Abstract
Purpose
Left ventricular (LV) remodeling and myocardial fibrosis have been linked to adverse heart failure outcomes. Mid wall late gadolinium enhancement (MW-LGE) on cardiac magnetic resonance (CMR) imaging is well-associated with non-ischemic cardiomyopathy (NICM), but prevalence in ischemic cardiomyopathy (ICM) and association with remodeling are unknown.
Methods
The population comprised patients with systolic dysfunction (LVEF≤40%). CMR was used to identify MW-LGE, conventionally defined as fibrosis of the mid-myocardial or epicardial aspect of the LV septum.
Results
285 patients were studied. MW-LGE was present in 12%, and was 10-fold more common with NICM (32%) vs. ICM (3%, p<0.001). However, owing to higher prevalence of ICM, 15% of patients with MW-LGE had ICM. LV wall stress was higher (p=0.02) among patients with, vs. those without, MW-LGE despite similar systolic blood pressure (p=0.24). In multivariate analysis, MW-LGE was associated with CMR-quantified LV end-diastolic volume (p=0.03) independent of LVEF and mass. Incorporation of clinical and imaging variables demonstrated MW-LGE to be associated with higher LV end-diastolic volume (OR=1.13 [CI 1.004–1.27] per 10 ml/m2, p=0.04) after controlling for presence of NICM (OR=16.0 [CI 5.8–44.1], p<0.001).
Conclusions
While more common in NICM, MW-LGE can occur in ICM and is a marker of LV chamber dilation irrespective of cardiomyopathic etiology.
Keywords: cardiomyopathy, cardiovascular magnetic resonance, myocardial fibrosis, remodeling
Introduction
Left ventricular (LV) chamber remodeling is an important prognostic marker that has been linked to heart failure and death [1–3]. Increased LV chamber size, an early adaptation to maintain cardiac output in the face of contractile dysfunction, can ultimately impair systolic function due to increased wall stress and altered myocardial architecture. Both LV chamber dilation and myocardial hypertrophy are associated with up-regulation of neurohormonal mediators [4–6], providing a physiologic mechanism for the relationship between pathologic remodeling and adverse prognosis. Consistent with this, LV remodeling parameters are established targets for heart failure therapies, with reverse remodeling shown to predict improved clinical outcomes [3, 7, 8].
Myocardial tissue substrate holds the potential to impact LV remodeling. Cardiac magnetic resonance (CMR) tissue characterization imaging is widely used to assess myopathic substrate for both ischemic (ICM) and non-ischemic (NICM) cardiomyopathies. In patients with ICM, infarct burden is an established predictor of LV dilation and dysfunction [7, 9, 10], as well as lack of LV remodeling response to heart failure medications [11]. CMR has also been used to demonstrate myocardial scar in the absence of coronary artery disease: NICM-associated scar; often termed mid wall late gadolinium enhancement (MW-LGE), characteristically involves the mid myocardial aspect of the interventricular septum [12, 13]. MW-LGE on CMR has been shown to correlate with actual fibrosis on histopathology [14, 15] and to predict increased longitudinal mortality risk [14, 16]. LV dysfunction and chamber dilatation have each been associated with MW-LGE [16–18]. However, although CMR can assess LV structure, function, and tissue characteristics within a single exam, the independent association of MW-LGE with LV remodeling parameters has not been tested. Additionally, while MW-LGE has been well described in NICM [13, 14, 16–18], uncertainty exists as to whether it can occur in the context of angiography-classified ICM.
This study examined prevalence and structural correlates of MW-LGE among a cross-sectional cohort of subjects with angiography-classified ICM and NICM. The goals were: (1) to determine the prevalence of MW-LGE among a broad population of patients with cardiomyopathy; and (2) to evaluate independent associations between MW-LGE and LV chamber remodeling.
Material and Methods
Population
The study population was retrospectively accrued from consecutive patients with CMR-evidenced advanced LV systolic dysfunction (LVEF ≤40%), in whom both blood pressure within 24 hours of CMR (for assessment of wall stress) and invasive x-ray angiography (for assignment of cardiomyopathic etiology) were available. In order to test associations between MW-LGE and LV remodeling, patients with conditions known to preclude assessment of localized fibrosis within the septal mid wall (i.e. amyloid, surgically repaired complex congenital heart disease, hypertrophic cardiomyopathy), or those with incomplete CMR exams (i.e. lack of delayed enhancement imaging necessary for MW-LGE) were excluded from this study.
CMR was performed at Weill Cornell Medical College (New York, NY) between May 2005 – November 2013. Transthoracic echocardiography (echo), if performed at Weill Cornell within 1 week of CMR, was used as a supplemental test for cardiac structure/function. Blood pressure was measured non-invasively as part of clinical care. Demographic indices were categorized using a standardized patient questionnaire, with results confirmed via review of medical records. In accordance with established criteria [19], patients were classified as having ICM if obstructive coronary artery disease (≥50% left main, ≥70% major epicardial vessel) was present on invasive angiography, or based on prior coronary revascularization. All other patients were classified as having NICM.
This study was conducted with approval of the Institutional Review Board (IRB) at Weill Cornell Medical College.
Imaging Protocol
Cardiac Magnetic Resonance
CMR was performed using 1.5 Tesla scanners (General Electric, Waukesha, WI). Exams consisted of two components: (1) cine-CMR for geometry/function and (2) delayed enhancement (DE-) CMR for tissue characterization. Cine-CMR was performed using a steady-state free precession sequence. DE-CMR was performed 10–30 minutes after administration of gadolinium (0.2 mmol/kg) using a segmented inversion recovery sequence [20], with inversion time tailored to null viable myocardium. Cine- and DE-CMR were obtained in matching short and long-axis planes: Short axis images were acquired throughout the LV from the mitral valve annulus through the apex. Long axis images were acquired in standard two-, three- and four-chamber orientations.
DE-CMR was used to identify MW-LGE, which was defined in accordance with established criteria as localized hyperenhancement within the mid myocardial aspect of the basal to mid inter-ventricular septum (Figure 1) [13]. MW-LGE was scored as a binary variable (present/absent) and, if present, total MW-LGE size was quantified via planimetery of hyperenhanced myocardium.
Figure 1. Typical Examples of Mid-Wall Fibrosis.
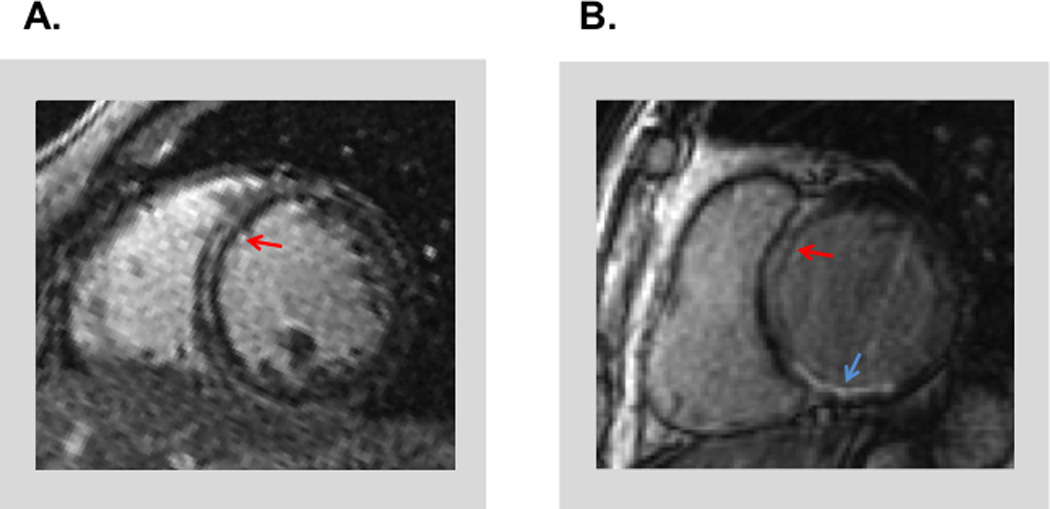
Representative examples of MW-LGE (red arrows) among patients with angiography-evidenced NICM (1A) and ICM (1B). Note presence MW-LGE with concomitant inferior wall myocardial infarction (blue arrow) in the context of ICM (1B).
Cine-CMR was used to assess cardiac function and chamber size. LV chamber volume at end-diastole and end-systole was quantified by planimetry of contiguous short axis images, with LV ejection fraction (LVEF) calculated based on chamber volumes. LV mass (myocardial volume * specific gravity) was measured at end-diastole; papillary muscle and trabeculae were included within myocardial contours [21, 22]with concentricity calculated as the ratio between mass and chamber volume. LV systolic wall stress (σ) was calculated at peak systole based on myocardial volume (VMYO), chamber volume (VLV) and systolic blood pressure (BP) [23]:
| (1) |
Echocardiography
Transthoracic 2D echocardiograms were performed by experienced sonographers using commercially available equipment (i.e. General Electric Vivid-7, Philips IE 33). Imaging was performed in parasternal long- and short-axis and apical 2-, 3-, and 4- chamber orientations. In accordance with American Society of Echocardiography guidelines for chamber quantification [24], LV linear dimensions at end diastole and end systole were measured in the parasternal long axis at the level of the LV minor axis.
Statistical Methods
Continuous variables (expressed as mean±standard deviation) were compared using Student’s t-tests. Categorical variables were compared using Chi-square or, when fewer than 5 expected outcomes per cell, Fisher’s exact test. Clinical and imaging parameters were compared using bivariate correlation coefficients, as well as univariable and multivariable logistic regression analyses. Two-sided p <0.05 was considered indicative of statistical significance. Statistical calculations were performed using SPSS 20.0 (SPSS Inc, Chicago, IL).
Results
Population characteristics
The study population consisted of 285 patients with advanced LV dysfunction (mean LVEF 27±8%). Two-thirds (68%) had ICM based on invasive coronary angiography. Mean blood pressure, as measured non-invasively within 1 day (4.7±7 hours) of CMR, was 118±20/68±13 mmHg. There was no difference in the prevalence of cardiovascular risk factors including diabetes and hypertension in relationship to MW-LGE presence (Table 1). Whereas hypertension, diabetes mellitus and hyperlipidemia were each more common among patients with ICM as compared to NICM (all p<0.01), none of these risk factors modified the likelihood for MW-LGE within either cardiomyopathic category (p=NS for all).
Table 1.
Clinical Characteristics
| Parameter | Overall (n=285) |
MW-LGE + (n=34) |
MW-LGE − (n=251) |
P |
|---|---|---|---|---|
| Age (year) | 60 ± 15 | 54 ± 16 | 61 ± 14 | 0.009 |
| Male gender | 73% (208) | 85% (29) | 71% (179) | 0.09 |
| Coronary Artery Disease Risk Factors | ||||
| Hypertension | 68% (194) | 59% (20) | 69% (174) | 0.22 |
| Hypercholesterolemia | 49% (140) | 41% (14) | 50% (126) | 0.32 |
| Diabetes Mellitus | 35% (100) | 32% (11) | 36% (89) | 0.72 |
| Tobacco Use | 53% (151) | 41% (14) | 55% (137) | 0.14 |
| Family History | 17% (49) | 12% (4) | 18% (45) | 0.47 |
| Prior Myocardial Infarction | 54% (152) | 9% (3) | 59% (149) | <0.001 |
| Coronary Revascularization | ||||
| Percutaneous Intervention | 31% (87) | 9% (3) | 34% (84) | 0.003 |
| Coronary Artery Bypass Grafting | 13% (36) | 3% (1) | 14% (35) | 0.10 |
| Cardiomyopathic Etiology | ||||
| Ischemic | 68% (194) | 15% (5) | 75% (189) | <0.001 |
| Non-Ischemic | 32% (91) | 85% (29) | 25% (62) | <0.001 |
| Hemodynamic Indices | ||||
| Heart Rate (bpm) | 79 ± 17 | 83 ± 19 | 79 ± 16 | 0.16 |
| Systolic blood pressure (mmHg) | 118 ± 20 | 122 ± 21 | 118 ± 20 | 0.24 |
| Diastolic blood pressure (mmHg) | 68 ± 13 | 72 ± 13 | 67 ± 13 | 0.04 |
| Mean arterial pressure (mmHg) | 85 ± 13 | 89 ± 14 | 84 ± 13 | 0.06 |
| Anthropomorphic indices | ||||
| Height (cm) | 171 ± 10 | 175 ± 9 | 171 ± 10 | 0.03 |
| Weight (kg) | 82 ± 20 | 88 ± 16 | 81 ± 21 | 0.07 |
| BSA (m2) | 1.9 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.3 | 0.02 |
Boldface type indicates p < 0.05
MW-LGE was present in 12% of the population; prevalence was over 10-fold higher among patients with NICM as compared to ICM (32% vs. 3%; p<0.001). However, owing to the predominance of ICM among the overall population, 15% of cases with MW-LGE actually occurred in patients with obstructive CAD. Figure 1B provides a representative example of MW-LGE in a patient with angiography-classified ICM.
Cardiac Function and Remodeling
Table 2 reports cardiac function and remodeling indices in relation to MW-LGE. As shown, MW-LGE was associated with increased LV chamber size and wall stress (both p<0.05). Among wall stress components, only chamber volume and myocardial mass differed between patients with and without with MW-LGE, whereas systolic blood pressure was similar between groups (p=NS). Regarding systolic function, Table 2 demonstrates that MW-LGE-associated decrement in CMR quantified LVEF (p=0.02) was not associated with reduction in either stroke volume (p=0.22) or cardiac output (p=0.09), consistent with the concept that end-diastolic chamber dilation was a compensation for lower systolic function. Regarding infarction, patients with concomitant ICM and MW-LGE (n=5) had smaller total infarct size as compared to those without (4.9 vs. 19.0% LV; p=0.003).
Table 2.
Imaging Parameters
|
Overall (n=285) |
MW-LGE + (n=34) |
MW-LGE − (n=251) |
P | |
| Cardiac Magnetic Resonance | ||||
| Left Ventricular Function | ||||
| Ejection fraction (%) | 27 ± 8 | 24 ± 9 | 28 ± 8 | 0.02 |
| Stroke volume (ml) | 60 ± 23 | 68 ± 40 | 59 ± 19 | 0.22 |
| (ml/m2) | 31 ± 11 | 34 ± 21 | 31 ± 9 | 0.42 |
| Cardiac Output (L/min) | 4.7 ± 1.9 | 5.6 ± 3.2 | 4.6 ± 1.6 | 0.09 |
| (L-m2/min) | 2.4 ± 0.9 | 2.8 ± 1.7 | 2.4 ± 0.7 | 0.20 |
| Left Ventricular Remodeling Indices | ||||
| End-diastolic volume (ml) | 230 ± 78 | 287± 93 | 223 ± 73 | <0.001 |
| (ml/m2) | 119 ± 37 | 141 ± 45 | 116 ± 35 | <0.001 |
| End-systolic volume (ml) | 170 ± 72 | 218 ± 90 | 163 ± 67 | 0.001 |
| (ml/m2) | 88 ± 35 | 107 ± 43 | 85 ± 33 | 0.001 |
| Myocardial mass (gm) | 192 ± 56 | 219 ± 56 | 188 ± 55 | 0.003 |
| (gm/m2) | 99 ± 26 | 108 ± 24 | 98 ± 26 | 0.04 |
| Concentricity (gm/ml) | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.12 |
| Anteroseptal wall thickness (cm) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.41 |
| Posterolateral wall thickness (cm) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.88 |
| Peak Systolic Wall stress (kPa) | 34 ± 8.8 | 37 ± 10 | 33 ± 9 | 0.02 |
| Left Atrial Geometry | ||||
| Diameter (cm) | 4.2 ± 0.7 | 4.3 ± 0.6 | 4.2 ± 0.7 | 0.38 |
| Area (cm2) | 27 ± 7 | 31 ± 7 | 26 ± 7 | <0.001 |
| (cm2/m2) | 14 ± 4 | 15 ± 3 | 14 ± 4 | 0.06 |
|
Overall (n=206) |
MW-LGE + (n=22) |
MW-LGE − (n=184) |
P | |
| Transthoracic Echocardiography* | ||||
| Left Ventricular Function | ||||
| Ejection fraction (%) | 26 ± 10 | 23 ± 10 | 27 ± 9 | 0.10 |
| Left Ventricular Remodeling Indices | ||||
| End-diastolic diameter (cm) | 6.2 ± 0.8 | 6.7 ± 1.1 | 6.2 ± 0.8 | 0.04 |
| End-systolic diameter (cm) | 5.5 ± 0.9 | 6.0 ± 1.2 | 5.4 ± 0.9 | 0.04 |
| Myocardial mass (gm) | 249 ± 70 | 288 ± 70 | 243 ± 68 | 0.004 |
| (gm/m2) | 128 ± 33 | 142 ± 31 | 126 ± 33 | 0.03 |
| Relative wall thickness (cm) | 0.30 ± 0.06 | 0.29 ± 0.09 | 0.30 ± 0.06 | 0.61 |
| Left Atrial Geometry | ||||
| Diameter (cm) | 4.4 ± 0.8 | 4.5 ± 1.0 | 4.3 ± 0.8 | 0.33 |
Available in 72% of the population (mean interval of 1.7 ± 3.0 days of CMR)
Integrated Clinical and Imaging Markers
Multivariate logistic regression analysis was performed to further assess structural and clinical markers of MW-LGE. Restricted to imaging indices, results (Table 3A) demonstrated LV end diastolic volume be independently associated with MW-LGE (OR=1.14, CI 1.02–1.29; p<0.05) after controlling for LV ejection fraction (OR=0.84, CI 0.50–1.42; p=0.51) and myocardial mass (OR=0.99, CI 0.84–1.17; p=0.90).
Table 3.
Multivariate Regression for Presence of Mid-Wall Fibrosis
| 3A. Imaging | |||
| (Model χ2 = 11.8, p = 0.008) | |||
| Variable (CMR) | Odds Ratio | 95% Confidence Interval | P |
| LV End-Diastolic Volume (per 10 ml/m2) | 1.14 | 1.02–1.29 | 0.03 |
| LV Ejection Fraction (per 10 % decrement) | 0.84 | 0.50–1.42 | 0.51 |
| Myocardial Mass (per 10 g/m2) | 0.99 | 0.84–1.17 | 0.90 |
| 3B. Clinical | |||
| (Model χ2 = 48.0, p < 0.001) | |||
| Variable | Odds Ratio | 95% Confidence Interval | P |
| Non-Ischemic Etiology | 17.80 | 6.3– 50.3 | <0.001 |
| Hypertension | 0.95 | 0.41 – 2.21 | 0.91 |
| Age (years) | 1.00 | 0.97 – 1.03 | 0.93 |
| 3C. Integrated Clinical/Imaging | |||
| (Model χ2 = 52.5, p < 0.001) | |||
| Variable | Odds Ratio | 95% Confidence Interval | P |
| Non-Ischemic Etiology | 15.99 | 5.80– 44.13 | <0.001 |
| LV End-Diastolic Volume (10 ml/m2) | 1.13 | 1.004 – 1.27 | 0.04 |
| LV Ejection Fraction (per 10 % decrement) | 1.17 | 0.68 – 2.02 | 0.57 |
Analysis of clinical variables (Table 3B) confirmed a strong association of MW-LGE with NICM (OR=17.8, CI 6.3–50.3; p<0.001), which was independent of age and clinical indices. As shown in Table 3C, a combined model incorporating both clinical and imaging variables demonstrated both NICM and LV chamber volume to be independently associated with MW-LGE even after controlling for LV ejection fraction. Overall strength (χ2 =52.5; p<0.001) of the combined clinical/imaging model for MW-LGE was higher than that of isolated clinical (χ2 = 48.0; p<0.001) and CMR imaging (χ2 = 11.8; p=0.008) models.
Prevalence and Size of Mid-Wall Fibrosis in relation to LV Volume
Figure 2 reports MW-LGE prevalence in relation to population-based stratification of LV chamber volume (2A) and wall stress (2B). As shown, MW-LGE was 4-fold more common among patients in the highest, as compared to those in the lowest tertile of LV end diastolic volume (7.7% vs. 1.8%), paralleling a 2-fold difference in MW-LGE prevalence among groups stratified based on LV wall stress (6.3% vs. 2.8%).
Figure 2. Mid-Wall Fibrosis Prevalence in Relation to LV Remodeling.
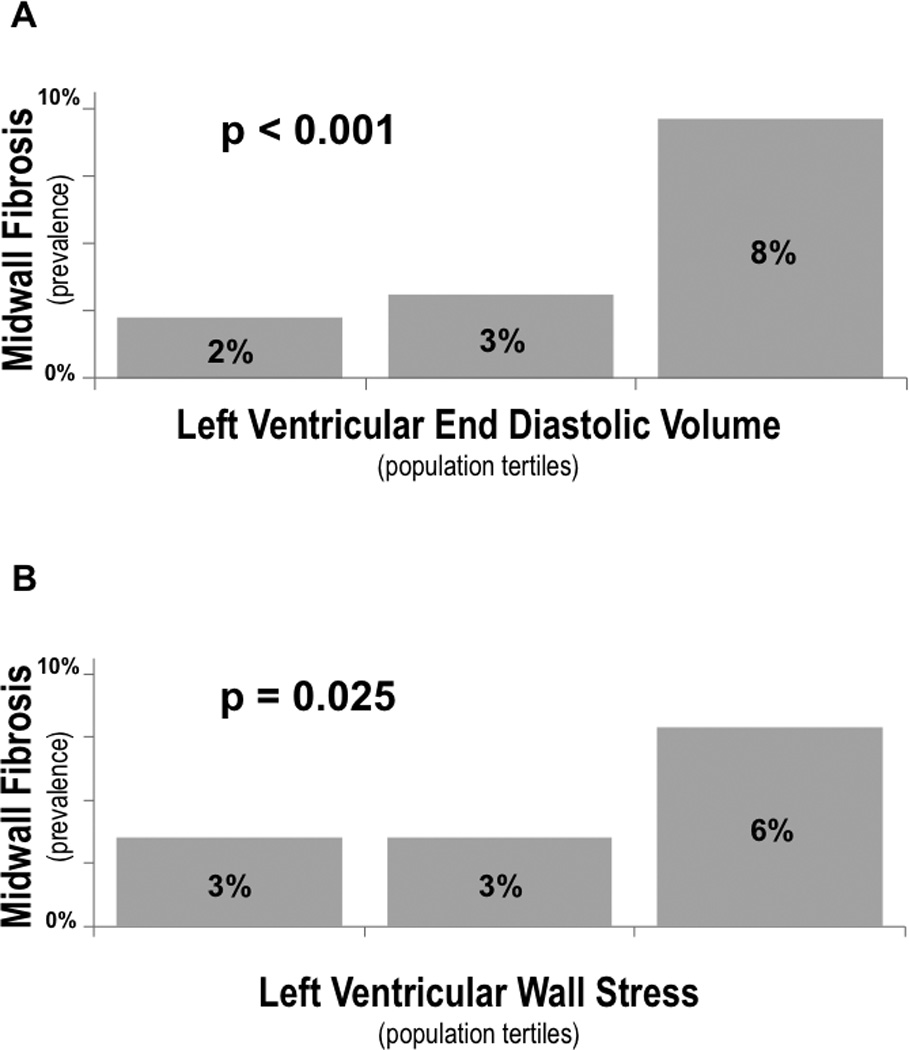
Prevalence of MW-LGE in relation to population-based tertiles of LV end-diastolic volume (2A) and wall stress (2B). As shown, MW-LGE prevalence increased stepwise in relation to both LV remodeling parameters (both p<0.05), although magnitude of difference between highest and lowest tertiles were greater for LV volume than for the aggregate parameter of LV wall stress.
MW-LGE constituted, on average, 1.9±1.4 (range 0.1–4.8) grams, corresponding to 0.9±0.7% (0.05–2.9%) LV myocardium. Figure 3 illustrates the relationship between MW-LGE size and LV chamber volume, with MW-LGE-affected patients partitioned into two groups based on fibrosis size. As shown, LV volume was similar (p=0.72) between MW-LGE-affected patients in the top, as compared to those in the bottom half of fibrosis size. Conversely, both MW-LGE-affected groups manifested similar magnitude of chamber dilation as compared to patients without MW-LGE (both p<0.01)
Figure 3. Mid-Wall Fibrosis Size in Relation to LV Remodeling.
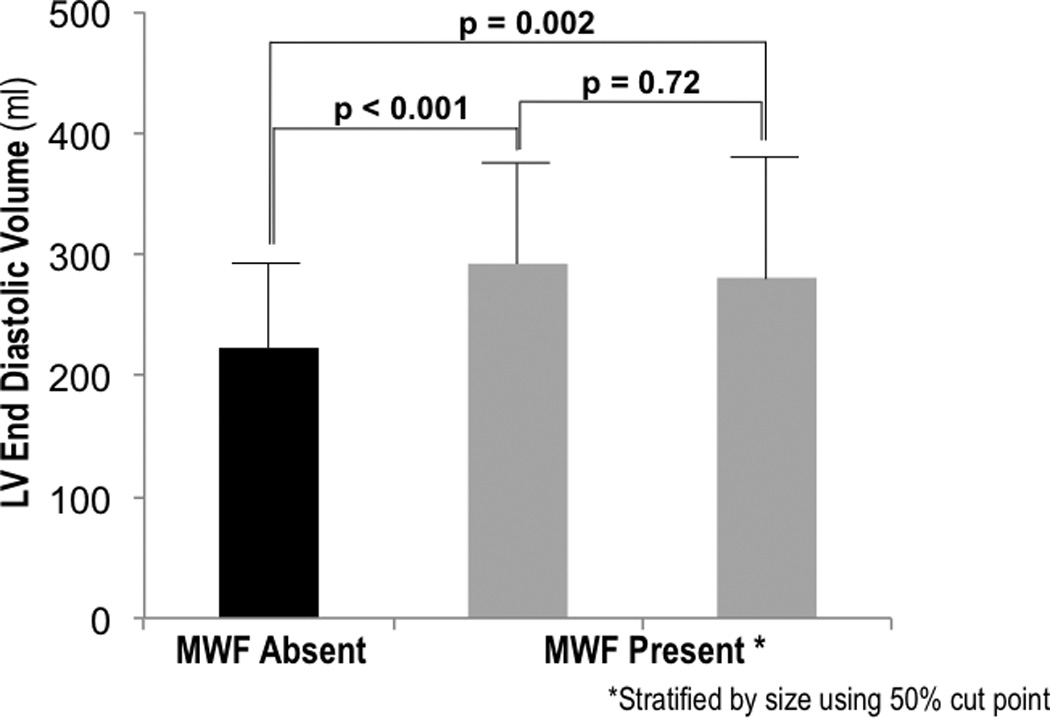
LV end-diastolic volume (mean ± SD) among patients without MW-LGE (black bar), as well as MW-LGE-affected patients (grey bars) stratified into two groups based on fibrosis size (right = top, left = bottom 50%). Note that both groups of MW-LGE-affected patients had larger LV chamber volumes than did those without MW-LGE, with non-significant differences between MW-LGE-affected patients stratified by fibrosis size.
Discussion
This study highlights the importance of myocardial tissue characterization for cardiomyopathy assessment: Among a broad cohort of patients with advanced LV dysfunction, 12% had MW-LGE by CMR. Whereas MW-LGE was far more common among patients with angiography-classified NICM (32%), this association was not exclusive, with MW-LGE present in a small percentage (3%) of patients with ICM. MW-LGE was strongly associated with LV chamber dilation by both CMR and echo, with multivariate analysis demonstrating an independent association between presence of MW-LGE and magnitude of LV chamber dilation even after controlling for cardiomyopathy etiology and severity of contractile dysfunction. While the multivariate model was performed using CMR alone so as to provide a single model for incorporation of LV geometry and tissue substrate, our observed relationship between LV remodeling parameters and MW-LGE on echo is of broad applicability to clinical practice as echo is widely used as the primary screening test for LV remodeling and has been shown to impact clinical outcomes independent of LV systolic function[2] [25] [7].
Our observation that MW-LGE can occur in the context of ICM sheds new light on earlier CMR literature, which suggested that this pattern of fibrosis is exclusively found among patients with NICM. For example, among a cohort of 90 cardiomyopathy patients, McCrohon et al. reported that no patients with ICM had MW-LGE [13]. Our results, derived from a larger cohort, demonstrate that MW-LGE can occur in the setting of ICM as classified based on invasive coronary angiography. Consistent with the known higher prevalence of ICM as compared to NICM [26], 15% of patients with MW-LGE in our study population had obstructive CAD. The notion that myocardial and coronary substrate can differ is well established. Endomyocardial biopsy studies have shown histologic findings consistent with myocarditis in heart failure patients with angiography-evidenced CAD [27]. Autopsy studies have demonstrated that pathology evidenced ischemic type scar can occur in the absence of obstructive CAD [28, 29]. Our findings demonstrate the converse – namely that non-ischemic scar can be present among patients with angiography evidenced CAD.
Our findings also extend upon prior research that has linked MW-LGE to LV remodeling [14, 17, 18]. Consistent with our results, studies have reported higher LV chamber size and myocardial mass, as well as lower EF among patients with MW-LGE [14, 17]. Higher LV wall stress – a measure of myocardial load directly proportional to chamber volume and blood pressure, and inversely proportional to myocardial mass – has also been shown to be associated with MW-LGE [23]. However, to the best of our knowledge, no study has simultaneously tested the independent relationship between MW-LGE and LV remodeling indices, raising uncertainty as to whether MW-LGE is primarily associated with alterations in LV volume, myocardial geometry, or contractile dysfunction. Our finding of an independent association between LV end-diastolic volume and MW-LGE supports the concept of a primary association between chamber remodeling and myocardial tissue damage manifested as MW-LGE.
Several possible mechanisms may explain our observed link between MW-LGE and LV chamber dilation. First, it is possible that increased chamber size, with concomitant increased wall stress as well as elevation of LV diastolic pressure impairs myocardial perfusion, resulting in localized myocardial ischemia or necrosis. Second, MW-LGE may reflect a localized manifestation of remodeling-associated alterations in systemic signaling pathways that serve to up-regulate expression of pro-fibrotic mediators. Indeed, patients with advanced LV dysfunction have been shown to manifest up-regulation of matrix metalloproteinases [30, 31], which can contribute to extracellular matrix degradation and fibrosis [32]. On the other hand, it is conceivable that MW-LGE itself reflects a cause rather than a consequence of LV remodeling, whereby fibrosis contributes to altered myocyte mechanics and contributes to secondary chamber enlargement.
To the best of our knowledge, no prior study has examined whether LV remodeling is altered in proportion to actual extent of MW-LGE. Despite our finding that presence of MW-LGE was associated with increased chamber size, results demonstrated no significant relationship between the extent of MW-LGE and chamber size. It is possible that our observed lack of correlation reflects a physiologic phenomenon, whereby MW-LGE develops when a given threshold of chamber dilation has been achieved, but does not vary in relation to progressive chamber enlargement. It is also plausible that different pharmacologic interventions may variably attenuate LV volume overload or MW-LGE, thereby blunting the correlation between the two indices. Further studies, employing serial CMR to examine time dependent changes in MW-LGE and LV remodeling, as well as the impact of therapeutic interventions, are needed to test these concepts.
In conclusion, this study demonstrates that MW-LGE, while far more common in NICM, can occur in ICM and is a marker of LV chamber dilation irrespective of cardiomyopathic etiology.
Study Limitations
Several limitations should be noted. First, whereas the interval between CMR and blood pressure measurement was short (mean 4.7±7.0 hours), both LV volume and afterload status can vary on a near instantaneous basis. However, interval between CMR and blood pressure did not differ between patients with and without MW-LGE (5.5±8.3 vs. 4.6±6.9 hours, p=0.49), supporting the notion that our observed association between MW-LGE and LV chamber enlargement was not influenced by this potential source of bias. Second, current analysis was limited to patients with LV systolic dysfunction, in context of recent data demonstrating MW-LGE to predict clinical prognosis in patients with systolic heart failure [14, 16]. While the results of the current study do not directly apply to MW-LGE in the absence of LV dysfunction, it is interesting to note that MW-LGE has been associated with right ventricular dilation among patients with pulmonary hypertension [33], suggesting that CMR-evidenced alterations in septal tissue characteristics may be a more generalized marker of myocardial tissue substrate changes that accompany chamber remodeling. Lastly, as demographic data including prior history of MI were obtained via a standardized patient survey, the exact date of MI could not be verified in a number of patients. Due to this limitation, temporal relationships between prior MI and MW-LGE formation could not be assessed.
Acknowledgments
Sources of Funding: K23 HL102249-01 (JWW)
Footnotes
Conflicts of Interests: None
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
All authors contributed significantly to the work. JK participated in the study’s design, image analysis, data collection and manuscript preparation. JDK, SG, AA, MP, SV, BD, PMO, EH, and RBD participated in data collection, image analysis and critical revision of the manuscript for important intellectual contents. JWW designed the study and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Migrino RQ, Young JB, Ellis SG, White HD, Lundergan CF, Miller DP, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO)-I Angiographic Investigators. Circulation. 1997;96:116–121. doi: 10.1161/01.cir.96.1.116. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fonarow GC, Child JS, et al. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol. 1993;72:672–676. doi: 10.1016/0002-9149(93)90883-e. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 4.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 5.Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999;100:2093–2099. doi: 10.1161/01.cir.100.20.2093. [DOI] [PubMed] [Google Scholar]
- 6.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci U S A. 2000;97:931–936. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SD, Skali H, Anavekar NS, Bourgoun M, Barvik S, Ghali JK, et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation. 2005;111:3411–3419. doi: 10.1161/CIRCULATIONAHA.104.508093. [DOI] [PubMed] [Google Scholar]
- 8.Wong M, Johnson G, Shabetai R, Hughes V, Bhat G, Lopez B, et al. Echocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI65–VI70. [PubMed] [Google Scholar]
- 9.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, et al. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 11.Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 12.Soriano CJ, Ridocci F, Estornell J, Pérez-Boscá JL, Pomar F, Trigo A, et al. Late gadolinium-enhanced cardiovascular magnetic resonance identifies patients with standardized definition of ischemic cardiomyopathy: a single centre experience. Int J Cardiol. 2007;116:167–173. doi: 10.1016/j.ijcard.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 13.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 14.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 15.de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14:299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 16.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 18.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 20.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 21.Janik M, Cham MD, Ross MI, Wang Y, Codella N, Min JK, et al. Effects of papillary muscles and trabeculae on left ventricular quantification: increased impact of methodological variability in patients with left ventricular hypertrophy. J Hypertens. 2008;26:1677–1685. doi: 10.1097/HJH.0b013e328302ca14. [DOI] [PubMed] [Google Scholar]
- 22.Codella NC, Cham MD, Wong R, Chu C, Min JK, Prince MR, et al. Rapid and accurate left ventricular chamber quantification using a novel CMR segmentation algorithm: a clinical validation study. J Magn Reson Imaging. 2010;31:845–853. doi: 10.1002/jmri.22080. [DOI] [PubMed] [Google Scholar]
- 23.Alter P, Rupp H, Adams P, Stoll F, Figiel JH, Klose KJ, et al. Occurrence of late gadolinium enhancement is associated with increased left ventricular wall stress and mass in patients with non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2011;13:937–944. doi: 10.1093/eurjhf/hfr082. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 26.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci A, Chimenti C, Maseri A. Global biventricular dysfunction in patients with asymptomatic coronary artery disease may be caused by myocarditis. Circulation. 1999;99:1295–1299. doi: 10.1161/01.cir.99.10.1295. [DOI] [PubMed] [Google Scholar]
- 28.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 29.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, et al. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 30.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 31.Tyagi SC, Campbell SE, Reddy HK, Tjahja E, Voelker DJ. Matrix metalloproteinase activity expression in infarcted, noninfarcted and dilated cardiomyopathic human hearts. Mol Cell Biochem. 1996;155:13–21. doi: 10.1007/BF00714328. [DOI] [PubMed] [Google Scholar]
- 32.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 33.Blyth KG, Groenning BA, Martin TN, Foster JE, Mark PB, Dargie HJ, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J. 2005;26:1993–1999. doi: 10.1093/eurheartj/ehi328. [DOI] [PubMed] [Google Scholar]


