Abstract
Background
PN patients are at increased risk of infectious complications compared to enteral feeding, which is in part explained by impaired mucosal immune function during PN. Adding GLN to PN has improved outcome in some clinical patient groups. Although GLN improves acquired mucosal immunity, its effect on innate mucosal immunity (defensins, mucus, lysozymes) has not been investigated.
Methods
Forty-eight hours following venous cannulation, male ICR mice were randomized to chow (n = 10), PN (n = 12), or PN+GLN (n = 13) for 5 days. Small intestine tissue and luminal fluid was collected for MUC2, lysozyme, cryptdin-4 analysis and luminal IL-4, IL-10 and IL-13 level measurement. Tissue was also harvested for ex-vivo intestinal segment culture to assess tissue susceptibility to enteroinvasive Escherichia coli.
Results
In both luminal and tissue samples, PN reduced MUC2 and lysozyme (P < 0.0001, respectively) compared with chow, whereas GLN addition increased MUC2 and lysozyme (luminal: P < 0.05; tissue: P < 0.0001, respectively) compared with PN alone. PN significantly suppressed cryptdin-4 expression, while GLN supplementation significantly enhanced expression. IL-4, IL-10 and IL-13 decreased significantly with PN compared with chow, whereas GLN significantly increased these cytokines compared with PN. Functionally, bacterial invasion increased with PN compared with chow (P < 0.05), while GLN significantly decreased enteroinvasion to chow levels (P < 0.05).
Conclusions
GLN supplemented PN improves innate immunity and resistance to bacterial mucosal invasion lost with PN alone. This work confirms a clinical rationale for providing glutamine for the protection of the intestinal mucosa.
Keywords: Parenteral nutrition, glutamine, innate immunity, intestinal mucosa, lysozyme, cryptdin 4
Introduction
Parenteral nutrition (PN) provides specialized nutrition support to patients with contraindications to enteral feeding. However, PN administered to postsurgical/trauma and critically ill patients results in an increased risk of infectious complications compared with patients fed enterally.1–4 These PN-induced vulnerabilities are undoubtedly multifactorial, but impaired mucosal defenses including innate and acquired immunity likely contribute to the observed infectious morbidity.5 While enteral feeding remains the preferred method of nutrition support, some patients cannot be fed enterally, so that the availability of a parenteral nutrient supplement which would stimulate mucosal immune defenses could be clinically advantageous; one supplement showing clinical promise is glutamine.
Glutamine (GLN) serves as a primary metabolic nutrient for the intestinal mucosa during stress and provides an important energy substrate for rapidly proliferating cells, such as enterocytes and immune cells, including T lymphocytes. PN with lack of enteral nutrition (EN) negatively impacts adaptive immunity, through a reduction in gut associated lymphoid tissue (GALT) lymphocytes and immunoglobulin A (sIgA) production. Our previous work demonstrates that GLN supports acquired mucosal defenses during PN. The addition of GLN to PN attenuates impaired acquired immunity following PN alone by preventing reductions in GALT cellularity - including the Peyer’s patches, lamina propria, and intraepithelial cell populations - maintaining IgA-stimulating cytokines, and preserving intestinal and respiratory IgA levels.6–8 Moreover, compared with PN alone, the addition of GLN to PN prevented impairment in upper respiratory tract immunity to the H1N1 virus and Pseudomonas aeruginosa.9, 10 No work has focused on the effect of GLN upon innate immunity during PN.
Innate immunity plays an important role in protecting the gastrointestinal tract mucosal surfaces. Specialized secretory epithelial cells, specifically goblet cells and Paneth cells within the mucosa, constitute the cellular components of innate mucosal immunity. A mucus layer consisting of glycoproteins released from the goblet cells coats the mucosal surface, concentrates the sIgA produced by the GALT (acquired immunity) and localizes antimicrobial compounds, such cryptdin 4 and lysozymes produced and released by the Paneth cells. This defensive layer forms a physiochemical layer against foreign antigens and pathogenic microorganisms.11, 12 Cryptdin 4 exhibits bactericidal activity against both gram-positive and gram-negative bacteria and displays the greatest antimicrobial activity compared with other crypdins.13, 14, 48 Lysozyme provides antimicrobial activity against gram-positive bacteria, but its function is potentiated in the presence of other gram-negative bactericidal molecules.14, 15 Deficiency in cryptdin 4, lysozyme, or mucin 2 (MUC2) results in susceptibility to enterocolitis and intestinal infections in mice and humans.16–19 In our previous work, PN reduced mRNA expression of cryptdin 4 and other antimicrobial molecules as well as the secretion of MUC2, which impairs the intestinal immune barrier function and increases susceptibility to bacteria invasision.20, 21 Others have shown that GLN supplementation during PN attenuates impaired intestinal permeability and intestinal inflammation in mice.22–23
The benefit of GLN is likely in part through modulation of intestinal cytokine levels. GLN increases Th2 lymphocyte derived cytokines – IL-4, IL-10, and IL-13 - in small intestinal tissue homogenates compared with PN alone.24, 25 IL-4 and IL-10 stimulate IgA secretion by plasma cells in support of adaptive immune function, but these cytokines also support innate immunity. IL-4 induces differentiation of glycoprotein-containing goblet cells, and injection of IL-10 into enteric tissue induces goblet cell hyperplasia.26. IL-10 deficient mice have lowered MUC2 synthesis and structurally altered goblet cells, due to impaired MUC2 protein folding responses, which leads to lethal colitis upon bacterial challenge.27 Accordingly, IL-10 protects animals from developing lethal intestinal infections following parasitic challenge.28 Both IL-4 and IL-13 increase MUC2 production in intestinal goblet cells.29 In addition, IL-13 induces Paneth cell hyperplasia and up-regulates gene expression ofcryptdin4.30 Our prior work demonstrated that stimulating Th2 cytokines with IL-25 maintained innate immunity by increasing the Paneth cell antimicrobial protein, sPLA2, and MUC2 production.20
With this substantial experimental background, we hypothesized that addition of GLN to PN would improve innate immunity by maintaining the Th2 response preserving the production of antimicrobial peptides cryptdin 4, lysozyme, and glycoprotein MUC2. Moreover, we speculated that the addition of GLN would increase intestinal barrier function against bacterial enteroinvasion using an ex-vivo intestinal segment culture (EVISC) compared with PN alone.
Materials and Methods
Animals
All animal experiment protocols were approved by Animal Care and Use Committee of the University of Wisconsin–Madison and the William S. Middleton Memorial Veterans Administration (VA) Hospital, Madison. Male Institute of Cancer Research (ICR) outbred mice were purchased from Harlan (Indianapolis, IN) and housed 5 per covered/filtered box in an American Association for Accreditation of Laboratory Animal Care–accredited conventional facility on the William S. Middleton Memorial VA Hospital Campus. The mice were fed standard mouse chow (Rodent Diet 5001, LabDiet; PMI Nutrition International, St Louis, MO) and water under controlled temperature, humidity, and light cycle (a 12:12-hour light: dark cycle) for 1 week (acclimatization) prior to initiation of the study protocol.
Experimental Design
In experiment 1, 35 male ICR mice (6–8 weeks old) were randomized to receive standard chow (chow, n=10), PN (n=12) or PN supplemented with glutamine (2%, PN+GLN, n=13). Animals were anesthetized intraperitoneal administration with a mixture of ketamine (100 mg/kg body weight) and acepromazine (10 mg/kg body weight). A silicone rubber catheter (0.012″ I.D. by 0.025″ O.D.; Helix Medical, Inc., Carpinteria, CA) was placed into the vena cava through the right jugular vein for intravenous infusion and the distal end of catheter was tunneled subcutaneously over the back to exit the midpoint of the tail. The mice were partially restrained by the tail to protect the catheter during infusions. This partial-restraint technique does not induce significant stress in the mice.31 0.9% saline was infused to all mice at a rate of 4 mL/d with free access to water and chow for 2 days after surgery. On day 3, animals were assigned to their respective treatments. Chow received 4 mL/d of intravenous 0.9% saline along with ad libitum chow and water. The PN and PN+GLN mice initially received 4 mL/day of their respective solutions on the third day and advanced to 7 mL/day on the fourth day, 10 mL/day for the remaining 3 days. The formulation of PN solution was described previously containing 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with 1440 kcal/L and a nonprotein calories:nitrogen ratio of 128:1. The PN+GLN solution replaced 2% of amino acid solution with L-Glutamine (Ajinomoto North America, Raleigh, NC). The PN and PN+GLN solutions were nearly isocaloric and isonitrogenous and they met the calculated nutrient requirements of mice weighing 25 to 30g. This GLN dose was chosen because a 30-gram mouse receiving 2% glutamine at rates of 4mL/day (day1), 7mL/day (day2), and 10mL/day (day3–5), will receive the metabolically scaled (7.1:1) levels of 0.38g/kg/day, 0.66g/kg/day, and 0.94g/kg/day, respectively, while a typical 70 kg human may receive 30 g GLN/day in the clinic (0.43 g/kg/day).
After 5 days of feeding (7 days post catheterization), mice were weighed, anesthetized as described above, and exsanguinated via left axillary artery transection. The small intestine was removed and the lumen rinsed with 20 mL buffer (HBSS; Bio Whittaker, Walkersville, MD). The luminal wash was centrifuged at 2000 × g for 10 minutes, and supernatant was aliquoted and frozen at −80°C for analysis of MUC2, lysozyme, and cytokines levels. Ileal tissue samples were taken from a 3 cm segment of ileum that excluded Peyer’s patches. These samples were stored at −80°C until subsequent analysis. The tissue samples for goblet cell and Paneth cell immunohistochemistry analysis were fixed in 4% paraformaldehyde overnight, transferred to 70% ethanol, and stored at 4°C until subsequent histology.
In experiment 2, twenty-two male ICR mice (6–8 weeks old) were randomized to receive standard chow (chow, n=8), PN (n=7) or PN supplemented with glutamine (2%, PN+GLN, n=7). All the procedures are the same as experiment 1. Two distal ileum segments of 2 centimeters excluding Peyer’s patches for each mouse were collected for the EVISC methodology to determine bacterial enteroinvasion.
Periodic Acid Schiff staining
The ileum tissue samples were fixed in formaldehyde, processed (Tissue-Tek V.I.P.; Sakura Finetek, Torrance, CA) and embedded in paraffin, after which tissue sections (5 µm thick) were cut. PAS staining was performed as previously reported.37 Goblet cells cells were counted by determining the average number of goblet cells present in 15 individual villi in represent active microscopic fields (original magnification, ×20) per mouse.
Western Blot for MUC2 in the Small Intestinal Wash Fluid and Ileum Tissue
Western blotting was performed as previously described.37 Briefly small intestinal wash fluid was separated on a 4–15% polyacrylamide gel (Ready Gel; Bio-Rad Laboratories, Hercules, CA) by electrophoresis. After transfer, membranes were equilibrated in TBS-Tween (Tris-buffered saline with 0.5% Tween-20) for 10 minutes and blocked (5%nonfat dry milk) for 1 hour at room temperature. The membranes were incubated overnight at 4°C with the primary antibody mouse anti-human MUC2 (ab-11197; AbcamInc, Cambridge, MA) diluted 1:2500 in blotto. Goat anti-mouse IgG-HRP conjugate (sc-2005; Santa Cruz Biotechnology, CA) antibody was diluted in (ratio: 1:20,000) and incubated for 1 hour with constant rocking. Membranes were detected using HRP substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) and the membrane was scanned using Image Quant LAS 4000 (GE Healthcare Bio-Sciences Corp. Piscataway, NJ). Densitometric analysis of target bands at the monomeric and dimeric MUC2 forms was determined by National Institutes of Health (NIH) Image J software. The internal controls were used to normalize the density among multiple membranes.
The tissue protein was homogenized from 3 centimeters of frozen small intestine segment using RIPA lysis buffer (Upstate, Lake Placid, NY) containing 1% protease inhibitor cocktail (Sigma-Aldrich). The homogenate was incubated for 20 minutes on ice followed by centrifugation at 16,000 × g at 4°C for 10 minutes. The supernatant was stored at −80°C until analysis. The protein concentration of the supernatant was determined by the Bradford method using bovine serum albumin (BSA) as a standard. 20 ug of total tissue protein was used to quantify ileum tissue MUC2 as described above.
Western Blot for lysozyme in the Small Intestinal Wash Fluid and Ileum Tissue 20 uL of SIWF was heated at 95°C for 5 minutes, separated on a 10% polyacrylamide gel (Ready Gel; Bio-Rad Laboratories, Hercules, CA) by electrophoresis at 150 V for 50 minutes and was transferred to a PVDF membrane (0.45um) using the standard transfer buffer at 80V for 30 minutes. After blocking in blotto as described above, the membranes were incubated with the primary antibody rabbit anti-Lysozyme (ab-108508; AbcamInc, Cambridge, MA) diluted 1:5000 in blotto overnight at 4°C. Goat anti-rabbit IgG-HRP conjugate (32260; Rockford, IL) was diluted (ratio: 1:15,000) for 1 hour at room temperature. Membranes were detected with horse radishperoxidase (HRP) substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) for 5 minutes, and the membranes were scanned using Image Quant LAS 4000 (GE Healthcare Bio-Sciences Corp. Piscataway, NJ). Densitometric analysis of target bands at 17 KD was determined by National Institutes of Health (NIH) Image J software. The internal controls were used to normalize the density among multiple membranes. The tissue protein was extracted as previously described and the 20ug of total tissue protein was used to quantify ileum tissue lysozyme.
Quantitative Cryptdin 4 PCR Analysis
RNA was extracted from frozen distal ileal tissue using the SV Total RNA isolation kit (V3100; Promega, Madison WI), according to the manufacturer’s protocol. The RNA purity and concentration was determined by Nanodrop (Thermo Fisher Scientific Inc.), and 1 µg of RNA was used as a template for reverse transcription using random primers and ImProm-II™ reverse transcriptase in a cDNA synthesis reaction according to the ImProm-II Reverse transcription kit’s instructions (Cat No. A3800,Promega). Quantitative real-time PCR (qPCR) was performed using the SYBRSelect Master MixSystem (Applied Biosystems, Foster City, CA). The primers for cryptdin 4 including forward primer (5'-CCAGGCTGATCCTATCCAAA-3') and reverse primer (5'-ATTCCACAAGTCCCACGAAC-3') were designed and composed by Invitrogen (Grand Island, NY). The cryptdin 4 mRNA expression level was determined using 7500 fast real-time PCR system software (applied biosystems, Foster City, CA) with a delta CT relative quantification model. The geometric mean of the expression levels of reference gene, β-actin, was calculated and used as a normalization factor.
Luminal Cytokine Quantitative Analysis
Concentrations of IL-4, IL-13, and IL-10 were determined in the small intestinal wash fluid using solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences, San Diego, CA), according to the manufacturer’s instructions as described in our previous work.7, 32 The Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA) was used to measure the absorbance values of samples at 450 nm. The cytokine concentrations in the samples were calculated by using a 4-parameter logistic fit standard curve (SOFTmax PRO software; Molecular Devices).
Immunohistochemistry
The fixed ileal tissue sections were processed (Tissue-Tek V.I.P.; Sakura Finetek, Torrance, CA) and embedded in paraffin. Sections were cut 5 µm thick and deparaffinized. Antigen retrieval was in sodium citrate buffer (Dako REAL Target Retrieval Solution; DakoCytomation). Sections were blocked in 10% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 1 hour and incubated with primary rabbit anti human lysozyme (A0099, DakoCytomation) with 1:1000 dilution in 1%BSA-PBS overnight at 4°C. Next, the goat anti-rabbit IgG-TR (SC 2780, Santa Cruz Biotech) with 1:400 dilution in 1%BSA-PBS was applied as an appropriate secondary antibody for 30 minutes at room temperature. Nuclei were counterstained with DAPI (Cat P36935, Invitrogen). The IHC features shown are representative of all tissue samples studied.
Bacterial Preparation
The ampicillin resistant E. coli 5011-Lux was prepared 72 hours prior to the day of EVISC experiment. It was cultured in 40 mL tryptose broth (LB, 100 µg/mL ampicillin) for 48 hours at 37°C under 5% CO2. 1 mL of aliquot from the surface was transferred to a new 40 mL LB broth containing 100 µg/mL ampicillin for 24 hours at 37°C under 5% CO2. Then the culture was centrifuged at 1,780×g for 11 minutes to obtain the pellet and the pellet was resuspended in 40 mL LB to wash twice. Finally it was resuspended in 1 mL Dulbecco’s phosphate-buffered saline (DPBS) to obtain a bacteria stock solution. The bacteria stock solution was diluted at the ratio of 1:100 to DPBS and the value was measured on a spectrophotometer (DU640B; Beckman, Brea, CA) at 450 nm wave length. Bacterial concentrations were calculated based on previously established growth curves and adjusted to 1×108 CFU/mL.
EX-Vivo Intestinal Segment Culture (EVISC)
The EVISC method was performed as previously described.43 Briefly, 2 cm of distal Ileum tissue were opened apical side up and kept hydrated in RPMI. One side of a plastic tissue disk (9 mm outer aperture and 6 mm internal aperture) was applied lightly with tissue glue (Dermabond; Ethicon, Cornelia, GA) and placed onto the mucosal side of intestinal tissue. Another tissue disc was glued on the serosal side of the intestinal segment. After covering the bottom of the serosal disc with a light layer of tissue glue, the intestinal segment was set into the bottom of a cell culture insert (Cat No. 3180, 0.4µM pore, 12-well format; BD Bioscience, Franklin Lakes, NJ) with gentle pressure.
400 µl of bacterial solution (1 × 108 CFU/mL) in RPMI containing ampicillin (100 µg/mL) was placed into the wells for 1 hour at 37°C. After washing, gentamicin (100 µg/mL) was added for 1 hour at 37°C to kill remaining bacteria in the well or adherent to the mucosal surface. RPMI containing gentamicin was removed and washed. 400 µL of 0.1% Triton-X in DPBS was added to each well and the plates were agitated on an orbital shaker (175 rpm; New Brunswick Scientific Classic Series C1 Shaker) for 30 minutes at room temperature. Serial dilutions (101–107) of the cell lysate in DPBS were plated on LB agar plates containing ampicillin (100µg/mL) and incubated for 18 hours at 37°C. Enteroinvasion was assessed by counting CFU of ExPEC grown on the plates.
Statistical Analysis
All the data are reported as means ± standard error of mean (SEM). A fixed-effects analysis of variance (ANOVA) model with the Fisher’s protected least-significant difference posthoc test (PLSD) was chosen for determining the statistical significance. All statistical calculations were performed with Stat View software (Abacus Concepts, Berkeley, CA). Statistical significance was accepted at P < 0.05.
Results
Analysis of goblet cell number
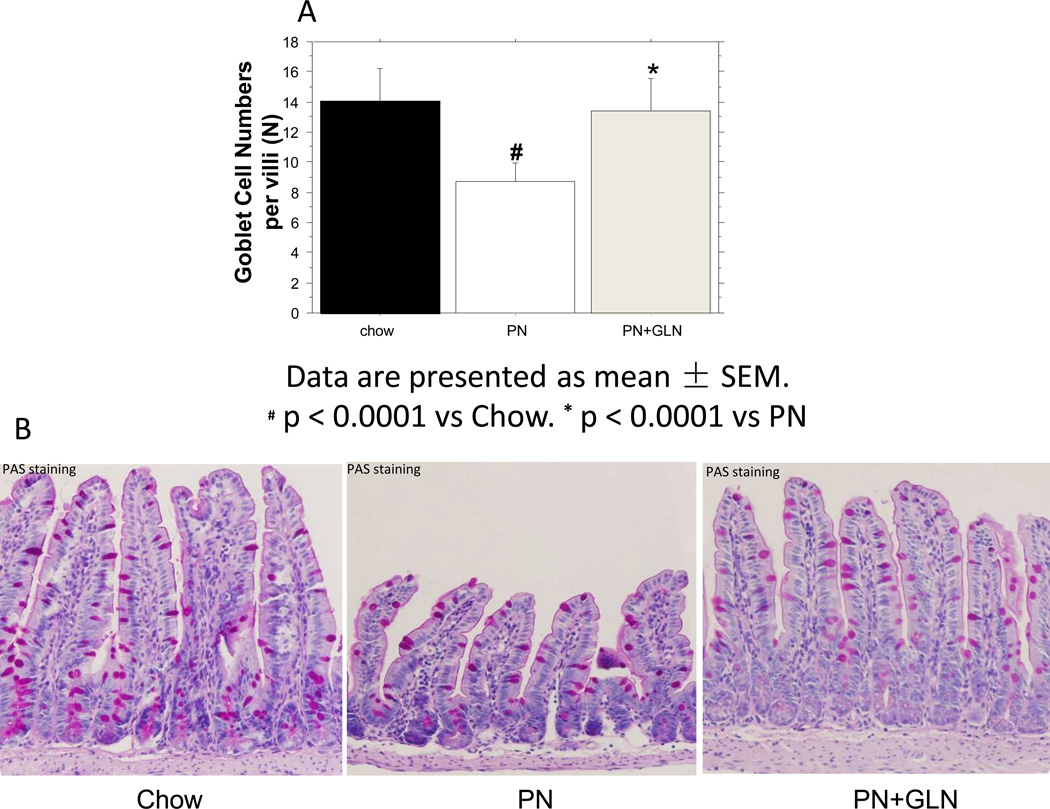
PN significantly reduced the number of goblet cells per villi compared with chow (Chow: 14.0 ± 0.70vs. PN: 8.74 ± 0.35, P < 0.0001). The addition of glutamine to PN significantly increased the number of goblet cells per villi compared with PN alone (PN+GLN: 13.4± 0.59vs. PN: 8.74 ± 0.35, P < 0.0001), to levels similar to chow (Chow: 14.0 ± 0.70vs.PN+GLN: 13.4± 0.59, P= 0.5). (Fig.1A) The average cell numbers per group are shown. (Fig.1B)
Figure.1. Morphological changes after feeding chow, PN and PN+GLN.
A. PN significantly decreased the number of goblet cells per villi in mice compared with chow. The addition of glutamine to PN significantly increased the number of goblet cells compared with PN alone. Data are presented as mean ± SEM. #p < 0.0001 versus chow. *p < 0.0001 versus PN. B. Representative images of periodic acid–Schiff (PAS) base-stained ileum tissue are shown for Chow, PN, and PN+GLN. Goblet cells are stained pink (original magnification, ×20).
Analysis of Intestinal Fluid MUC-2
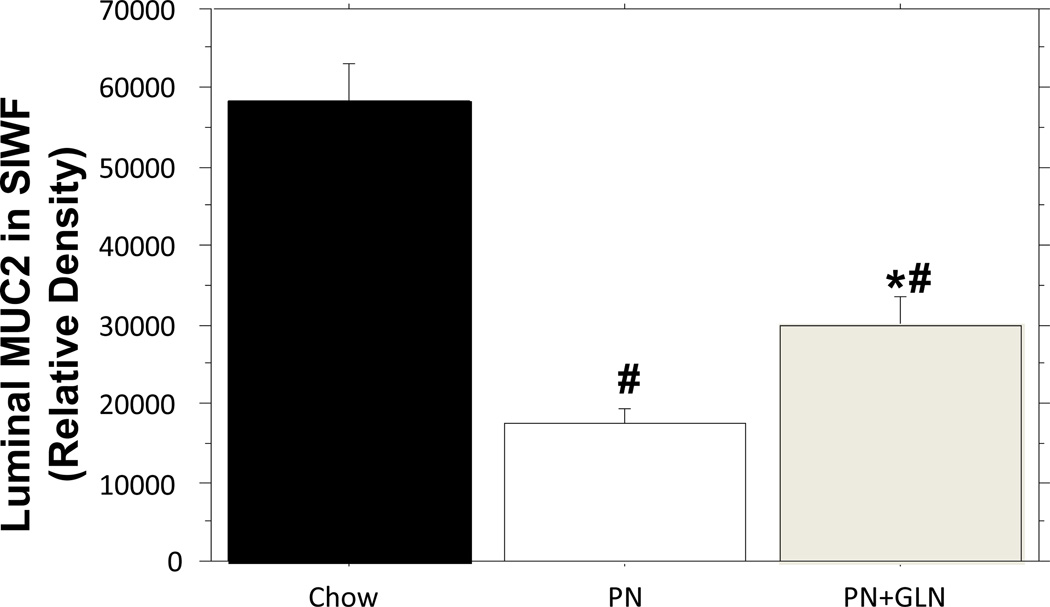
PN significantly lowered the relative density of intestinal fluid MUC2 compared with chow mice (Chow: 58,244 ± 4700 vs. PN: 17,400 ± 1921 vs. P < 0.0001). The addition of glutamine to PN significantly increased the luminal level of MUC2 in the SIWF compared with PN alone (PN+GLN: 29,771± 3701 vs. PN: 17,400 ± 1921, P = 0.01), however, the level of MUC2 remained significantly lower than that in chow (PN+GLN: 29,771 ± 3701 vs. Chow:58,244 ± 4700, P < 0.0001)(Fig.2).
Figure.2. Relative density of MUC2 from the small intestinal wash fluid (SIWF).
PN significantly decreased luminal MUC2 levels compared with chow. Glutamine supplemented PN significantly increased luminal MUC2 levels compared with PN alone. Data are presented as mean ± SEM. #p < 0.0001 versus chow. *p = 0.01 versus PN.
Analysis of Intestinal Fluid lysozyme
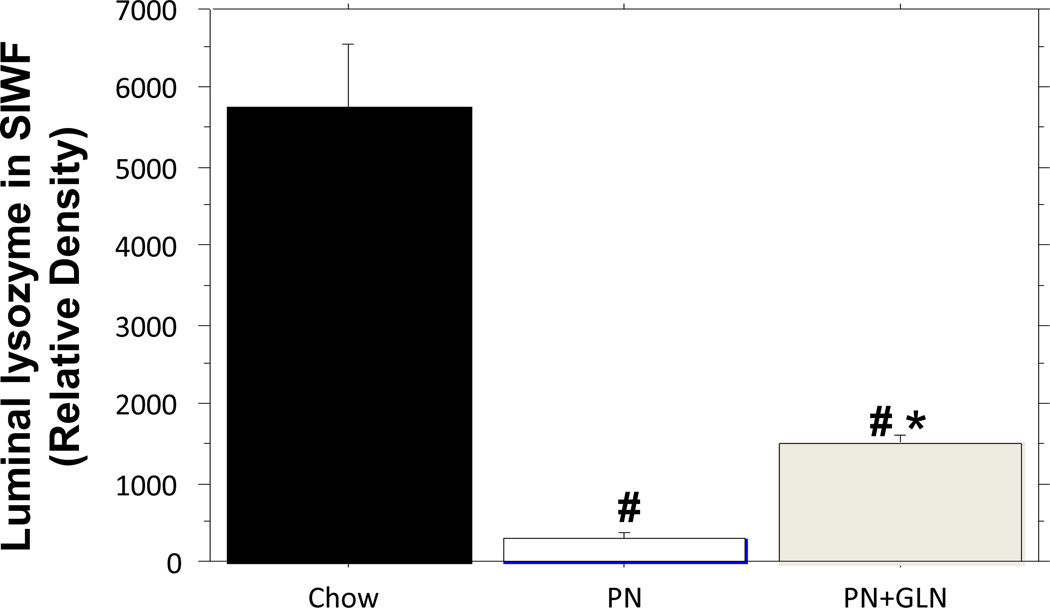
PN significantly decreased lysozyme (relative density) in the intestinal fluid compared with chow (PN: 293 ± 88.7 vs. Chow: 5742 ± 813, P < 0.0001). The addition of glutamine to PN significantly increased lysozyme levels in the intestinal fluid compared with PN alone (PN+GLN: 1507± 108 vs. PN: 293 ± 88.7, P =0.04), however, lysozyme levels remained significantly lower than chow (PN+GLN: 1507 ± 108 vs. Chow: 5742 ± 813, P < 0.0001)(Fig.3).
Figure.3. Relative density analysis of lysozyme from the small intestinal wash fluid (SIWF).
PN significantly decreased luminal lysozyme density compared with chow. Glutamine supplemented PN significantly increased luminal lysozyme density compared with PN alone. Data are presented as mean ± SEM. #p < 0.0001 versus chow. *p < 0.05 versus PN.
Analysis of Small Intestinal Wash Levels of IL-4, IL-13 and IL-10
IL-4 and IL-13 levels in the small intestinal washes of the PN group were significantly lower than those in the chow group (P = 0.02 and P < 0.05, respectively) (Table 1). Both levels in the PN+GLN group were significantly higher than those in the PN group (P = 0.02 and P = 0.005, respectively). There were no significant differences of IL-4 and IL-13 levels between the chow and PN+GLN groups (IL-4: P = 0.9; IL-13: P = 0.4). PN significantly reduced IL-10 in the small intestinal washes compared to chow (P = 0.0008). The addition of glutamine to PN significantly increased the luminal levels of IL-10 compared with PN alone (P = 0.007) and IL-10 was statistically similar to the chow group (P = 0.3).(Table 1)
Table 1.
Luminal IL-4, IL-13 and IL-10 concentrations in Small intestinal wash fluid
| Cytokines | Chow | PN | PN+GLN |
|---|---|---|---|
| IL-4, pg/mL | 155.7 ± 15.3 | 109.3 ± 9.6# | 157.7 ± 12.6* |
| IL-13, pg/mL | 8.6 ± 0.5 | 5.6 ± 1.0# | 9.7 ± 1.1* |
| IL-10, pg/mL | 1366.5± 114.1 | 812.8 ± 76.3# | 1216.0± 107.2* |
Data are presented as mean ± SEM.
p < 0.05 vs Chow.
p < 0.01 vs PN
Analysis of Ileum Tissue MUC-2
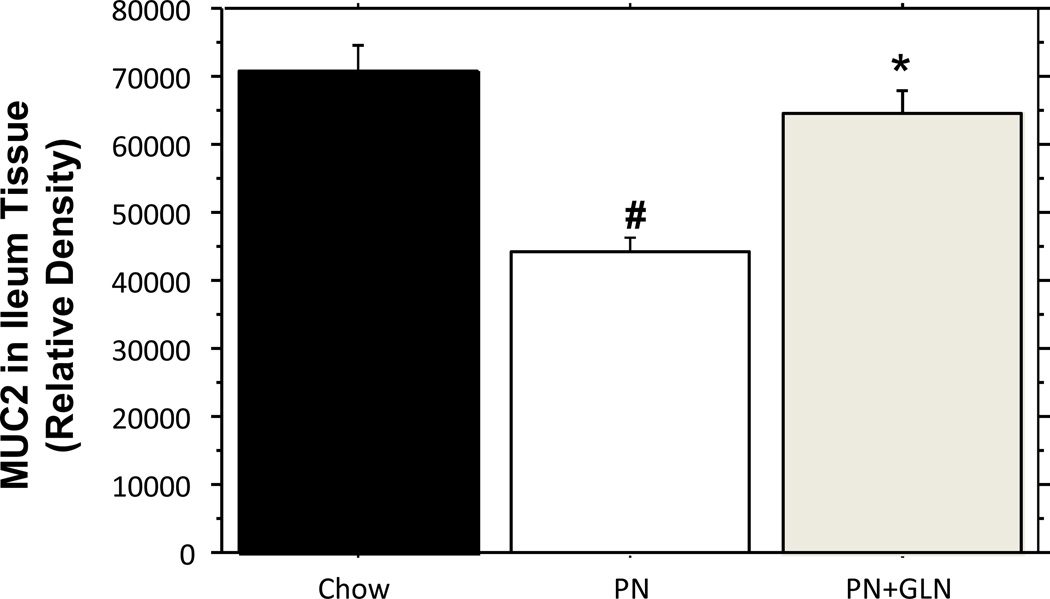
Consistent with the change of goblet cell numbers, the relative density of ileum tissue MUC2 was significantly decreased in PN alone group than that in chow mice (Chow: 70,762 ± 3844 vs. PN: 44,008 ± 2385, P < 0.0001). The addition of glutamine to PN significantly increased the tissue level of MUC2 compared with PN alone (PN: 44,008 ± 2385vs.PN+GLN: 64,565± 3484, P < 0.0001), and the MUC2 relative level was statistically similar to the chow group (Chow: 70,762 ± 3844 vs. PN+GLN: 64,565 ± 3484, P = 0.2) (Fig.4).
Figure.4. Relative density of MUC2 in ileum tissue.
The band density for MUC2 in the ileum of PN mice was significantly decreased compared with chow mice. The relative density of MUC2 was significantly increased in the ileum of glutamine supplemented PN compared to PN alone. There were no different changes in tissue MUC2 protein between the chow mice and PN+GLN mice. Data are presented as mean ± SEM. #p < 0.0001 versus chow. *p < 0.0001 versus PN.
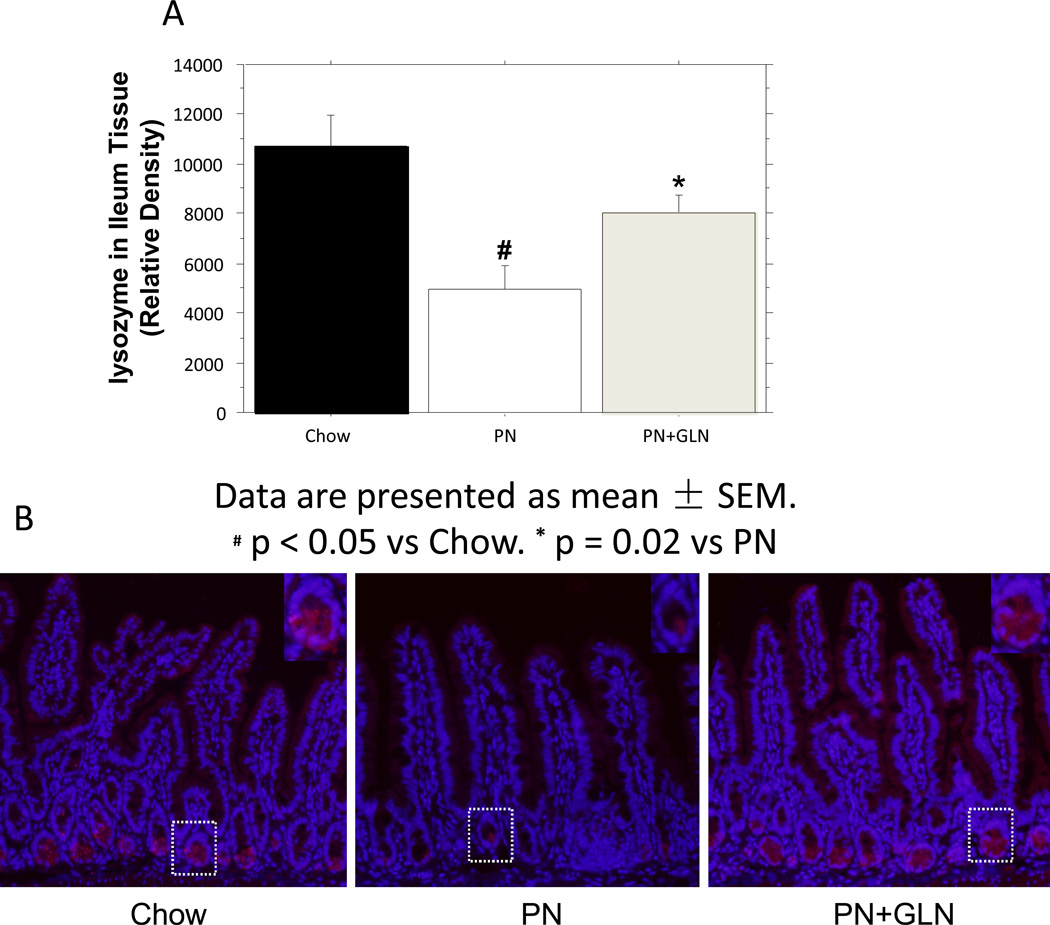
Analysis of Ileum Tissue lysozyme
PN significantly decreased the ileum tissue lysozyme (relative density) compared with chow (PN: 4977± 936 vs. Chow: 10,717± 1206, P = 0.0002). The addition of glutamine to PN significantly enhanced the tissue level of lysozyme compared with PN alone (PN: 4977 ± 936vs. PN+GLN: 8051± 687, P =0.02), and the level of tissue lysozyme remained similar to the chow group (PN+GLN: 8051 ± 687vs. Chow: 10,717 ± 1206, P > 0.05) (Fig.5A). IHC for lysozyme in the ileum showed that PN reduced the expression of lysozyme compared with chow. The overall expression of lysozyme was increased after 5 days of glutamine supplementation to PN (Fig.5B).
Figure.5. Relative density of lysozyme in ileum tissue.
A. PN significantly decreased the band density for lysozyme in the ileum tissue compared with chow. Glutamine supplemented PN significantly increased lysozyme density in the ileum tissue compared with PN. Data are presented as mean ± SEM. #p < 0.0001 versus chow. *p < 0.0001 versus PN. B. Representative immunohistochemistry for lysozyme in ileum tissue from chow (left panel) versus PN (middle panel) showed a reduced expression of lysozyme in Paneth cells from PN mice. The addition of glutamine to PN (right panel) resulted in significant increased lysozyme expression in Paneth cells compared with PN alone (middle panel) (original magnification, ×20). Inserts: Magnifications of single crypts from the three groups (original magnification, ×40). The histological features are representative for all tissue samples studied.
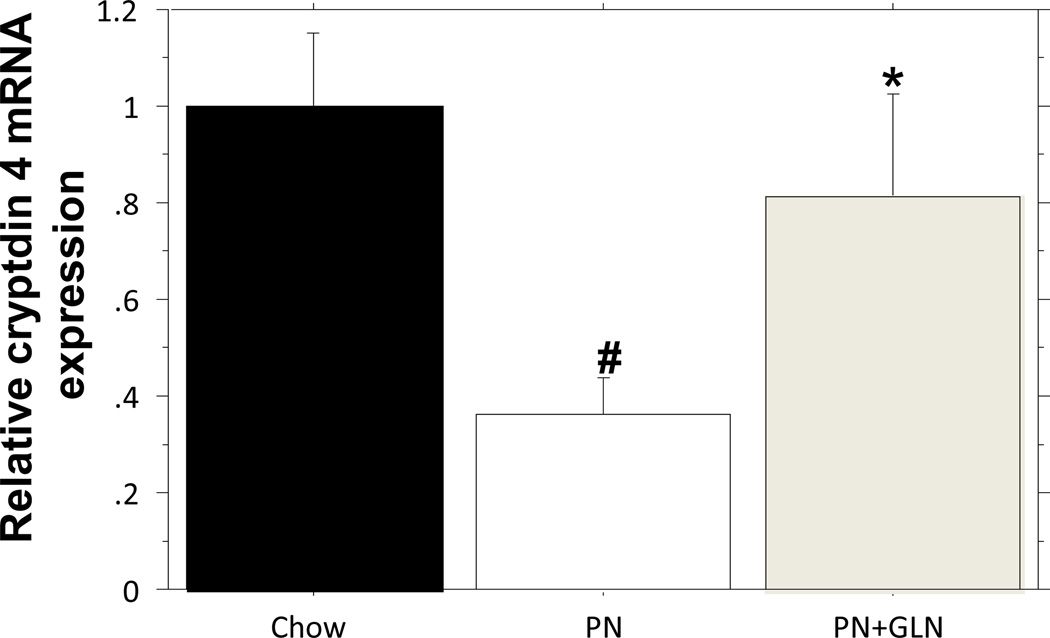
Analysis of Ileum cryptdin 4 mRNA expression
qPCR analysis of cryptdin 4 showed relative mRNA expression was significantly depressed (Chow: 1.0 ± 0.15 vs. PN: 0.36 ± 0.08, P = 0.006) after PN, compared with chow. Glutamine supplementation to PN significantly increased cryptdin 4 mRNA expression compared with PN alone (PN+GLN: 0.81 ± 0.21 vs. PN:0.36 ± 0.08, P = 0.039), and the cryptdin 4 mRNA expression was not significantly different from chow group (Chow: 1.0 ± 0.15 vs. PN+GLN: 0.81 ± 0.21, P = 0.4) (Fig.6)
Figure.6. qPCR analysis of cryptdin 4 expression in ileum tissue.
PN significantly suppressed the cryptdin4 mRNA level compared with chow. The addition of glutamine to PN significantly enhanced the cryptdin 4 mRNA expression compared with PN alone. #p < 0.01 versus chow. *p < 0.04 versus PN.
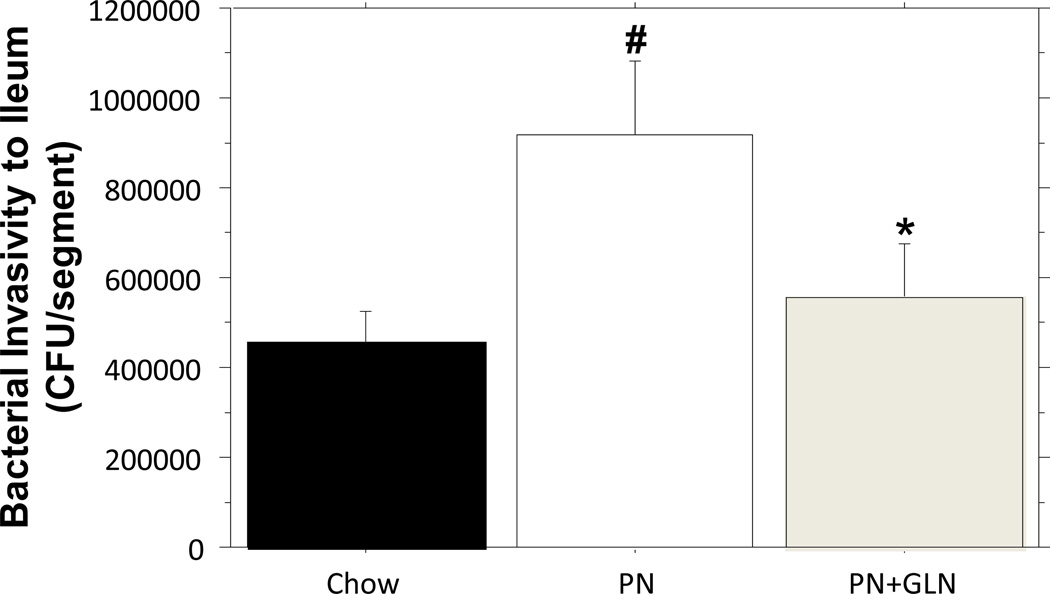
Bacterial (ExPEC) Invasion after EVISC
Compared with chow, the number of invaded E.coli recovered from intestinal explants significantly increased following PN (Chow: 457462.500 ± 65679.411 vs. PN: 919800.000 ± 158808.832, p = 0.01). The addition of glutamine to PN significantly reduced the number of invaded E.coli compared with PN alone (PN: 919800.000 ±158808.832vs. PN+GLN: 556200.000 ± 118085.619, P = 0.04). The number of invaded E.coli in the GLN group was limited to levels observed in chow (Chow: 457462.500 ± 65679.411 vs. PN+GLN: 556200.000 ± 118085.619, P > 0.05) (Fig.7).
Figure.7. Effect of chow, PN and PN+GLN- feeding on susceptibility of ileal tissue to invasion by bacteria.
The results of EVISC model showed PN significantly increased the recovered CFUs of ExPEC in the ileum segment compared with chow. The glutamine supplemented PN significantly reduced the recovered CFUs compared with PN alone and remained similar levels to those recoverd from chow mice. Data are presented as mean ± SEM. #p < 0.05 versus chow. *p < 0.05 versus PN.
Discussion
Several clinical studies reported that perioperative glutamine-supplemented parenteral nutrition (GLN-PN) reduces the morbidity from infectious complications in patients undergoing abdominal surgery or trauma compared to the standard PN.34–36 Our work has established that PN impairs mucosal immunity providing a cogent explanation for this vulnerability. The mucosal immune system is comprised of both innate and adaptive immune arms. Both systems respond to potentially harmful antigens within the intestinal lumen, including environmental, bacterial, viral, and dietary antigens. The adaptive immune system consists of specialized T & B lymphocytes, macrophages, and dendritic cells that coordinate production and transport of secretory immunoglobulin-A (sIgA) from the lamina propria onto mucosal surfaces. Our prior work demonstrated that glutamine supplementation improved adaptive immunity impaired with PN feeding alone through several mechanisms.8, 21 Experimentally, GLN added to PN improved survival in immunized mice after intratracheal administration of Pseudomonas aeruginosa compared to PN alone through maintenance of sIgA in the upper respiratory tract.6, 36 The second arm of mucosal immunity - innate immunity - provides non-specific, immediate, and continuous defense against bacterial invasion of epithelial surfaces. To our knowledge, this work is the first investigation of glutamine effects upon this more teleologically ancient line of host defense.
Innate immunity consists in part of specialized secretory epithelial cells, including goblet and Paneth cells, which reside within the mucosal epithelial layer and respond to pathogens through secretion of a physicochemical mucous barrier. These secretions consist of a complex fluid rich in mucin glycoproteins that concentrate a wide variety of antimicrobial peptides released from the Paneth cells as well as the sIgA transported from the lamina propria. In the small intestine, this layer covers the epithelium and helps prevent enteric pathogens from reaching the underlying epithelial layer. Glutamine maintains several aspects of intestinal defense compared with PN alone. The most abundant mucin glycoprotein in this extracellular mucus barrier is MUC2 and, consistent with our previous studies, PN significantly decreases the luminal and tissue levels of MUC2.37 In this work, PN significantly decreased the absolute number of goblet cells per villi in the small intestinal epithelium suggesting one reason for the loss of luminal and tissue MUC2. These goblet cell number and mucus changes mirror those of Khan and Ekelund’s studies, although the later examined jejunum38,39. In the present work GLN maintained the number of goblet cells per ileum villi and normalized the ileal luminal tissue homogenates MUC2 compared with PN alone, findings consistent with Khan et al where PN was supplemented with alanyl-glutamine. Conour et al, however, used a model of PN in neonatal piglets and demonstrated goblet cell expansion in PN compared with enteral nutrition control group40. In contrast to the present study, an important factor in Conour’s work was that the enteral control group received formula milk replacer. This constitutes a diet considerably different in complexity and composition than our control group, which was fed standard rodent chow. Furthermore, compared with adult animals, neonatal animals have considerably different adaptive immune cell compartments, so differences are not surprising between neonatal and adult animals.
Paneth cells reside at the base of each small intestinal crypt. These cells secrete a battery of antimicrobial products, including defensins, lysozyme and sPLA2, that form an immunological defense against luminal pathogens. Enteric α-defensins released from murine Paneth cells were named cryptdins (“crypt defensins”). Cryptdin 4 is an important active form that exhibits a broad-spectrum of bacterial activities and has the greatest antimicrobial activity of the described cryptdins.15, 16, 41, 48 Cryptdin 4 eliminates Gram-positive bacteria by binding lipid II and inhibiting bacterial cell wall biosynthesis and attacks gram-negative bacteria by forming membrane pores leading to bacterial lysis. In concert with cryptdin 4, another Paneth product, lysozyme, assaults gram-positive bacteria through hydrolyzing the b(1–4) glycosidic bonds between N-acetylglucosamine and N-acetylmuramic acid to induce bacterial lysis. Lysozyme functions most effectively when combined with cryptdin 4 against gram-negative bacteria since cryptdin 4 helps degrade the outer membrane of bacteria. Together, the combined activity prevents the attachment of luminal bacteria and reduces the invasion of bacteria into the intestinal mucous layer. In our study, PN significantly reduced the cryptdin 4 mRNA expression as well as both the luminal and tissue protein levels of lysozyme, consistent with our hypothesis that PN compromises Paneth cell function. Paneth cells constitute a comparably long lived population of epithelial cells (~21 days). In our model, we observed no evidence of decreased Paneth cell numbers during the 5 days of PN feeding but only a decrease in the level of intracellular Paneth cell granules during PN 42. Functionally, others showed that Paneth cell dysfunction results in intestinal inflammation and may lead to lethal colitis in mice.17 Furthermore, our study reveals that glutamine partially restores the protein expression of lysozyme in small intestinal washing fluid and completely restores tissue levels of lysozme in mouse ileum. Together, these data support the hypothesis that GLN up-regulates innate immunity during PN.
Since we demonstrated improvements in innate immunity and adaptive immunity following GLN compared with PN alone, we further hypothesized that GLN would provide protective effects on barrier function. In prior work, we developed an ex vivo intestinal segment culture that simulates host-pathogen interactions occurring at the mucosal surface.43 This model demonstrated that PN without enteral stimulation significantly increases the susceptibility of murine ileum to enteroinvasion by an extra-intestinal pathogenic Escherichia coli (E.coli) isolated from a patient with recurrent urinary tract infections resistant to therapy. The results demonstrate that glutamine significantly attenuates susceptibility of ileal segments to enteroinvasive E.coli compared to PN alone to levels observed in chow This finding is consistent with enhanced innate immune expression of cryptdin 4, and levels of lysozyme and MUC2 observed in vivo.21 Cryptdins represented nearly 70% of secreted bactericidal compounds from Paneth cells.17, 44 Interestingly, the exogenous supplementation of glutamine failed to increase luminal sPLA2 activity in this study (data not shown), a compound with important antimicrobial functions. Our prior work demonstrated PN significantly reduced sPLA2 within the intestinal lumen. This suggests that not all antimicrobial compounds produced by Paneth cells are stimulated by glutamine and that improvement in barrier functionwas not dependent upon increases in all antibacterial molecules.
On a side note, we previously reported that PN significantly decreases the luminal and tissue levels of Th2 cytokines, and the addition of GLN to PN significantly improved IL-4 in small intestine homogenates and partly restored IL-10 levels compared to PN alone.23, 32 In this work we found that adding GLN to PN completely restored levels of IL-4, IL-10, and IL-13 in the small bowel wash fluid to levels found in chow mice. We speculated in prior work that luminal cytokines could act as paracrine signals to stimulate the epithelium, affecting the epithelial cell physiology and function. These signals may have been restored with GLN, since this compound provides fuel for rapidly dividing cells (particularly lymphocytes and enterocytes) and intestinal epithelial cells.45–47 The implications of these finding remain unclear but are a focus of further investigations in our laboratory.
In conclusion, this work supports our hypothesis that PN without EN feeding decreases Paneth cell function, through decreased lysozyme and cryptdin 4 expression, and number and function of goblet cells. Taken together, these changes impair innate immunity and increase susceptibility of the mucosa to bacterial invasion. Glutamine supplementation improves innate immunity by improving Paneth and goblet cell parameters, normalizing Th2 cytokines, and increasing resistance to bacterial mucosal invasion. This study confirms the importance of enteral feeding upon innate immunity as well as the potential importance of providing parenteral nutrient supplements for the protection of the intestinal mucosa.
Acknowledgments
Funding: The project described was supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and by NIH Surgical Oncology Research Training Program T32CA090217-13. The contents of this article do not represent the views of the Veterans Affairs or the United States Government.
References
- 1.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–511. doi: 10.1097/00000658-199205000-00013. discussion 511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Moore EE, Haenel JB, Waring BJ, Parsons PE. Post-traumatic pulmonary pseudocyst in the adult: pathophysiology, recognition, and selective management. J Trauma. 1989;29(10):1380–1385. doi: 10.1097/00005373-198910000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Mazaki T, Ebisawa K. Enteral versus parenteral nutrition after gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials in the English literature. J Gastrointest Surg. 2008;12(4):739–755. doi: 10.1007/s11605-007-0362-1. [DOI] [PubMed] [Google Scholar]
- 4.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74(4):534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 5.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132(12):1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Kudsk KA, Janu P, Renegar KB. Effect of glutamine-enriched total parenteral nutrition on small intestinal gut-associated lymphoid tissue and upper respiratory tract immunity. Surgery. 1997;121(5):542–549. doi: 10.1016/s0039-6060(97)90109-4. [DOI] [PubMed] [Google Scholar]
- 7.Kudsk KA, Wu Y, Fukatsu K, Zarzaur BL, Johnson CD, Wang R, Hanna MK. Glutamine-enriched total parenteral nutrition maintains intestinal interleukin-4 and mucosal immunoglobulin A levels. JPEN J Parenter Enteral Nutr. 2000;24(5):270–274. doi: 10.1177/0148607100024005270. discussion 274-5. [DOI] [PubMed] [Google Scholar]
- 8.Zarzaur BL, Ikeda S, Johnson CD, Le T, Sacks G, Kudsk KA. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN J Parenter Enteral Nutr. 2002;26(5):265–270. doi: 10.1177/0148607102026005265. discussion 270. [DOI] [PubMed] [Google Scholar]
- 9.Li J, King BK, Janu PG, Renegar KB, Kudsk KA. Glycyl-L-glutamine-enriched total parenteral nutrition maintains small intestine gut-associated lymphoid tissue and upper respiratory tract immunity. JPEN J Parenter Enteral Nutr. 1998;22(1):31–36. doi: 10.1177/014860719802200131. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt RC, Wu Y, Renegar KB, Kudsk KA. Glutamine-enriched total parenteral nutrition preserves respiratory immunity and improves survival to a Pseudomonas Pneumonia. J Surg Res. 1999;84(1):13–18. doi: 10.1006/jsre.1999.5592. [DOI] [PubMed] [Google Scholar]
- 11.Van Klinken BJ, Tytgat KM, Büller HA, Einerhand AW, Dekker J. Biosynthesis of intestinal mucins: MUC1, MUC2, MUC3 and more. Biochem Soc Trans. 1995;23(4):814–818. doi: 10.1042/bst0230814. [DOI] [PubMed] [Google Scholar]
- 12.Van Klinken BJ, Dekker J, Buller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269(5 Pt 1):G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 13.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 14.Ellison RR, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics. 2010;125(6):1237–1247. doi: 10.1542/peds.2009-3289. [DOI] [PubMed] [Google Scholar]
- 17.Stappenbeck TS. Paneth cell development, differentiation, and function: new molecular cues. Gastroenterology. 2009;137(1):30–33. doi: 10.1053/j.gastro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Lu P, Burger-van Paassen N, van der Sluis M, Witte-Bouma J, Kerckaert JP, van Goudoever JB, Van Seuningen I, Renes IB. Colonic gene expression patterns of mucin Muc2 knockout mice reveal various phases in colitis development. Inflamm Bowel Dis. 2011;17(10):2047–2057. doi: 10.1002/ibd.21592. [DOI] [PubMed] [Google Scholar]
- 19.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heneghan AF, Pierre JF, Gosain A, Kudsk KA. IL-25 Improves Luminal Innate Immunity and Barrier Function During Parenteral Nutrition. Ann Surg. 2013 doi: 10.1097/SLA.0b013e318284f510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heneghan AF, Pierre JF, Tandee K, Shanmuganayagam D, Wang X, Reed JD, Steele JL, Kudsk KA. Parenteral Nutrition Decreases Paneth Cell Function and Intestinal Bactericidal Activity While Increasing Susceptibility to Bacterial Enteroinvasion. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113497514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.dos Santos Rd, Viana ML, Generoso SV, Arantes RE, Davisson Correia MI, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. JPEN J Parenter Enteral Nutr. 2010;34(4):408–413. doi: 10.1177/0148607110362530. [DOI] [PubMed] [Google Scholar]
- 23.Fukatsu K, Kudsk KA, Zarzaur BL, Wu Y, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8(6):339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie GJ, Emson CL, Bell SE, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9(3):423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 26.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162(10):6233–6237. [PubMed] [Google Scholar]
- 27.Schwerbrock NM, Makkink MK, van der Sluis M, Büller HA, Einerhand AW, Sartor RB, Dekker J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10(6):811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr, Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168(5):2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189(10):1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182(8):4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 31.Sitren HS, Heller PA, Bailey LB, Baumgartner TG, Cerda JJ. Total parenteral nutrition in the mouse: body composition and plasma chemistries. JPEN J Parenter Enteral Nutr. 1985;9(5):600–604. doi: 10.1177/0148607185009005600. [DOI] [PubMed] [Google Scholar]
- 32.Heneghan AF, Pierre JF, Gosain A, Kudsk KA. IL-25 Improves Luminal Innate Immunity and Barrier Function During Parenteral Nutrition. Ann Surg. 2013 doi: 10.1097/SLA.0b013e318284f510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue C, Tian W, Wang W, Huang Q, Zhao R, Zhao Y, Li Q, Li J. The impact of perioperative glutamine-supplemented parenteral nutrition on outcomes of patients undergoing abdominal surgery: a meta-analysis of randomized clinical trials. Am Surg. 2013;79(5):506–513. [PubMed] [Google Scholar]
- 34.Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, Merle V, Mazerolles M, Samba D, Guillou YM, Petit J, Mansoor O, Colas G, Cohendy R, Barnoud D, Czernichow P, Bleichner G. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34(3):598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. [DOI] [PubMed] [Google Scholar]
- 35.Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131(9 Suppl):2543S–2549S. doi: 10.1093/jn/131.9.2543S. discussion 2550S-1S. [DOI] [PubMed] [Google Scholar]
- 36.Sacks GS, Kudsk KA. Maintaining mucosal immunity during parenteral feeding with surrogates to enteral nutrition. Nutr Clin Pract. 2003;18(6):483–488. doi: 10.1177/0115426503018006483. [DOI] [PubMed] [Google Scholar]
- 37.Pierre JF, Heneghan AF, Feliciano RP, Shanmuganayagam D, Roenneburg DA, Krueger CG, Reed JD, Kudsk KA. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN J Parenter Enteral Nutr. 2013;37(3):401–409. doi: 10.1177/0148607112463076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan J, Iiboshi Y, Cui L, Wasa M, Sando K, Takagi Y, Okada A. Alanyl-glutamine-supplemented parenteral nutrition increases luminal mucus gel and decreases permeability in the rat small intestine. JPEN J Parenter Enteral Nutr. 1999;23(1):24–23. doi: 10.1177/014860719902300124. [DOI] [PubMed] [Google Scholar]
- 39.Ekelund M, Kristensson E, Ekelund M, Ekblad E. Total parenteral nutrition causes circumferential intestinal atrophy, remodeling of the intestinal wall, and redistribution of eosinophils in the rat gastrointestinal tract. Dig Dis Sci. 2007;52(8):1833–1839. doi: 10.1007/s10620-006-9678-z. [DOI] [PubMed] [Google Scholar]
- 40.Conour JE, Ganessunker D, Tappenden KA, Donovan SM, Gaskins HR. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. Am J Physiol Gastrointest Liver Physiol. 2002 Nov;283(5):G1185–G1196. doi: 10.1152/ajpgi.00097.2002. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt P, Wilmes M, Pugnière M, Aumelas A, Bachère E, Sahl HG, Schneider T, Destoumieux-Garzón D. Insight into invertebrate defensin mechanism of action: oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J Biol Chem. 2010;285(38):29208–29216. doi: 10.1074/jbc.M110.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omata J, Pierre JF, Heneghan AF, Tsao FH, Sano Y, Jonker MA, Kudsk KA. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery. 2013 Jan;153(1):17–24. doi: 10.1016/j.surg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre JF, Heneghan AF, Meudt JM, Shea MP, Krueger CG, Reed JD, Kudsk KA, Shanmuganayagam D. Parenteral nutrition increases susceptibility of ileum to invasion by E coli. J Surg Res. 2013;183(2):583–591. doi: 10.1016/j.jss.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23(2):115–120. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 45.Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J Immunol. 2006;176(10):5805–5814. doi: 10.4049/jimmunol.176.10.5805. [DOI] [PubMed] [Google Scholar]
- 46.Leon F, Sanchez L, Camarero C, Roy G. Cytokine production by intestinal intraepithelial lymphocyte subsets in celiac disease. Dig Dis Sci. 2005;50(3):593–600. doi: 10.1007/s10620-005-2480-5. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues RS, Oliveira RA, Li Y, Zaja-Milatovic S, Costa LB, Braga Neto MB, Kolling GL, Lima AA, Guerrant RL, Warren CA. Intestinal epithelial restitution after TcdB challenge and recovery from Clostridium difficile infection in mice with alanyl-glutamine treatment. J Infect Dis. 2013;207(10):1505–1515. doi: 10.1093/infdis/jit041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouellette AJ, Hsieh MM, Nosek MT, Cano-Gauci DF, Huttner KM, Buick RN, Selsted ME. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994 Nov;62(11):5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]