Abstract
A reaction center of photosystem II was isolated from Pisum sativum by using immobilized metal affinity chromatography. This reaction center is photochemically active and has a room temperature Qgamma chlorophyll (Chl) absorption band peaking at 677.5 nm. From HPLC analysis, the pigment stoichiometry was suggested to be 5 Chls per 1 beta-carotene per 2 pheophytins. Low-temperature absorption measurements at 77 K were consistent with the removal of one of the Chls associated with the usual form of the reaction center isolated by using ion-exchange chromatography. Transient absorption spectroscopy on the picosecond time scale indicated that the Chl removed belongs to a pool of Chl absorbing at approximately 670 nm (C670II) that transfers energy relatively slowly to the primary donor P680 in support of our recently proposed model. The results also support the previous conclusion that radical pair formation is largely associated with a 21-ps time constant when P680 is directly excited and that the identity of C670II is likely to be peripherally bound Chls possibly ligated to conserved His residues at positions 118 on the D1 and D2 proteins.
Full text
PDF
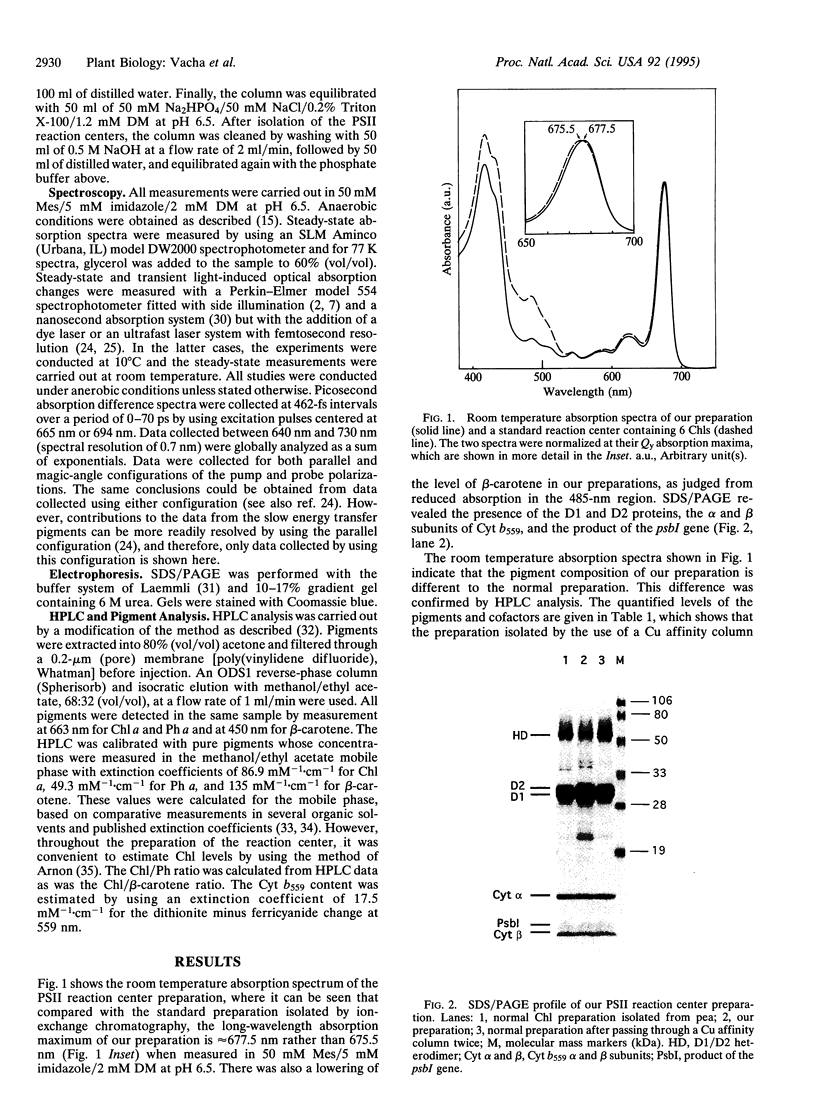
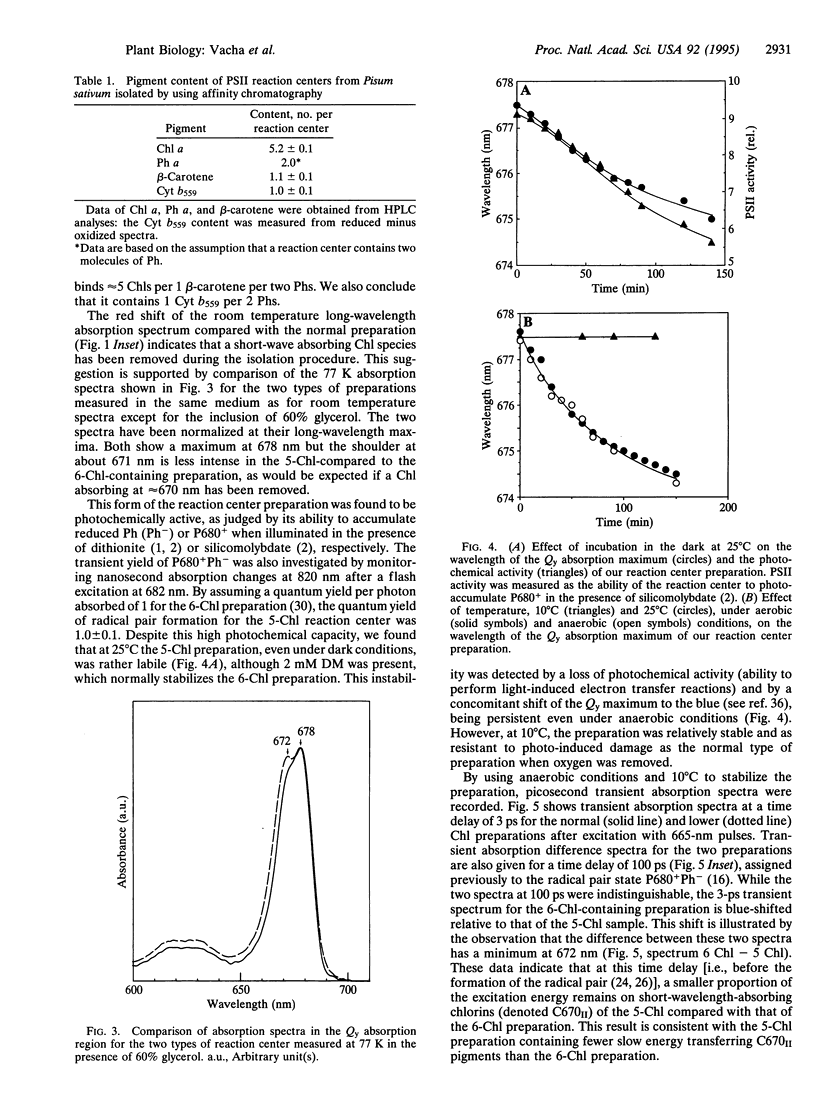
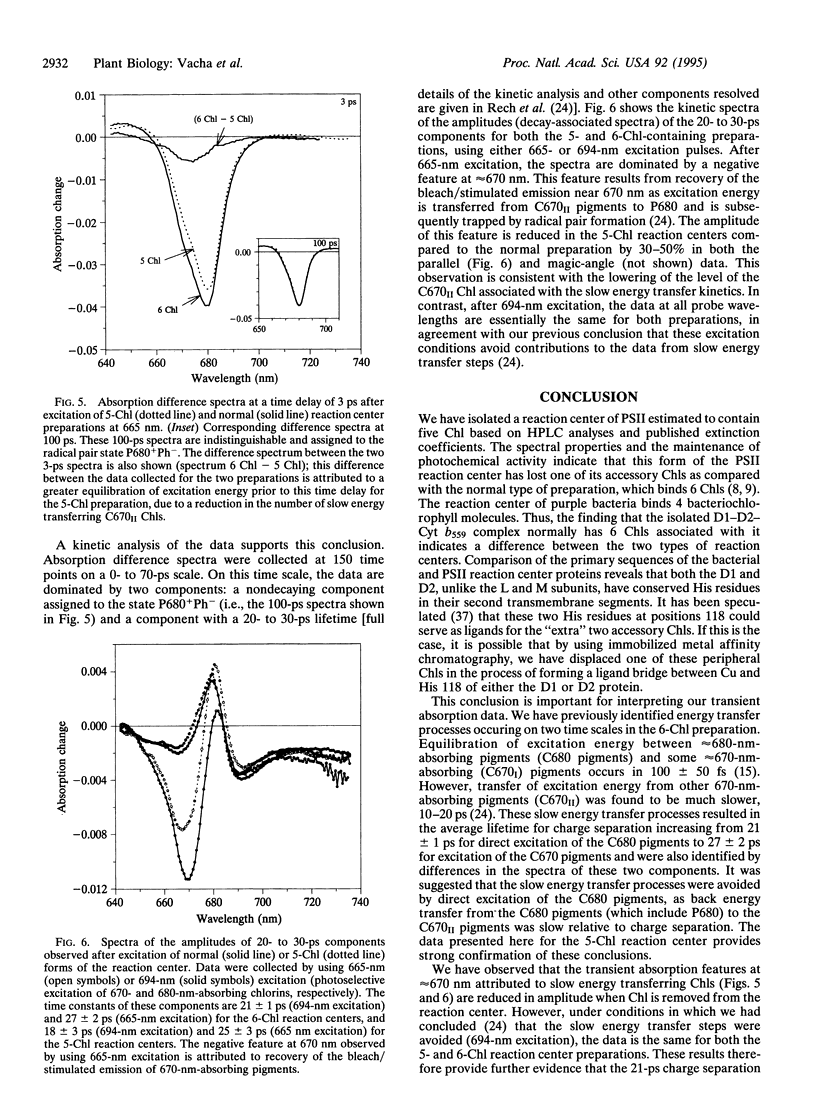

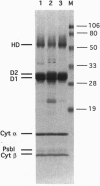
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth P. J., Crystall B., Ahmad I., Barber J., Porter G., Klug D. R. Observation of multiple radical pair states in photosystem 2 reaction centers. Biochemistry. 1991 Jul 30;30(30):7573–7586. doi: 10.1021/bi00244a029. [DOI] [PubMed] [Google Scholar]
- Braun P., Greenberg B. M., Scherz A. D1-D2-cytochrome b559 complex from the aquatic plant Spirodela oligorrhiza: correlation between complex integrity, spectroscopic properties, photochemical activity, and pigment composition. Biochemistry. 1990 Nov 13;29(45):10376–10387. doi: 10.1021/bi00497a012. [DOI] [PubMed] [Google Scholar]
- Durrant J. R., Hastings G., Joseph D. M., Barber J., Porter G., Klug D. R. Subpicosecond equilibration of excitation energy in isolated photosystem II reaction centers. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11632–11636. doi: 10.1073/pnas.89.23.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanotakis D. F., de Paula J. C., Demetriou D. M., Bowlby N. R., Petersen J., Babcock G. T., Yocum C. F. Isolation and characterization of the 47 kDa protein and the D1-D2-cytochrome b-559 complex. Biochim Biophys Acta. 1989 Apr 17;974(1):44–53. doi: 10.1016/s0005-2728(89)80164-1. [DOI] [PubMed] [Google Scholar]
- Gounaris K., Chapman D. J., Booth P., Crystall B., Giorgi L. B., Klug D. R., Porter G., Barber J. Comparison of the D1/D2/cytochrome b559 reaction centre complex of photosystem two isolated by two different methods. FEBS Lett. 1990 Jun 4;265(1-2):88–92. doi: 10.1016/0014-5793(90)80890-u. [DOI] [PubMed] [Google Scholar]
- Hastings G., Durrant J. R., Barber J., Porter G., Klug D. R. Observation of pheophytin reduction in photosystem two reaction centers using femtosecond transient absorption spectroscopy. Biochemistry. 1992 Aug 25;31(33):7638–7647. doi: 10.1021/bi00148a027. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Montoya G., Yruela I., Picorel R. Pigment stoichiometry of a newly isolated D1-D2-Cyt b559 complex from the higher plant Beta vulgaris L. FEBS Lett. 1991 Jun 3;283(2):255–258. doi: 10.1016/0014-5793(91)80601-x. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech T., Durrant J. R., Joseph D. M., Barber J., Porter G., Klug D. R. Does slow energy transfer limit the observed time constant for radical pair formation in photosystem II reaction centers? Biochemistry. 1994 Dec 13;33(49):14768–14774. doi: 10.1021/bi00253a015. [DOI] [PubMed] [Google Scholar]
- Seibert M., Picorel R., Rubin A. B., Connolly J. S. Spectral, Photophysical, and Stability Properties of Isolated Photosystem II Reaction Center. Plant Physiol. 1988 Jun;87(2):303–306. doi: 10.1104/pp.87.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A., Dhami S., Bishop S. M., Phillips D., Barber J. beta-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry. 1994 Dec 6;33(48):14469–14474. doi: 10.1021/bi00252a013. [DOI] [PubMed] [Google Scholar]
- Wasielewski M. R., Johnson D. G., Seibert M., Govindjee Determination of the primary charge separation rate in isolated photosystem II reaction centers with 500-fs time resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(2):524–528. doi: 10.1073/pnas.86.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G. P., Seibert M., Govindjee, Wasielewski M. R. Femtosecond photodichroism studies of isolated photosystem II reaction centers. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8999–9003. doi: 10.1073/pnas.91.19.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yruela I., van Kan P. J., Müller M. G., Holzwarth A. R. Characterization of a D1-D2-cyt b-559 complex containing 4 chlorophyll a/2 pheophytin a isolated with the use of MgSO4. FEBS Lett. 1994 Feb 14;339(1-2):25–30. doi: 10.1016/0014-5793(94)80377-3. [DOI] [PubMed] [Google Scholar]