non-small cell lung cancer (NSCLC) in 2007 (1). Approximately 3–5% of NSCLC tumors harbor ALK rearrangements. In addition to EML4, other fusion partners such as TFG, KIF5B, and KLC1 have been identified in NSCLC (2–4). Fusion with any of these partner proteins is believed to mediate oligomerization of ALK, which then leads to constitutive activation of the tyrosine kinase, and aberrant downstream signaling (5). As a result, ALK fusions function as potent “oncogenic drivers” and cancers harboring these rearrangements are highly sensitive to ALK kinase inhibition.
Crizotinib is a potent small molecule inhibitor targeting cMET, ALK and ROS1 tyrosine kinases. Based on its activity in phase 1, 2, and 3 clinical trials, crizotinib is approved in many countries for the treatment of advanced, ALK-rearranged NSCLC (6–8). While crizotinib often induces marked and durable responses, most patients will relapse within one to two years due to the development of resistance.
Multiple different mechanisms of resistance to crizotinib have been reported (9–14). In approximately one-third of crizotinib-resistant cases, resistance is mediated by a genetic alteration in ALK itself, typically a missense mutation in the tyrosine kinase (TK) domain, though amplification of the ALK fusion gene has also been observed. In contrast to EGFR-mutant NSCLC, in which T790M represents the sole EGFR resistance mutation in the clinic, crizotinib resistance can be mediated by a variety of different secondary mutations in ALK. To date, eight different crizotinib resistance mutations have been identified, including the gatekeeper L1196M substitution (9, 15). In some cases, resistant tumors have been found to harbor multiple non-overlapping mutations within the ALK-TK domain (9, 11, 16). Additionally, some cancers may develop resistance because the crizotinib fails to fully suppress ALK signaling despite the absence of an ALK resistance mutation (17), possibly secondary to inadequate drug exposures. Other mechanisms of crizotinib resistance appear to be independent of ALK and involve activation of alternative signaling pathways, so-called bypass tracks, such as EGFR and cKIT (11, 13, 14).
To overcome acquired resistance to crizotinib, a number of structurally distinct, next generation ALK inhibitors have been developed and are in various phases of clinical development. In general, these drugs are more potent inhibitors of ALK and may be effective against many of the known resistance mutations, including L1196M (10, 18). Alectinib (RO5424802/CH5424802) is one of the most advanced next-generation ALK inhibitors. In preclinical studies, alectinib demonstrated strong antitumor activity against cancer cells harboring ALK fusions, both in vitro and in vivo (18). In a phase 1/2 study conducted in Japan, alectinib was found to be highly effective and safe in crizotinib-naïve, ALK-rearranged NSCLC, inducing responses in 94% of treated patients (19). Alectinib has also been tested in a phase 1/2 study in the United States. Preliminary data from this study suggest that alectinib is also highly active in crizotinib-resistant patients, with a reported response rate of 55% (24 of 44 patients)(20). A similarly high response rate in crizotinib-resistant disease has also been reported with the next generation ALK inhibitor ceritinib (LDK378)(21). Based on these promising results, alectinib received Breakthrough Therapy Designation by the US FDA, and ceritinib was recently approved by the US FDA for ALK-positive patients with crizotinib-resistant or crizotinib-intolerant disease.
As with crizotinib, patients eventually develop resistance to next generation ALK inhibitors. In this study, we have explored acquired resistance to alectinib in a cell line model and in a primary tumor specimen from an alectinib-refractory patient. We have identified two novel secondary mutations within the ALK TK domain, both of which mediate resistance to alectinib: V1180L, which functions as a gatekeeper like L1196M, and I1171T, which resides in the αC helix within the ALK TK domain. The thermodynamic stability of different alectinib-ALK complexes suggests that both V1180L and I1171T substitutions cause resistance by decreasing the binding affinity of alectinib for the mutated kinases. While cancer cells expressing either V1180L- or I1171T-mutated EML4-ALK are resistant to alectinib as well as crizotinib, they remain sensitive to other structurally distinct ALK inhibitors and to hsp90 inhibitors. Thus, two different therapeutic strategies may be effective in overcoming resistance to alectinib, including the use of an alternative next generation ALK inhibitor in tumors with susceptible resistance mutations like V1180L or I1171T.
Materials and Methods
Patients
The ALK-positive NSCLC patient with acquired alectinib resistance underwent biopsy of a resistant tumor in October 2012. Standard histopathology was performed to confirm the diagnosis of malignancy and the histological subtype. Total nucleic acid was isolated as described. We also performed FISH and IHC studies as described below. The cell line was also established when we obtained sufficient tissue. The established ALK-positive patient-derived cell line has been previously tested for mutation status to confirm their authenticity. The electronic medical record was reviewed retrospectively to obtain clinical information under an IRB approved protocol.
Reagents
Alectinib and LDK378 were purchased from ActiveBiochem (HongKong), 17-AAG was from LC-Laboratories (Woburn, MA), NVP-TAE-684 and ASP3026 were from ChemieTek (Indianapolis, IN), crizotinib was from ShangHai Biochempartner (ShangHai, China). AP26113 was from Selleck (Cambridge, MA). Each compound was dissolved in DMSO for cell culture experiments.
Isolation of gDNA or total RNA preparation, sequencing of ALK fusion gene
Genomic DNA was isolated from cell pellets or fresh frozen specimen with a DNeasy Blood & Tissue Kit (QIAGEN) according to the manufacturer’s protocol. Total RNA was isolated from cell pellets or fresh frozen specimen with an RNeasy mini kit (QIAGEN) according to the manufacturer’s instruction. Each exon of ALK kinase domain (Exon21 to 27) or ALK kinase domain was PCR-amplified from genomic DNA or cDNA synthesized from total RNA with Oligo dT using KOD Plus ver.2 (TOYOBO, Osaka, Japan), and sequenced bidirectionally by Sanger sequencing.
Fluorescence in situ hybridization
Two-color, break-apart FISH to detect ALK rearrangements was performed using formalin-fixed paraffin-embedded tissues with in-house ALK FISH probes (ALK-5′ terminal (red) / ALK-3′ terminal (green)) made from BAC clones. Isolated or split signals indicate ALK rearrangement, while overlapping signals indicate a non-rearranged ALK gene. Images were captured with an Olympus BX51 fluorescent microscope equipped with a charge-coupled device camera (DP71; Olympus).
Reagents and cell culture conditions
The H3122 human NSCLC cell line was obtained as previously described (22). PC9 cells were kindly provided by Dr. Kazuto Nishio and have been previously characterized (23). HCC827 was kindly provided by Dr. Adi Gazdar and has been previously characterized (24). H460 and A549 human NSCLC cells were obtained from American Type Culture Collection. Ba/F3, immortalized murine bone marrow derived pro-B cells were obtained from the RIKEN BRC Cell Bank (RIKEN BioResource Center). H3122, H3122-derived alectinib resistant cells (H3122 CHR-A1), HCC827, PC9, H460 and A549 cells were cultured in RPMI1640 supplemented with 10% FBS (R-10). Human embryonic kidney 293FT cells were cultured in DMEM supplemented with 10% FBS (D-10). Ba/F3, immortalized murine bone marrow derived pro-B cells, were cultured in D-10 with or without 0.5ng/mL IL-3 (Invitrogen).
Generation of H3122 CHR-A1 cells
H3122 CHR-A1 cells were established in the same manner as H3122 CR1 (10). Briefly, H3122 cells were seeded at ~50% confluence in 15-cm dishes in R-10. Alectinib was added at a starting concentration of 10 nM, and cells were maintained in fresh drug-containing medium changed every 72 to 96 hours. Cells were passaged once they reached confluence. After every two passages at a given concentration of drug, the concentration of alectinib was increased until a final concentration of 1 μM was achieved. The resulting pool of resistant cells (designated H3122 CHR-A1) were maintained in R-10 with 1 μM alectinib.
Survival assays
For 72 hr drug treatments, 2000 to 3000 cells were plated in replicates of three to six into 96-well plates. Following drug treatments, cells were incubated with CellTiter-Glo assay reagent (Promega) for 10 min and luminescence was measured using a Centro LB 960 microplate luminometer (Berthold Technologies). The data were graphically displayed using GraphPad Prism version 5.0 (GraphPad Software). IC50 value was determined by a nonlinear regression model with a sigmoidal dose response in GraphPad.
Immunoblot analysis
Lysates were prepared as described previously (Engelman (Science) and Katayama et al (STM)). Equal amounts of lysates were electrophoresed and immunoblotted with the antibodies against phospho-ALK (Tyr1604), ALK (C26G7), phospho-p42/44 ERK/MAPK (Thr202/Tyr204), p42/44 ERK/MAPK, phospho-Akt (Ser473) (D9E), panAkt (C67E7), phospho-S6 Ribosomal Protein (Ser240/244, D68F8) and S6 Ribosomal Protein (54D2) (Cell Signaling Technology), GAPDH (6C5, Millipore), and β-actin (Sigma).
Retroviral infection
cDNAs encoding EML4-ALK variant1, EML4-ALK variant1 V1180L or I1171T mutants were cloned into 1520 retroviral expression vectors (pLenti), and virus was produced as previously described (Engelman Cancer Res 2007). After retroviral infection, Ba/F3 cells were selected in puromycin (1.0 μg/mL) for 2 weeks. For Ba/F3 cells infected by EML4-ALK variants, IL-3 was withdrawn from the culture medium for at least 2 wks before experiments.
Statistical analysis
All data are shown as mean ± SD. Statistical analysis was performed using two-tailed Student’s t test. Significance was established for p values < 0.05.
Results
Generation and characterization of alectinib-resistant cells
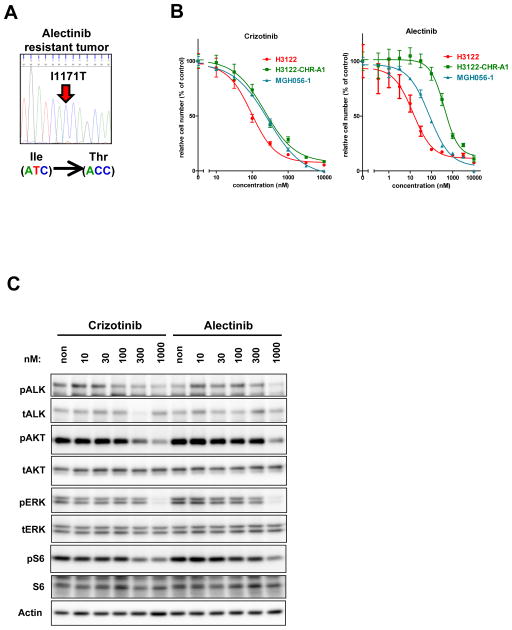
The EML4-ALK-expressing NSCLC cell line H3122 is more sensitive to alectinib than crizotinib, as reported previously (Supplementary Figure S1, (10)). To explore mechanisms of alectinib resistance, we generated resistant H3122 cells by exposing the sensitive parental cells to increasing concentrations of alectinib for 7 months. The fully resistant cells, H3122 CHR-A1 cells, were maintained in 1 μM of alectinib. H3122 CHR-A1 cells were as resistant to alectinib as cancer cell lines that do not harbor ALK rearrangement (Fig 1A, B). In contrast to parental H3122 cells, H3122 CHR-A1 cells maintained ALK phosphorylation and downstream AKT and ERK phosphorylation in the presence of 300 nM of alectinib (Fig 1C). As amplification of ALK fusion genes has been shown to mediate crizotinib resistance (10), we examined ALK gene copy number by fluorescence in situ hybridization (FISH). Like the parental H3122 line, CHR-A1 cells showed no evidence of amplification of the ALK fusion gene (Fig 1D).
Fig. 1. H3122 CHR-A1 cells are resistant to alectinib and harbor a V1180L mutation in the ALK kinase domain.
(A) Cells were seeded in 96-well black plates and treated with increasing concentrations of alectinib for 72 hrs. Cell survival was analyzed using the CellTiter-Glo assay. While H3122 cells showed high sensitivity to alectinib (red line), H3122 CHR-A1 cells (green line) were as insensitive to alectinib as non-ALK rearranged cell lines. (B) Table summarizing IC50 values (nM) of each cell line against alectinib. (C) Parental H3122 and CHR-A1 cells were treated with alectinib at the indicated concentrations for 6 hrs. Cell extracts were immunoblotted to detect the indicated proteins. (D) Two-color FISH (ALK-5′ region (red) / ALK-3′ region (green)) analysis was performed on parental H3122 and CHR-A1 cells. Most of the cells harbor isolated or split green signals (arrowhead, EML4-ALK). Shown on the right are electrophoretograms of EML4-ALK cDNA from parental H3122 and CHR-A1 cells. The g3538c mutation within exon 23 results in a V1180L substitution which corresponds to a gatekeeper mutation in ALK.
We next examined the entire coding sequencing of EML4-ALK in both H3122 parental and CHR-A1 resistant cells. In the resistant cells, we detected a C to A substitution at nucleotide 3538 of EML4-ALK variant1 which was not detected in the parental line (Fig 1D). This 3538 G to C substitution results in a Valine to Leucine change at codon 1180 within the ALK TK domain. Based on structural modeling studies of ALK (25), V1180 is predicted to lie near the L1196 gatekeeper residue, which is mutated in a subset of cell lines and patient biopsies with acquired resistance to crizotinib (9–11, 14, 16). No other TK mutations were detected in resistant cells.
Identification of a novel ALK resistance mutation in a crizotinib- and alectinib-refractory patient
To identify mechanisms of alectinib resistance that develop in patients, we examined a resistant tumor sample from a patient with advanced ALK-positive NSCLC who had relapsed on alectinib. This patient had previously received three lines of chemotherapy and had initially responded to crizotinib, as indicated by an improvement in disease burden on CT scans. After 8 months of crizotinib therapy, the patient developed disease progression and was then treated with alectinib (300 mg twice daily). Restaging CT scans demonstrated a response to alectinib, but after 4 months, the patient again experienced disease progression including worsening liver metastases. The patient was discontinued from alectinib, and underwent biopsy of a resistant liver lesion. Cytopathology of the tumor cells demonstrated adenocarcinoma histology, and ALK FISH analysis confirmed the presence of ALK rearrangement in the resistant tumor specimen. In addition, a cell line was established directly from the biopsy specimen which we designated MGH056-1.
To determine whether a secondary mutation within ALK might underlie the development of alectinib resistance in this patient, we extracted total nucleic acid from a flash frozen specimen, and the entire TK domain of ALK was amplified from cDNA and sequenced. We identified a T to C missense mutation at codon 3512, leading to an I1171T amino acid substitution (Fig. 2A). The I1171 residue is located in the αC helix. Mutation of I1171 has been reported previously in neuroblastoma (26), but not in ALK-rearranged NSCLC. The MGH056-1 cell line also harbored the I1171T mutation.
Fig. 2. Discovery of the ALK I1171T secondary mutation in a patient with acquired resistance to crizotinib and alectinib.
(A) Secondary I1171T mutation was only detected in the post-alectinib treated specimen. Shown are electrophoretograms of ALK kinase domain cDNA from the post-alectinib treatment. (B) Sensitivity of MGH056-1 cells to crizotinib or alectinib. MGH056-1 cells, parental H3122 and CHR-A1 cells were treated with indicated concentrations of crizotinib or alectinib. Cell survival was analyzed with CellTiter-Glo. (C) MGH056-1 cells were exposed to increasing concentrations of crizotinib or alectinib for 6 hrs. Cell lysates were immunoblotted to detect the indicated proteins.
To determine whether MGH056-1 cells are resistant to ALK TKI therapy, we compared the sensitivity of the parental H3122, H3122 CHR-A1, and MGH056-1 cells to crizotinib and alectinib. As shown in Fig. 2B, both H3122 CHR-A1 and MGH056-1 cells were similarly resistant to crizotinib in cell viability assays. With respect to alectinib, H3122 CHR-A1 cells were highly resistant, whereas the MGH056-1 cells displayed intermediate resistance (Fig. 2B). Furthermore, compared to parental H3122 cells, MGH-056-1 cells required substantially higher concentration of alectinib (or crizotinib) to suppress the phospho-ALK and downstream signaling intermediates (compare Figs. 1C and 2C). These results suggest that I1171T mutant ALK is relatively insensitive to both crizotinib and alectinib.
ALK I1171T and V1180L mutations mediate resistance to alectinib in Ba/F3 models
To determine whether the I1171T and V1180L mutations are sufficient to cause resistance to alectinib, we engineered Ba/F3 cells expressing EML4-ALK harboring each mutation. In cell survival assays, Ba/F3 lines expressing the mutant EML4-ALK proteins were significantly less sensitive to alectinib than those expressing wild-type EML4-ALK (Fig. 3A). Ba/F3 cells with EML4-ALK V1180L were more resistant than Ba/F3 cells with EML4-ALK I1171T (Fig. 3A), consistent with greater resistance observed in the H3122 CHR-A1 cells compared to the MGH056-1 cells. We next examined the effect of alectinib on ALK phosphorylation in the presence or absence of each resistance mutation. Consistent with the cell survival assays and the immunoblotting results with H3122 CHR-A1 and MGH056-1 cells, alectinib was much less potent at suppressing the phosphorylation of EML4-ALK harboring V1180L and, to a lesser extent, I1171T, compared to wild-type EML4-ALK (Fig. 3B). Thus, in Ba/F3 cells, both the V1180L and I1171T mutations confer resistance to alectinib, with V1180L appearing to confer a higher degree of resistance than I1171T.
Fig. 3. Biochemical and structural basis of alectinib resistance mediated by ALK I1171T and V1180L mutations.
(A) Ba/F3 cells expressing WT, I1171T or V1180L EML4-ALK were seeded in 96-well plates and treated with the indicated concentrations of alectinib for 72 hrs. Cell viability was analyzed using the CellTiter-Glo assay. (B) Inhibition of phospho-ALK by alectinib in Ba/F3 models. WT or mutated EML4-ALK-expressing Ba/F3 cells were exposed to increasing concentrations of alectinib for 2 hrs. Cell lysates were immunoblotted to detect the indicated proteins. (C) Estimated interaction energies of the indicated complexes (alectinib with WT ALK (green), ALK I1171T (cyan) or ALK V1180L (magenta)) by MP-CAFEE methods are shown. In the MP-CAFEE methods, the free energy value (ΔG) is estimated by summation of coulomb and van-der-Waals (vdw) potential energies. (D) Shown are the complex average structures in the equilibrated molecular dynamics simulation for wild-type ALK (green), ALK I1171T (cyan) and ALK V1180L (magenta).
Structural analysis of resistance mutations by computational simulation with MP-CAFEE
To understand the structure-function relationship of the two alectinib-resistance mutations in the ALK kinase domain, we performed computational simulation analysis with MP-CAFEE. To evaluate the thermodynamic stability of each complex of alectinib with wild-type, I1171T and V1180L mutated ALK, we performed molecular dynamics simulation and estimated the free energies of each complex using MP-CAFEE method (27). Supplementary Figure S2 shows the comparison of experimental IC50 values and calculated free energy values. A linear correlation between experimental and calculated values was observed. This result indicates that the molecular dynamic simulation and free energy estimation by MP-CAFEE could correctly predict the resistant mechanism of each resistant mutant. In the MP-CAFEE method, the free energy value is estimated by summation of Coulomb and van-der-Waals (vdw) potential energies. Figure 3C shows the estimated interaction energies of alectinib with wild-type, I1171T or V1180L ALK. Interestingly, the decrease in binding affinities of I1171T-alectinib and V1180L-alectinib, compared with wild-type ALK-alectinib, are caused by decreases in Coulomb and vdw interactions, respectively. As shown in Fig. 3D, the I1171T mutation distorts the C-helix, shifting the position of the glutamic acid at 1167 inferiorly. As the cyano group of alectinib forms a hydrogen bond with E1167, this downward shift may disrupt binding of alectinib to the I1171T mutant. In contrast, for the V1180L mutant, our modeling predicts that the methyl group of the leucine residue clashes with the multi-cyclic rings of alectinib (Fig. 3D). This steric interference likely decreases the vdw interaction between V1180L and alectinib. Taken together, these results suggest that for both I1171T and V1180L mutations, drug resistance is due to decreased binding affinity of alectinib for the mutant kinases.
Structurally distinct next generation ALK inhibitors overcome alectinib-resistant mutations
In addition to alectinib, several other ALK TKIs are currently in clinical development. To determine whether these next-generation ALK inhibitors may have activity in alectinib-resistant cancers, we used the Ba/F3 cells expressing EML4-ALK harboring the I1171T or V1180L mutation. As controls, we also tested Ba/F3 cells expressing wild-type EML4-ALK and parental, IL-3-dependent Ba/F3 cells. We focused on four ALK inhibitors: the tool compound NVP-TAE684 (28), and three drugs currently in clinical trials or clinically available for ALK-rearranged NSCLC, ceritinib (LDK378) (17, 21), AP26113 and ASP3026. As shown in Figure 4A, NVP-TAE684 demonstrated potent activity against Ba/F3 cells expressing V1180L EML4-ALK or the I1171T EML4-ALK. The clinically available ALK inhibitors ceritinib and AP26113 showed slightly different selectivity profiles against the different ALK resistance mutations. Ceritinib was highly active against V1180L, even more so than against WT EML4-ALK (Figure 4A, Supplementary Figure S3). The I1171T mutation was also sensitive to ceritinib, demonstrating a dose-response similar to WT EML4-ALK. In contrast, AP26113 and ASP3026 were similarly active against both V1180L mutant and wildtype EML4-ALK, but AP26113 was a little less active against I1171T mutated EML4-ALK while ASP3026 was inactive against I1171T (Supplementary Figures S3 and S4). The suppression of phospho-ALK by the different inhibitors across the various mutations was consistent with the potencies observed in these Ba/F3 studies (Supplementary Figure S3 and S5). These results suggest that at least two of the next-generation ALK inhibitors – ceritinib and AP26113 – may be able to overcome resistance to alectinib mediated by either I1171T or V1180L mutations.
Fig. 4. Ceritinib overcomes alectinib-resistance in Ba/F3 models and H3122 CHR-A1 cells.
(A) Parental Ba/F3 cells and Ba/F3 cells expressing WT, I1171T or V1180L EML4-ALK were seeded in 96-well plates and treated with the indicated concentrations of TAE-684 (left), crizotinib (center), or ceritinib (right) for 72 hrs. Cell viability was analyzed using the CellTiter-Glo assay. (B) Cancer cell lines, including parental H3122 and alectinib-resistant H3122 CHR-A1 cells, were seeded in 96-well plates and treated with increasing concentrations of TAE-684 (left), crizotinib (center) or ceritinib (right) for 72 hrs. Cell viability was measured using CellTiter-Glo. Both parental H3122 cells (red line) and H3122 CHR-A1 cells (green line) showed marked sensitivity to TAE-684 and ceritinib. Non-ALK rearranged cell lines (A549, H460, HCC827 and PC-9 cells) showed minimal growth inhibition when exposed to ALK inhibitors. (C) Suppression of ALK signaling by ALK inhibitors (TAE684, crizotinib or ceritinib) in parental H3122 and CHR-A1 cells. Cells were exposed to increasing concentrations of TAE684, crizotinib or ceritinib for 6 hrs. Cell lysates were immunoblotted to detect the indicated proteins.
We next tested the efficacy of several ALK inhibitors (TAE-684, crizotinib and ceritinib) in alectinib resistant H3122 CHR-A1 cells harboring the V1180L EML4-ALK mutation. Parental H3122 cells, as well as KRAS or EGFR mutant cancer cell lines (A549, H460, PC-9, and HCC827) were used as controls. As shown in Fig. 4B, TAE-684 markedly suppressed cell growth in both sensitive H3122 and alectinib-resistant H3122 CHR-A1 cells, but had almost no effect on the viability of other, non ALK-dependent cancer cell lines. TAE-684 suppressed ALK phosphorylation and downstream signaling (Fig 4C, left), and induced apoptosis (Supplementary Fig. S6). In contrast, crizotinib treatment was significantly less effective against H3122CHR-A1 cells compared with H3122 parental cells (Fig 4B and Fig 4C). Like TAE-684, ceritinib demonstrated potent activity against both parental H3122 and alectinib-resistant H3122 CHR-A1 cells, decreasing cell growth (Fig. 4B), suppressing ALK phosphorylation (Fig. 4C), and inducing apoptosis (Supplementary Fig. S6).
The hsp90 inhibitor 17-AAG overcomes alectinib resistance in H3122 CHR-A1 cells
A number of hsp90 inhibitors have demonstrated clinical activity in ALK-rearranged NSCLC, including in one patient with acquired resistance to crizotinib (29, 30). We therefore tested whether the alectinib-resistant H3122 CHR-A1 cells might be sensitive to the hsp90 inhibitor 17-AAG. Compared to KRAS or EGFR mutant cancer cell lines (A549, H460, PC-9, and HCC827), CHR-A1 cells were highly sensitive to 17-AAG treatment, and nearly as sensitive as parental H3122 cells (Supplementary Fig. S7). Based on immunoblotting, 17-AAG treatment reduced EML4-ALK protein levels in both parental H3122 and H3122CHR-A1 cells to similar extents, as well as downstream signaling. Thus, Hsp90 inhibition may represent an alternative therapeutic strategy for overcoming resistance to alectinib due to acquisition of a resistance mutation.
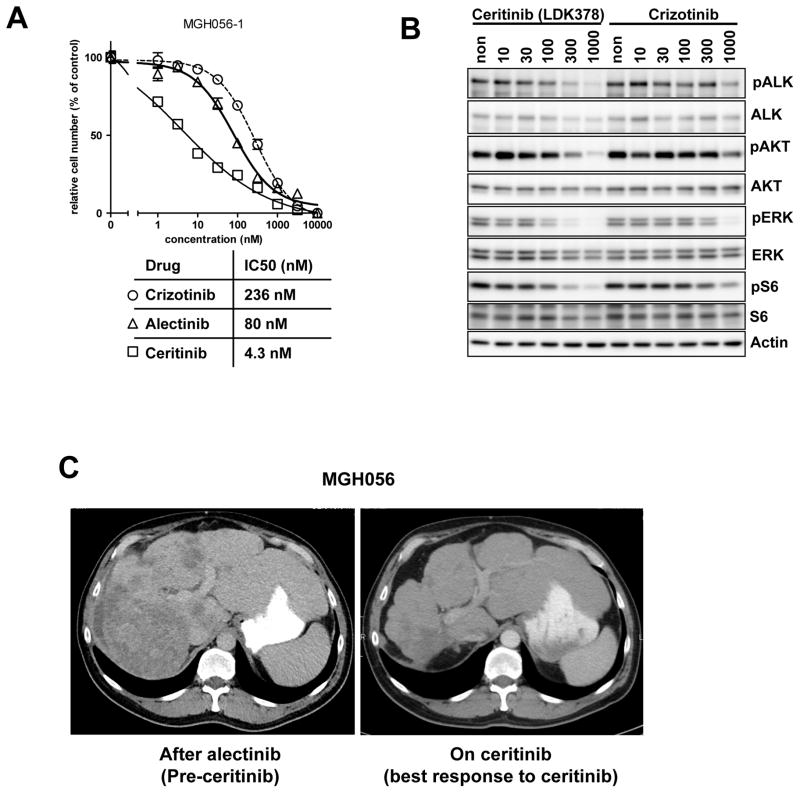
Ceritinib is active to crizotinib and alectinib resistant cells and patient
We also tested the efficacy of different ALK inhibitors in the MGH056-1 cells. As shown in Fig. 5A, ceritinib, but not crizotinib or alectinib, markedly suppressed the cell growth of MGH056-1 cells. Ceritinib also suppressed ALK phosphorylation and downstream AKT and ERK phosphorylation to a greater extent than crizotinib and alectinib (Fig 5B and 2C). Consistent with these results, treatment of patient MGH056 with ceritinib led to significant tumor regression (Fig. 5C), with a confirmed partial response lasting over 7 months. These preclinical and clinical results suggest that ceritinib may be effective in treating cancers that have become resistant to alectinib due to a secondary mutation such as I1171T or V1180L.
Fig. 5. Preclinical and clinical activity of ceritinib in alectinib-resistant cancer cells harboring the I1171T mutation.
(A) MGH056-1 cells were seeded in 96-well plates and treated with the indicated concentration of crizotinib, alectinib, or ceritinib for 72 hrs. Cell viability was measured using CellTiter-Glo. (B) MGH056 cells were exposed to increasing concentrations of crizotinib or ceritinib for 6 hrs. Cell lysates were immunoblotted to detect the indicated proteins. (C) Axial CT scan images of patient MGH056. Shown on the left is an axial slice through this patient’s liver after he had relapsed on alectinib and prior to ceritinib. Shown on the right is a comparable axial image after 18 weeks of ceritinib treatment demonstrating marked improvement in his liver metastases.
Discussion
ALK-rearranged NSCLC represents one of the newest oncogene-addiction paradigms in clinical oncology. As with other oncogene-addicted cancers, ALK-rearranged lung cancers are initially sensitive to the first generation targeted agent, but eventually become resistant through a variety of different mechanisms. Remarkably, the vast majority of crizotinib-resistant tumors remain ALK-dependent and re-respond to more potent, next generation ALK inhibitors such as alectinib and ceritinib (21, 31). However, despite their promising activity in early phase studies, resistance to next generation ALK inhibitors invariably develops and ultimately limits the clinical benefit afforded by these new agents.
In this study, we focused on acquired resistance to alectinib, one of the most advanced of the next generation ALK inhibitors in the clinic. We identified two novel ALK mutations, V1180L in a cell line made resistant to alectinib, and I1171T in a tumor specimen from a patient who had relapsed on alectinib. Both mutations confer resistance to crizotinib as well as alectinib, and hence add to the growing list of secondary ALK mutations that can mediate crizotinib resistance. Mutation of the V1180 residue has not yet been reported in patients, but was observed at very low frequency in an in vitro mutagenesis screen for crizotinib-resistant mutants in EML4-ALK (25). Based on the crystal structure of ALK (32), V1180 resides at the back of the ATP pocket and likely makes direct contact with crizotinib and alectinib, similar to the L1196 gatekeeper residue. Our computational modeling revealed weaker binding of alectinib to the V1180L mutant compared to wild-type ALK, supporting the notion that substitution of leucine for valine at this residue interferes with the ability of alectinib to bind effectively to the kinase.
The second alectinib-resistant mutation, I1171T, has not been previously reported in crizotinib-resistant patients, but mutation of this residue has been described in two patients with neuroblastoma (26). In neuroblastoma, mutation at this residue results in an I1171N amino substitution and leads to activation of ALK, though the mutant kinase is unable to transform Ba/F3 cells (33). Based on our computational modeling studies, we would predict that I1171T disrupts a hydrogen bond between alectinib and E1167 (Fig. 3D), destabilizing the complex of alectinib with the mutant kinase. Whether ALK I1171T is weakly oncogenic like I1171N and whether this could contribute to crizotinib resistance is unknown.
Compared with V1180L which conferred high level TKI resistance, the I1171T mutation was associated with intermediate resistance to alectinib in cell line studies. This mutation was discovered in a patient who had relapsed after four months of alectinib therapy. Prior to alectinib, the patient had received crizotinib with a response lasting eight months. While no tumor specimens were available prior to or after crizotinib therapy for genetic analyses, we suspect that the I1171T mutation emerged during the course of alectinib treatment, given his previous durable response to crizotinib followed by a re-response to alectinib. Of note, this patient was treated with alectinib at a dose of 300 mg twice daily. This represents half of the recommended phase 2 dose (RP2D) of alectinib established in a recent phase 1 study (NCT01588028, (20)). The relatively low drug exposure at this dose may have been a factor in selecting and/or expanding a clone with intermediate resistance to alectinib. As higher drug exposures are predicted at the RP2D, we speculate that patients treated at this dose could develop more highly resistant mutations like V1180L.
Recently, several other mechanisms of resistance have been reported in patients who have relapsed on next generation ALK inhibitors. The solvent front mutation G1202R, first discovered in a crizotinib-resistant tumor (11), appears to mediate high-level resistance to both alectinib and ceritinib. In one case, a patient who had relapsed on crizotinib was treated with alectinib at the RP2D and showed no evidence of response, consistent with intrinsic resistance (34). Molecular studies performed on a resistant specimen revealed the G1202R mutation. Similarly, in a series of 11 ceritinib-resistant tumors, three were found to harbor a new G1202R mutation and two had acquired a mutation at residue F1174 (17). Importantly, neither of these mutations was detectable in biopsies taken prior to ceritinib treatment. In the absence of a secondary ALK mutation, activation of alternative signaling pathways could also mediate resistance to alectinib. Indeed, in one patient who had relapsed on alectinib, amplification of cMET was reported in a resistant specimen, although it is unknown if it was driving resistance (35). In the case of our alectinib-resistant patient with I1171T, cMET was likely not driving the development of resistance, since ceritinib, which has no anti-cMET activity, was able to induce a durable response lasting over 7 months.
The observation that other structurally distinct, next generation ALK inhibitors may overcome alectinib resistance in vitro and in vivo is clinically significant. Currently, nine next generation ALK inhibitors have entered the clinic, with several showing potent activity in both crizotinib-naïve and crizotinib-resistant patients (19–21). One of the nine next generation ALK inhibitors has already been approved by the FDA for the treatment of advanced, crizotinib-resistant, ALK-positive NSCLC. Until now, it was unknown whether patients could continue to derive benefit from ALK inhibition after failure of a next generation ALK inhibitor. Our results suggest that patients may benefit from multiple, sequential ALK inhibitor therapies, depending on the underlying mechanism of resistance. In those cases with susceptible resistance mutations, like V1180L and I1171T, ceritinib may be highly effective, even in a third-line, post-crizotinib, post-alectinib setting. However, in cases where resistance is mediated by a highly recalcitrant mutation such as G1202R or by a completely different tyrosine kinase, ceritinib may not be helpful, and alternative treatment strategies such as hsp90 inhibition or combinatorial therapeutics may be required. Overall, these findings highlight the importance of serial biopsies to track the dynamic evolution of drug resistance, and to enable the rational selection of therapies most likely to be effective based on the underlying molecular alterations.
Supplementary Material
Statement of translational relevance.
The ALK tyrosine kinase inhibitor (TKI) crizotinib is a standard therapy for patients with ALK-rearranged NSCLC. However, cancers invariably develop resistance to crizotinib and this has spurred the clinical development of multiple next-generation ALK-TKIs, including alectinib (RO5424802/CH5424802) and ceritinib (LDK378). To determine how cancers develop resistance to alectinib, we established a cell line model of acquired resistance to alectinib and analyzed a resistant tumor specimen from a patient who had relapsed on alectinib. We identified two novel secondary ALK mutations, both of which were still sensitive to ceritinib in vitro. Importantly, we found that in the patient, ceritinib was able to overcome resistance to alectinib, suggesting a potential role for sequential therapy with multiple next generation ALK TKIs.
Acknowledgments
We thank S. Baba at Japanese Foundation for Cancer Research (JFCR) for helping with the FISH analysis. Use of the “K” Supercomputer from RIKEN is also acknowledged.
Grant Support
The study was supported in part by R01CA137008, R01CA140594, National Cancer Institute Lung SPORE (to J.A. E.), R01CA164273 (to A.T.S. and J.A.E.) and JSPS KAKENHI grant number 24300344 and 22112008 (to N. Fujita) and 25710015 (to R. Katayama).
Footnotes
Disclosure of Potential Conflicts of Interest
Engelman JA.: Consultant for Genentech, Chugai, Novartis. Sponsored research from Novartis
Shaw AT.: Consultant for: Pfizer, Novartis, Chugai, Ariad, Ignyta, Daiichi-sankyo, and Genentech
References
- 1.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi K, Soda M, Togashi Y, Ota Y, Sekiguchi Y, Hatano S, et al. Identification of a novel fusion, SQSTM1-ALK, in ALK-positive large B-cell lymphoma. Haematologica. 2011;96:464–7. doi: 10.3324/haematol.2010.033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Togashi Y, Soda M, Sakata S, Sugawara E, Hatano S, Asaka R, et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PLoS One. 2012;7:e31323. doi: 10.1371/journal.pone.0031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer. 2013;13:772–87. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 9.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 10.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–43. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–60. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–82. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovly CM, Pao W. Escaping ALK inhibition: mechanisms of and strategies to overcome resistance. Sci Transl Med. 2012;4:120ps2. doi: 10.1126/scitranslmed.3003728. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Kim TM, Kim DW, Go H, Keam B, Lee SH, et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol. 2013;8:415–22. doi: 10.1097/JTO.0b013e318283dcc0. [DOI] [PubMed] [Google Scholar]
- 17.Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–73. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–90. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–8. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 20.Ou SH, Gadgeel S, Chiappori A, Riely G, Lee R, Garcia L, et al. Safety and efficacy analysis of RO5424802/CH5424802 in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) patients who have failed crizotinib in a dose-finding phase I study (AF-002JG, NCT01588028). The European Cancer Congress; 2013. p. Abst 44 LBA. [Google Scholar]
- 21.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono M, Hirata A, Kometani T, Miyagawa M, Ueda S, Kinoshita H, et al. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Molecular cancer therapeutics. 2004;3:465–72. [PubMed] [Google Scholar]
- 24.Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–35. [PubMed] [Google Scholar]
- 25.Zhang S, Wang F, Keats J, Zhu X, Ning Y, Wardwell SD, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des. 2011;78:999–1005. doi: 10.1111/j.1747-0285.2011.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujitani H, Tanida Y, Matsuura A. Massively parallel computation of absolute binding free energy with well-equilibrated states. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:021914. doi: 10.1103/PhysRevE.79.021914. [DOI] [PubMed] [Google Scholar]
- 28.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proc Natl Acad Sci U S A. 2007;104:270–5. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sang J, Acquaviva J, Friedland JC, Smith DL, Sequeira M, Zhang C, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3:430–43. doi: 10.1158/2159-8290.CD-12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a Novel Heat-Shock Protein 90 Inhibitor, in Patients With Molecularly Defined Non-Small-Cell Lung Cancer. J Clin Oncol. 2010;28:4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad MM, Katayama R, McTigue M, Liu W, Deng YL, Brooun A, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossi RT, Saccardo MB, Ardini E, Menichincheri M, Rusconi L, Magnaghi P, et al. Crystal structures of anaplastic lymphoma kinase in complex with ATP competitive inhibitors. Biochemistry. 2010;49:6813–25. doi: 10.1021/bi1005514. [DOI] [PubMed] [Google Scholar]
- 33.Schonherr C, Ruuth K, Yamazaki Y, Eriksson T, Christensen J, Palmer RH, et al. Activating ALK mutations found in neuroblastoma are inhibited by Crizotinib and NVP-TAE684. Biochem J. 2011;440:405–13. doi: 10.1042/BJ20101796. [DOI] [PubMed] [Google Scholar]
- 34.Ou SH, Azada M, Hsiang DJ, Herman JM, Kain TS, Siwak-Tapp C, et al. Next-Generation Sequencing Reveals a Novel NSCLC ALK F1174V Mutation and Confirms ALK G1202R Mutation Confers High-Level Resistance to Alectinib (CH5424802/RO5424802) in ALK-Rearranged NSCLC Patients Who Progressed on Crizotinib. Journal of Thoracic Oncology. 2014;9:549–53. doi: 10.1097/JTO.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 35.Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib can overcome acquired resistance to CH5424802: is amplification of the MET gene a key factor? J Thorac Oncol. 2014;9:e27–8. doi: 10.1097/JTO.0000000000000113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.