Abstract
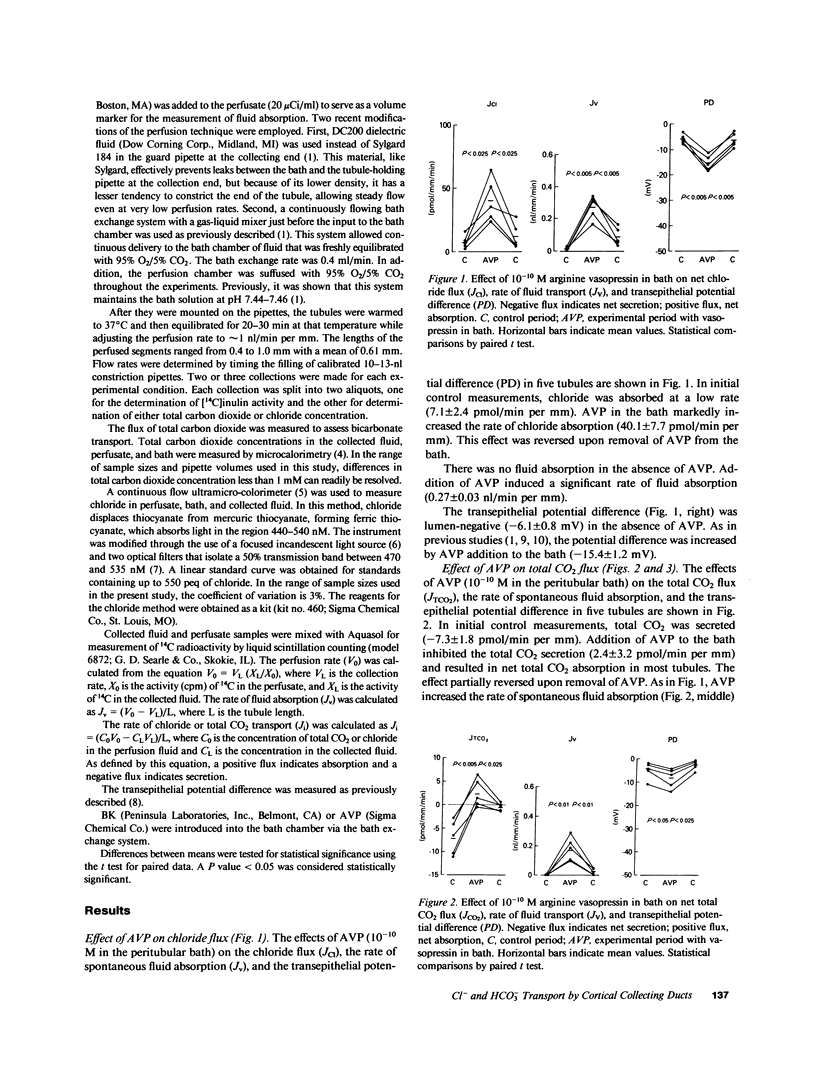
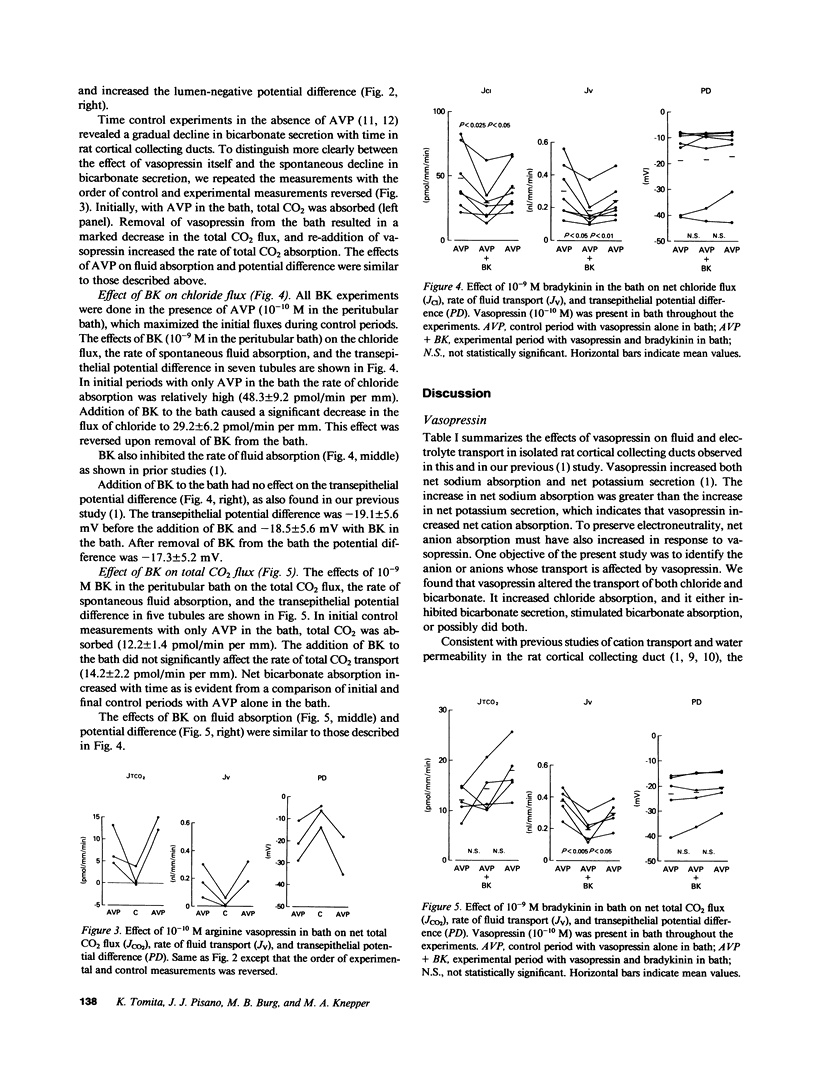
Our previous studies in cortical collecting ducts isolated from rat kidneys have shown that vasopressin increases both sodium absorption and potassium secretion, while bradykinin inhibits sodium absorption without affecting potassium transport. To determine which anions are affected by these agents, we perfused cortical collecting ducts from rats treated with deoxycorticosterone and measured net chloride flux, net bicarbonate flux (measured as total CO2), transepithelial voltage, and the rate of fluid absorption. Arginine vasopressin (10(-10) M in the peritubular bath) caused a sustained sixfold increase in net chloride absorption and a two- to threefold increase in the magnitude of the lumen negative transepithelial voltage. Before addition of vasopressin, the tubules secreted bicarbonate. Vasopressin abolished the bicarbonate secretion, resulting in net bicarbonate absorption (presumably due to proton secretion) in many tubules. Bradykinin (10(-9) M added to the peritubular bath) caused a reversible 40% inhibition of net chloride absorption, but did not affect the transepithelial voltage or the bicarbonate flux. We concluded: (a) that arginine vasopressin stimulates absorption of chloride and inhibits bicarbonate secretion (or stimulates proton secretion) in the rat cortical collecting duct; and (b) that bradykinin inhibits net chloride absorption in the rat cortical collecting duct without affecting transepithelial voltage or bicarbonate flux. Combining these results with the previous observations on cation fluxes described above, we conclude that bradykinin inhibits electroneutral NaCl absorption (or stimulates electroneutral NaCl secretion) in the rat cortical collecting duct.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. L., Burg M. B. Bicarbonate transport by isolated perfused rat collecting ducts. Am J Physiol. 1985 Oct;249(4 Pt 2):F485–F489. doi: 10.1152/ajprenal.1985.249.4.F485. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Friedman P. A., Andreoli T. E. CO2-stimulated NaCl absorption in the mouse renal cortical thick ascending limb of Henle. Evidence for synchronous Na +/H+ and Cl-/HCO3- exchange in apical plasma membranes. J Gen Physiol. 1982 Nov;80(5):683–711. doi: 10.1085/jgp.80.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MYLLE M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959 Apr;196(4):927–936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Kokko J. P. Study of chloride transport across the rabbit cortical collecting tubule. J Clin Invest. 1978 Jul;62(1):39–44. doi: 10.1172/JCI109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper M. A., Good D. W., Burg M. B. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985 Dec;249(6 Pt 2):F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Good D. W., Burg M. B. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol. 1984 Nov;247(5 Pt 2):F729–F738. doi: 10.1152/ajprenal.1984.247.5.F729. [DOI] [PubMed] [Google Scholar]
- Knepper M. A. Urea transport in isolated thick ascending limbs and collecting ducts from rats. Am J Physiol. 1983 Nov;245(5 Pt 1):F634–F639. doi: 10.1152/ajprenal.1983.245.5.F634. [DOI] [PubMed] [Google Scholar]
- Levine D. Z. An in vivo microperfusion study of distal tubule bicarbonate reabsorption in normal and ammonium chloride rats. J Clin Invest. 1985 Feb;75(2):588–595. doi: 10.1172/JCI111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am J Physiol. 1982 Oct;243(4):F335–F341. doi: 10.1152/ajprenal.1982.243.4.F335. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate transport by rabbit cortical collecting tubules. Effect of acid and alkali loads in vivo on transport in vitro. J Clin Invest. 1977 Sep;60(3):766–768. doi: 10.1172/JCI108830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil R. G., Boulpaep E. L. Ionic conductive properties and electrophysiology of the rabbit cortical collecting tubule. Am J Physiol. 1982 Jul;243(1):F81–F95. doi: 10.1152/ajprenal.1982.243.1.F81. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Reif M. C., Troutman S. L., Schafer J. A. Sustained response to vasopressin in isolated rat cortical collecting tubule. Kidney Int. 1984 Nov;26(5):725–732. doi: 10.1038/ki.1984.208. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B. A., Biemesderfer D., Wade J. B., Giebisch G. Structural and functional study of the rat distal nephron: effects of potassium adaptation and depletion. Kidney Int. 1981 Jan;19(1):36–48. doi: 10.1038/ki.1981.5. [DOI] [PubMed] [Google Scholar]
- Star R. A., Burg M. B., Knepper M. A. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest. 1985 Sep;76(3):1123–1130. doi: 10.1172/JCI112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz P. R., Andersen O. S. Electrogenic proton transport in epithelial membranes. J Membr Biol. 1982;65(3):155–174. doi: 10.1007/BF01869960. [DOI] [PubMed] [Google Scholar]
- Stetson D. L., Beauwens R., Palmisano J., Mitchell P. P., Steinmetz P. R. A double-membrane model for urinary bicarbonate secretion. Am J Physiol. 1985 Oct;249(4 Pt 2):F546–F552. doi: 10.1152/ajprenal.1985.249.4.F546. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Sodium chloride absorption by the urinary bladder of the winter flounder. A thiazide-sensitive, electrically neutral transport system. J Clin Invest. 1984 Jul;74(1):7–16. doi: 10.1172/JCI111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Tomita K., Pisano J. J., Knepper M. A. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest. 1985 Jul;76(1):132–136. doi: 10.1172/JCI111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez H., Good D. W., Wright F. S. Mutual dependence of sodium and chloride absorption by renal distal tubule. Am J Physiol. 1984 Dec;247(6 Pt 2):F904–F911. doi: 10.1152/ajprenal.1984.247.6.F904. [DOI] [PubMed] [Google Scholar]
- Vurek G. G. Calcium measurement: picomole quantitation by continuous-flow colorimetry. Anal Biochem. 1981 Jul 1;114(2):288–293. doi: 10.1016/0003-2697(81)90483-8. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Knepper M. A. A colorimeter for measurement of picomole quantities of urea. Kidney Int. 1982 Apr;21(4):656–658. doi: 10.1038/ki.1982.74. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- WIRZ H. Der osmotische Druck in den corticalen Tubuli der Rattenniere. Helv Physiol Pharmacol Acta. 1956;14(3):353–362. [PubMed] [Google Scholar]
- Woodhall P. B., Tisher C. C. Response of the distal tubule and cortical collecting duct to vasopressin in the rat. J Clin Invest. 1973 Dec;52(12):3095–3108. doi: 10.1172/JCI107509. [DOI] [PMC free article] [PubMed] [Google Scholar]



