Abstract
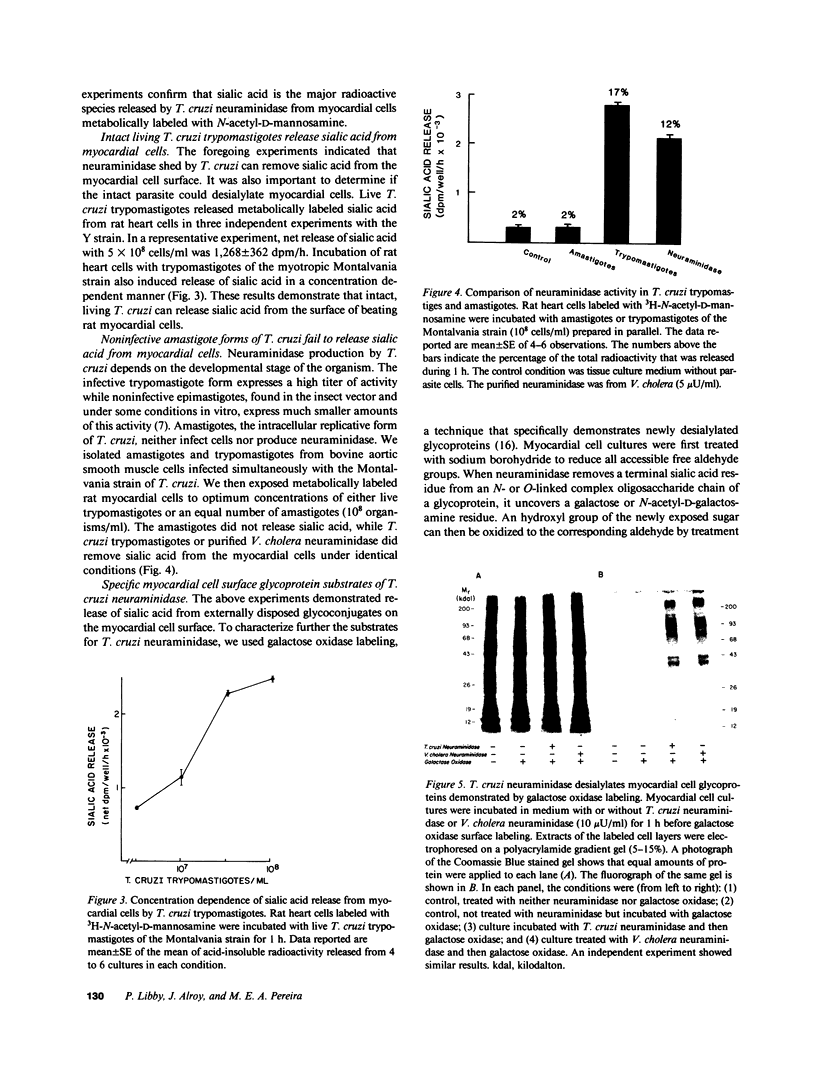
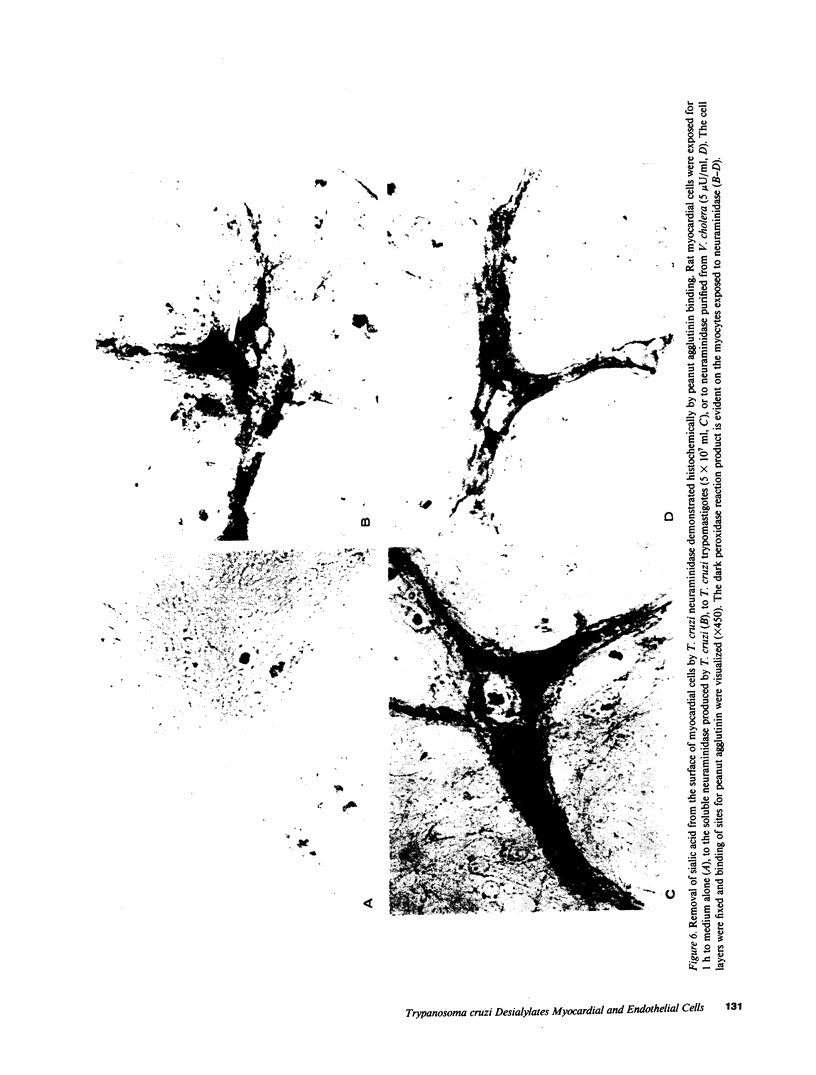
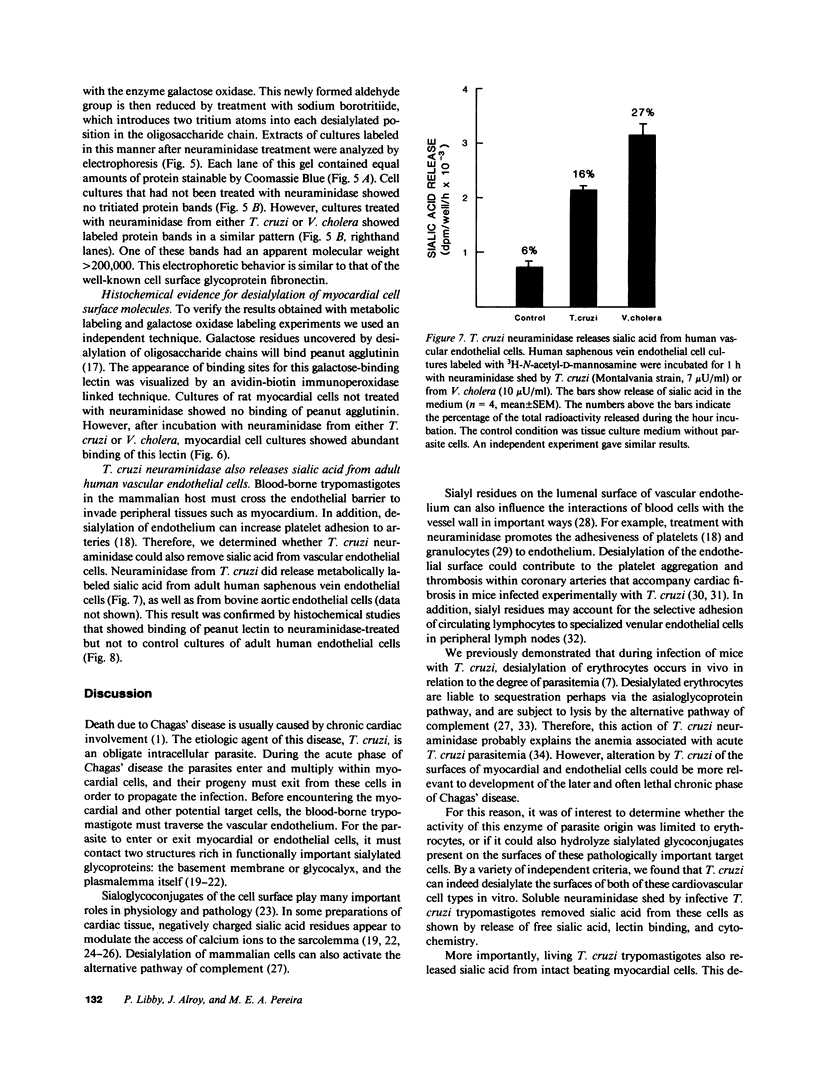
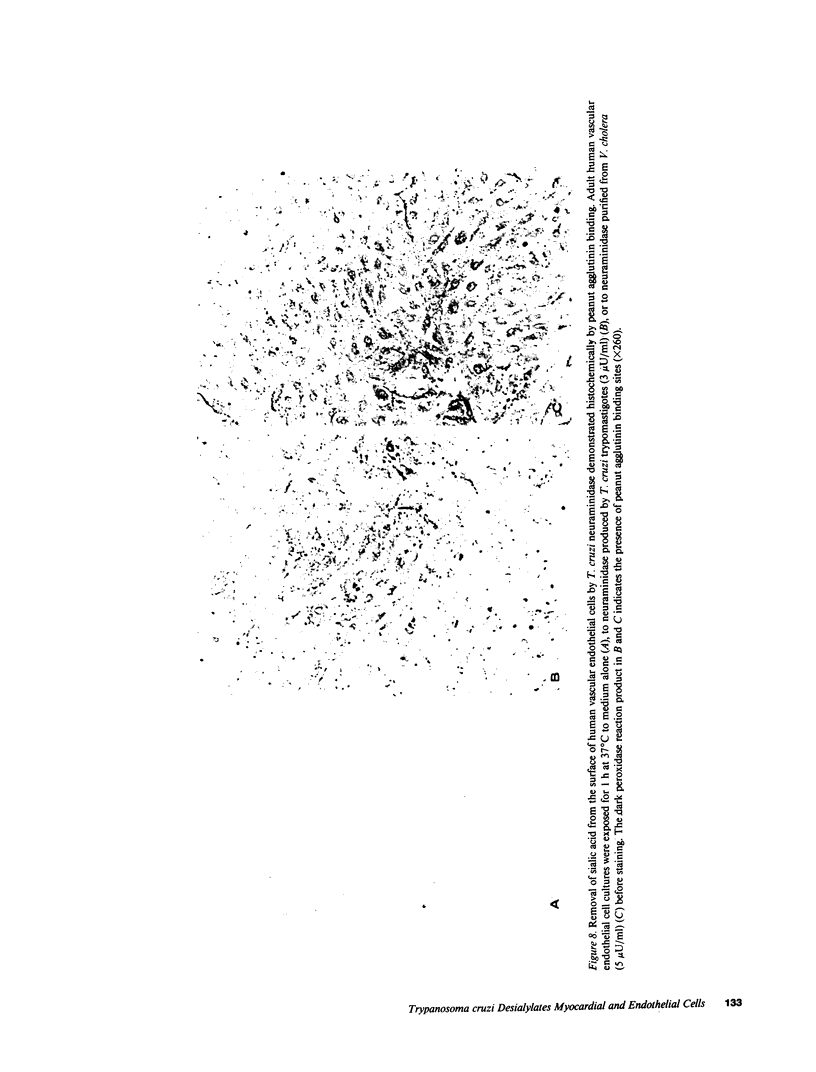
Trypanosoma cruzi causes Chagasic heart disease, a major public health problem in Latin America. The mechanism of interaction of this protozooan parasite with host cells is poorly understood. We recently found that the infective trypomastigote form a T. cruzi exhibits neuraminidase activity and can desialylate mammalian erythrocytes. However, it is not known if T. cruzi can also modify the surfaces of cardiovascular cells that are directly involved in the most important clinical manifestations of this disease. Accordingly, this study determined whether T. cruzi can remove sialic acid from cultured rat myocardial or human vascular endothelial cells. Sialic acid was labeled metabolically with the precursor 3H-N-acetyl-D-mannosamine. Soluble neuraminidase, isolated from intact T. cruzi trypomastigotes, caused significant release of labeled material from myocardial cells (e.g., 2,174 +/- 27 dpm/h vs. spontaneous release of 306 +/- 30 dpm/h, n = 4, P less than 0.001). Chromatographic analysis showed that the bulk of the radioactivity released by T. cruzi neuraminidase was sialic acid. Intact T. cruzi trypomastigotes also released sialic acid from metabolically labeled myocardial cells in a concentration-dependent manner. In contrast, a noninfective form of T. cruzi, the amastigote, did not desialylate these cells. Galactose oxidase labeling demonstrated newly desialylated glycoproteins on the surface of myocardial cells treated with T. cruzi neuraminidase. Desialylation of myocardial cells was confirmed histochemically by the appearance of binding sites for peanut agglutinin, a lectin that binds to complex oligosaccharide moieties after removal of the terminal sialyl residue. T. cruzi neuraminidase also removed sialic acid from adult human saphenous vein endothelial cells, as determined by both histochemical and metabolic labeling studies. Thus, infective forms of T. cruzi can chemically modify the surfaces of myocardial and vascular endothelial cells by desialylation. This alteration may play a role in the initial interaction of this parasite with these important target cells of the host cardiovascular system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Bioul-Marchand M., Jadin J. M., Steiger R. F., Boon T. Multiplication of Trypanosoma cruzi in a mouse myocardial cell line. J Parasitol. 1980 Dec;66(6):1050–1052. [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Frank M. M. Interaction of desialated guinea pig erythrocytes with the classical and alternative pathways of guinea pig complement in vivo and in vitro. J Clin Invest. 1983 Jun;71(6):1710–1719. doi: 10.1172/JCI110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso J. E., Brener Z. Hematological changes in mice experimentally infected with Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1980 Jul-Dec;75(3-4):97–104. doi: 10.1590/s0074-02761980000200009. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Cho S., Wittner M., Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am J Trop Med Hyg. 1985 Mar;34(2):246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gilfix B. M., Sanwal B. D. Metabolic labelling of oligosaccharide chains of glycoproteins in a rat myoblast cell line and its lectin resistant mutants. Can J Biochem Cell Biol. 1983 Oct;61(10):1129–1132. doi: 10.1139/o83-145. [DOI] [PubMed] [Google Scholar]
- Görög P., Pearson J. D. Surface determinants of low density lipoprotein uptake by endothelial cells. Atherosclerosis. 1984 Oct;53(1):21–29. doi: 10.1016/0021-9150(84)90101-1. [DOI] [PubMed] [Google Scholar]
- Görög P., Schraufstätter I., Born G. V. Effect of removing sialic acids from endothelium on the adherence of circulating platelets in arteries in vivo. Proc R Soc Lond B Biol Sci. 1982 Mar 22;214(1197):471–480. doi: 10.1098/rspb.1982.0022. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Halliday J. Removal of sialic acid from cardiac sarcolemma does not affect contractile function in electrically stimulated guinea pig left atria. Nature. 1980 Aug 21;286(5775):819–821. doi: 10.1038/286819a0. [DOI] [PubMed] [Google Scholar]
- Henriquez D., Piras R., Piras M. M. The effect of surface membrane modifications of fibroblastic cells on the entry process of Trypanosoma cruzi trypomastigotes. Mol Biochem Parasitol. 1981 Apr;2(5-6):359–366. doi: 10.1016/0166-6851(81)90087-6. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Hudson L. Trypanosoma cruzi: the immunological consequences of infection. J Cell Biochem. 1983;21(4):299–304. doi: 10.1002/jcb.240210406. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Jancik J., Schauer R. Sialic acid--a determinant of the life-time of rabbit erythrocytes. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):395–400. doi: 10.1515/bchm2.1974.355.1.395. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The structure and function of the myocardial cell surface. Am J Physiol. 1978 Nov;235(5):H461–H468. doi: 10.1152/ajpheart.1978.235.5.H461. [DOI] [PubMed] [Google Scholar]
- Libby P. Long-term culture of contractile mammalian heart cells in a defined serum-free medium that limits non-muscle cell proliferation. J Mol Cell Cardiol. 1984 Sep;16(9):803–811. doi: 10.1016/s0022-2828(84)80004-8. [DOI] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- NEVA F. A., MALONE M. F., MYERS B. R. Factors influencing the intracellular growth of Trypanosoma cruzi in vitro. Am J Trop Med Hyg. 1961 Mar;10:140–154. doi: 10.4269/ajtmh.1961.10.140. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N. Host and parasite factors affecting the invasion of mononuclear phagocytes by Trypanosoma cruzi. Ciba Found Symp. 1983;99:52–73. doi: 10.1002/9780470720806.ch4. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Pelikan P., Gimbrone M. A., Jr, Cotran R. S. Distribution and movement of anionic cell surface sites in cultured human vascular endothelial cells. Atherosclerosis. 1979 Jan;32(1):69–80. doi: 10.1016/0021-9150(79)90148-5. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science. 1983 Mar 25;219(4591):1444–1446. doi: 10.1126/science.6338592. [DOI] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Sadigursky M., Acosta A. M., Santos-Buch C. A. Muscle sarcoplasmic reticulum antigen shared by a Trypanosoma cruzi clone. Am J Trop Med Hyg. 1982 Sep;31(5):934–941. doi: 10.4269/ajtmh.1982.31.934. [DOI] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Silbert J. E., Palade G. E. Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J Cell Biol. 1981 Sep;90(3):614–621. doi: 10.1083/jcb.90.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology. 1980 Mar;101(2):440–449. doi: 10.1016/0042-6822(80)90457-2. [DOI] [PubMed] [Google Scholar]
- Woods W. T., Imamura K., James T. N. Electrophysiological and electron microscopic correlations concerning the effects of neuraminidase on canine heart cells. Circ Res. 1982 Feb;50(2):228–239. doi: 10.1161/01.res.50.2.228. [DOI] [PubMed] [Google Scholar]