Abstract
Introduction
Tocomin® represents commercially available mixture of naturally occurring tocotrienols (T3s) and tocopherol (Ts) extracted from palm oil/palm fruits that possess powerful antioxidant, anticancer, neuro/cardioprotective and cholesterol lowering properties. Cellular autophagy represents a defense mechanism against oxidative stress and several anticancer compounds. Recently, we reported that tocotrienols induce apoptosis and endoplasmic reticulum stress in breast cancer cells.
Methodology
We studied the effects of Tocomin® on MCF-7 and MDA-MB 231 breast cancer cells and non-tumor MCF-10A cells.
Results
Tocomin® inhibited cell proliferation and induced apoptosis in both MCF-7 and MDA-MB 231 breast cancer cell lines without affecting the viability of MCF-10A cells. We also showed that Tocomin® negatively modulates PI3K and mTOR pathways and induces cytoprotective autophagic response in triple negative MDA-MB 231 cells. Lastly, we demonstrate that autophagy inhibitor 3-methyladenine (3-MA) potentiated the apoptosis induced by Tocomin® in MDA-MB 231 cells.
Conclusion
Together our data indicates anticancer effects of Tocomin® in breast cancer cells which is potentiated by the autophagy inhibitor 3-MA.
Keywords: Vitamin E, Tocomin®, Tocotrienols, Breast Cancer, Apoptosis, Autophagy
Introduction
Vitamin E is composed of Tocopherols (Ts) and Tocotrienols (T3s) that have been shown to possess anti-cancer properties. Tocomin® represents commercially available mixture of naturally occurring tocotrienols (78%) and tocopherols (22%) extracted from palm oil/palm fruits. It also contains other phytonutrients such as plant squalene, phytosterols, co-enzyme Q10 and mixed carotenoids extracted along with tocotrienols from palm fruits. Palm oil is predominantly rich in tocotrienols and has been demonstrated to possess more powerful antioxidant, anticancer, neuro/cardioprotective and cholesterol lowering properties than tocopherols [1–3]. Both tocopherols and Tocotrienols exist as four isoforms each (α, β, γ and δ). The accumulation of T3s in the cells is much greater than tocopherols and might be one of the reasons of a more significant physiological effects of tocotrienols than tocopherols [4].
T3s have been shown to inhibit the growth of various cancer cells including breast cancer without affecting the growth of normal cells [5–8]. T3s have been evaluated in vitro and in vivo as powerful cancer chemotherapeutic/preventive agents, yet their exact mechanisms of action on cell death and other inhibitory pathways are unknown [9,10,7,11,12]. Various mechanisms including blocking oxidative stress or radiation-induced DNA damage [7,13], modulation of immune response [14,15], suppression of multiple oncogenic signaling molecules and pathways such as PI3/AKT/β-catenin, NF-κB, ERK and cyclinD1 [16–21] and ceramide synthesis [22] have been suggested. Also, studies have shown that tocotrienols inhibit cell migration and invasion by modulating matrix metalloproteinases and their inhibitors [23] as well as negatively modulate VEGF dependent angiogenesis [24]. Tocotrienols exhibit cell inhibitory effects in breast cancer cell lines irrespective of their ER status, gene expression profiling in estrogen receptor (ER) positive, p53 wild type MCF-7 and ER negative, p53 mutant MDA-MB 231 cells treated with tocotrienol rich fraction (TRF) of palm oil suggested different mechanisms in the two cell lines [25]. Other mechanisms including activation of proapoptotic pathways including caspase-8 activation and mitochondrial dependency modulation of p53, Bax/Bcl2 [26,17,27,28] have been reported. Recent studies from our laboratory have suggested the role of ATF3 in the apoptosis induced by γ-T3 [29]. Also, we demonstrated the modulation PERK and IRE1α dependent endoplasmic reticulum-stress (ER-stress) and unfolded protein response (UPR) related pathways in MCF-7 and MDA-MB 231 cells when treated with γ-T3 [29]. ER-stress activates unfolded protein response (UPR) that can reestablish endoplasmic reticulum homeostasis through autophagy; however, persistent UPR can also lead to apoptosis. We demonstrated earlier that γ-T3 activates PERK signaling, which has also been shown to induce autophagy as a protective response to cellular insults, such as hypoxia and nutrient deprivation [30–32,29]. Similarly, IRE1α has also been implicated in autophagic response [33,34,31]. In the present study we used commercially available Tocomin® as a source of naturally occurring dietary tocotrienols and studied its effects on inducing autophagy and apoptosis. Further, we used 3-Methyladenine (3-MA), a widely used autophagy inhibitor to study whether combined treatment of 3-MA with Tocomin® modulates apoptosis in breast cancer cells.
1. Materials and Methods
2.1. Cell Culture and Media
Human breast cancer cells (MCF-7 and MDA-MB 231) and human nontransformed mammary epithelial cells (MCF-10A) were obtained from Lombardi Comprehensive Cancer Center cell repository and cultured as recommended.
2.2. Chemicals, Reagents and Antibodies
Tocomin® 50% (here after used as Tocomin®) is a mixture of natural tocotrienols (78%) and Tocopherols (22%), gifted by Carotech International (Edison, NJ) and dissolved in DMSO. WST-1 reagent used for cytotoxicity assay and protease inhibitor cocktail tablets (Roche Applied Science, Indianapolis, IN); 3-Methyladenine or 3-MA (Sigma, St. Louis, MO). ECL Plus Western blotting detection system (GE Healthcare, Piscataway, NJ); and Coomassie protein assay reagent (BioRad, Hercules, CA). All the antibodies were from Cell Signaling Technology Inc., Danvers, MA. Secondary antibodies conjugated with horseradish peroxidase included goat anti-mouse IgG, goat anti-rabbit IgG and rabbit anti-goat IgG (Jackson ImmunoResearch, West Grove, PA).
2.3. Cell Viability and Proliferation Assay
Effects of Tocomin® on cell viability and proliferation were determined as described earlier [29] using a cell viability detection kit (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3-benzene disulfonate, WST-1) according to the manufacturer’s instructions (Roche Applied Science, Indianapolis, IN).
2.4. MDC staining
Monodansylcadaverine (MDC) staining was used as a marker of autophagic vacuoles and lysosomes using Autophagic/cytotoxicity dual staining kit (Cayman Chemical Company, Ann Arbor, MI). Autophagic vacuole staining intensity was measured using an excitation wavelength of 335nm and an emission wavelength of 512nm following the manufacturer’s instructions.
2.5. Western Blotting
Immunoblotting was performed essentially as described previously [29]. Each experiment was performed at least 2 times. Representative Western blots are shown.
2.6. Statistical Analyses
Cell proliferation experiments were performed in 6 replicates. Student’s T test was used to analyze treated vs. untreated cells. Results were expressed as averages ± SD. P<0.05 was considered significant.
3. Results
3.1. Dietary tocotrienols Tocomin® inhibit the proliferation of human breast cancer cells
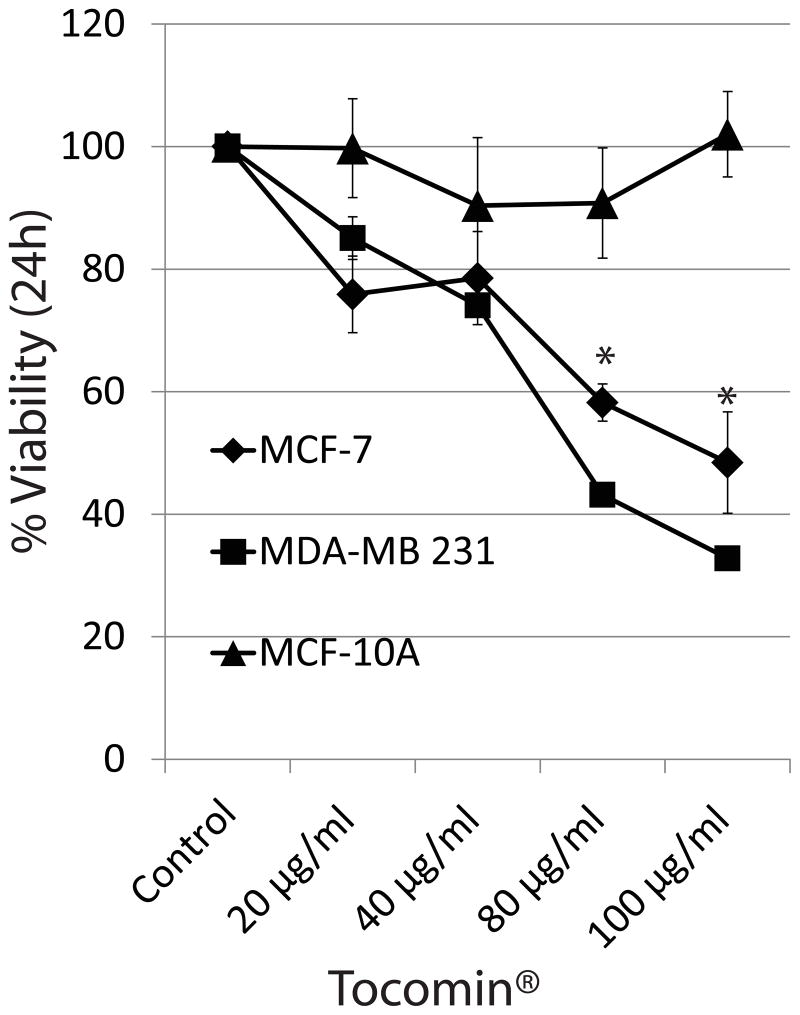
We compared the effects of Tocomin® on the viability of MCF-10A mammary epithelial cells; and MCF-7 and MDA-MB 231 breast cancer cells using WST-1 assay. As shown in Figure 1, cell viability was significantly reduced in cells treated with 80μg/ml and 100μg/ml Tocomin® as compared to the vehicle treated control cells after 24h in both the cell lines. No significant effect on cell viability was observed in MCF-10A cells. Three independent batches of Tocomin® were used with similar results.
Fig. 1.
Tocomin® inhibits the proliferation of MCF-7 and MDA-MB 231 breast cancer cells. Cells were seeded at a density of 3000 cells/well in a 96 well plate and treated with DMSO control or indicated concentrations of Tocomin® for 24h. Cell proliferation was determined by WST-1 assay (Roche). A representative of 3 experiments performed in six replicates each is shown. P<0.05 (*) was considered significant.
3.2. Tocomin® induces apoptosis
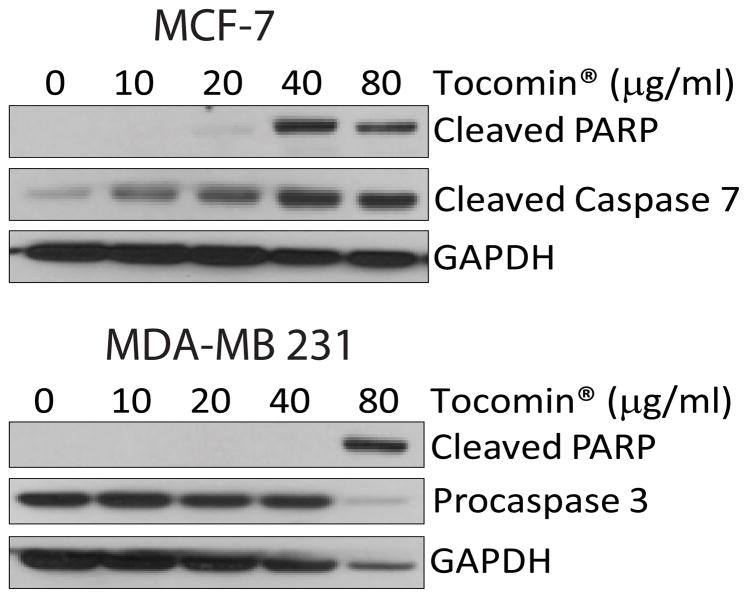
Next we studied the cell death mechanism and whether this mixture of dietary tocotrienols Tocomin® can induce apoptosis. We treated MCF-7 and MDA-MB 231 cells with Tocomin® at various concentrations ranging from 10μg/ml to 80μg/ml and analyzed the cleavage of PARP and caspases as apoptotic markers. In MDA-MB 231 cells we assessed the cleavage of terminal caspase, caspase-3. MCF-7 cells are deficient in caspase-3, thus we analyzed caspase-7 cleavage. Western blotting analysis demonstrated PARP cleavage as indicator of apoptosis in both the cell lines. Also, we observed caspase-7 and caspase-3 cleavage starting at 40μg/ml and 80μg/ml Tocomin® in MCF-7 and MDA-MB 231 respectively after 24h treatment. The results have shown that Tocomin® induces apoptosis in MCF-7 and MDA-MB 231 breast cancer cells after 24h of treatment (Figure 2).
Fig. 2.
Tocomin® induces apoptosis in MCF-7 and MDA-MB 231 cells. Cells were treated with indicated concentrations of Tocomin® for 24h and the expression levels of cleaved-PARP, cleaved caspase-7 and pro-caspase-3 were determined by immunoblotting as an indicator of apoptosis. GAPDH was used as loading control.
3.3. Tocomin® induces autophagic response in MDA-MB 231 cells
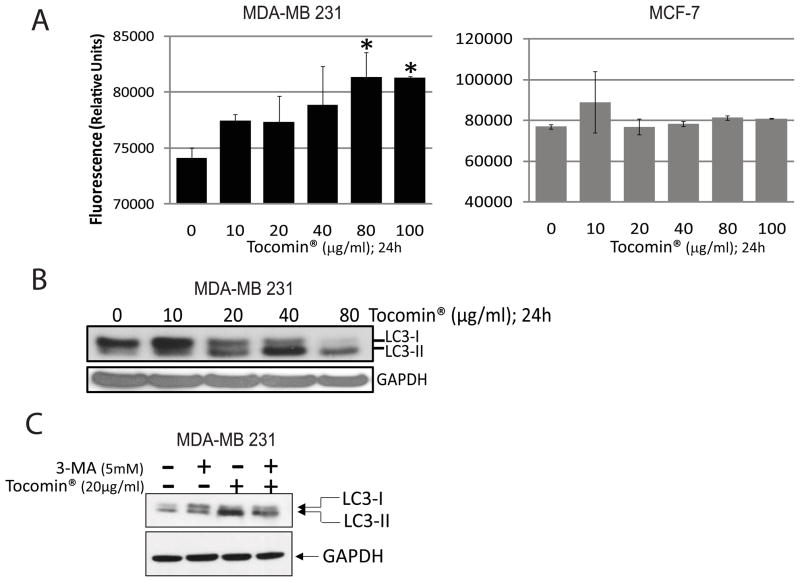
Autophagy provides certain advantage to tumor cells and an autophagic response has been shown to be associated with almost all anti-cancer regimens as tumor cell-intrinsic, resistance mechanism [35–38]. Paradoxically, Autophagy is also induced by dietary compounds with cancer chemo preventative properties and an important role of autophagy has been suggested in chemoprevention of cancer [34,37,31]. Autophagic response also precedes an apoptotic response in certain cases of autophagic cell death. To obtain clues as to the mechanism by which Tocomin® induces apoptosis and whether, an autophagic response is part of Tocomin® induced cell signaling, we performed MDC fluorescent staining to measure autophagy in response to Tocomin®. MCF-7 and MDA-MB 231 cells were treated with Tocomin® ranging from 10–100 μg/ml in a 96-well plate. Cells were incubated with the autofluorescent drug Monodansylcadaverine (MDC), a specific autphagosome marker to detect autophagy. Figure 3A revealed that Tocomin® increased MDC fluorescence intensity only in MDA-MB 231 but not in MCF-7, cells indicating that Tocomin® causes an increase in autophagy in MDA-MB 231 cells but not in MCF-7 cells. Furthermore, we also confirmed the results by detecting the LC3β-II conversion by Western blotting. Both the cell lines were treated with indicated concentrations of Tocomin® from 10 to 80μg/ml for 24h followed by Western blotting with antibodies to the autophagic marker LC3β. As shown in Figure 3B, LC3β-I to II conversion was observed starting at 40μg/ml in MDA-MB 231 cells. No changes in LC3β expression was observed in MCF-7 cells (data not shown). 3-MA is a widely used autophagy inhibitor that inhibits class III phosphoinositide 3-kinase (PI3K). We examined whether 3-MA can inhibit the autophagy induced by Tocomin®. As shown in figure 3C, co-treatment with 3-MA inhibited the autophagy induced by Tocomin® in MDA-MB 231 cells.
Fig. 3.
Induction of autophagy by Tocomin® in MDA-MB 231 cells. A. Indicated cells were seeded at a density of 6000 cells/well in a 96 well plate and treated with DMSO control or indicated concentrations of Tocomin® for 24h. Fluorescent autophagic Monodansylcadaverine (MDC) staining (Cayman Chemical Company) was used as a marker of autophagic vacuoles. A representative of 3 experiments performed in six replicates each is shown. P<0.05 (*) was considered significant. Tocomin® induced autophagy was observed only in MDA-MB 231 cells (3A left panel) and not in MCF-7 cells (3A right panel). B. MDA-MB 231 cells were treated with indicated concentrations of Tocomin® for 24 h and autophagy was determined by Western Blotting using LC3 antibody; C. Pretreatment with 3-MA inhibits tocomin induced autophagy. Cells were treated with 3-MA for 30 minutes followed by treatment with indicated concentrations of Tocomin® for 24h. Autophagy was determined by Western Blotting using LC3 antibody. GAPDH was used as loading control.
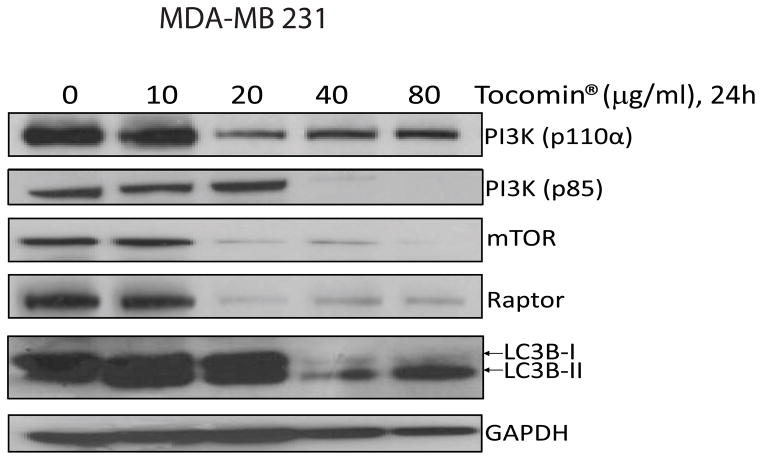
3.4. Tocomin® negatively modulates mTOR pathway
To investigate the mechanisms of Tocomin® induced autophagy, we studied the effects of Tocomin® on components of the mTOR pathway including the PI3 kinase class I that regulates mTOR signaling. The PI3K class I is mainly activated via the insulin receptor leading to the activation of AKT and mTOR complex 1 leading to the suppression of autophagy. As shown in Figure 4, both p85 and 110α subunits were downregulated after Tocomin® treatment. Furthermore, the expression levels of mTOR and Raptor were also downregulated as the concentration of Tocomin® was increased.
Fig. 4.
Tocomin® modulates the expression of proteins involved in the PI3K/mTOR pathway. MDA-MB 231 Cells were treated with indicated concentrations of Tocomin® for 24h and the expression of indicated proteins was determined by immunoblotting. GAPDH was used as loading control.
3.5. 3-MA modulates Tocomin® induced apoptosis
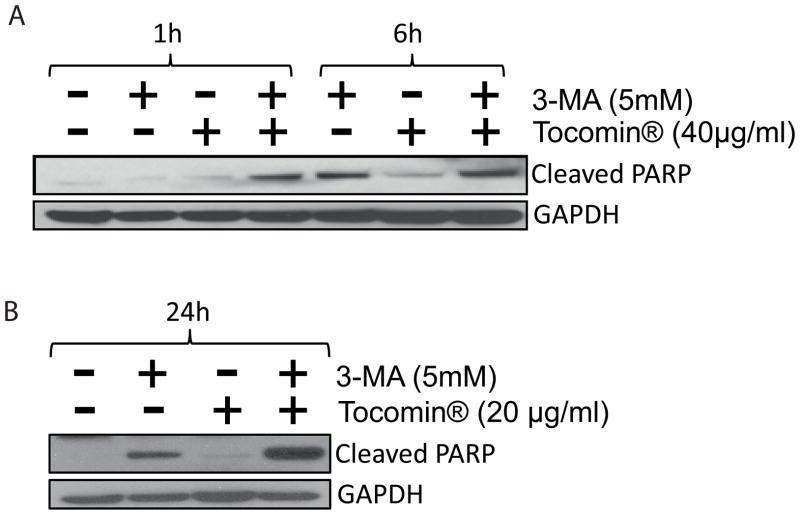
Previous studies have shown that during drug-induced protective autophagy, inhibition of the autophagy often leads to enhanced apoptotic response. We reasoned that if in our case, the autophagy is a resistance mechanism to Tocomin® induced apoptosis, we should also get a similar effect of autophagy inhibition on Tocomin® induced apoptosis. To test this hypothesis, we used 3-MA as an inhibitor of autophagy. MDA-MB 231 cells were starved overnight followed by treatment with 5mM 3-MA for 30 minutes before the combined treatment with sub-effective dose of 20μg/ml Tocomin® for 1, 6 and 24 h. As shown in figure 5, treatment with 3-MA and Tocomin® exhibited an increased apoptotic response as evident by PARP cleavage starting very early at 1h of the combined treatment with profound synergistic apoptotic effect evident at 24h. The results indicated that 3-MA mediated inhibition of autophagy modulates Tocomin® induced apoptosis.
Fig. 5.
Pretreatment with 3-MA modulates Tocomin® induced apoptosis in MDA-MB 231 cells. MDA-MB 231 cells were starved overnight and treated with 5mM 3-MA for 30 minutes followed by 20μg/ml Tocomin® for 1, 6 and 24 h. Apoptosis was determined by detecting PARP cleavage by immunoblotting. GAPDH was used as loading control.
4. Discussion
Vitamin E is composed of tocopherols and Tocotrienols which are two structurally similar compounds with diverse physiological functions. Much of the studies on the effects of Vitamin E have been performed predominantly on tocopherols. Recently several studies have indicated a much prominent role of Tocotrienols in human health including their compelling anti-cancer properties. Studies on purified Tocotrienols have shown that in addition to being pro-apoptotic, T3s also inhibit epithelial mesenchymal transition, angiogenesis and exhibit chemosensitizing as well as anti-cancer stem cell properties [8]. Earlier studies in our lab have shown that γ-T3 induced apoptosis is dependent on ATF3 and is also associated with unfolded protein response leading to endoplasmic reticulum stress (ER-stress) [29]. Autophagy is often activated as a cell survival mechanism followed by ER-stress and UPR [31] with its inhibition leading to sensitization to chemotherapeutic drugs [39–41,36]. In the present study we utilized Tocomin® as a source of primarily dietary tocotrienols (T3s) and studied its effects on MDA-MB 231 and MCF-7 breast cancer cells in inducing apoptosis and autophagy. Studies from our lab and others performed on purified tocotrienols and tocotrienol rich fraction from palm oil have demonstrated their apoptotic effects on various cancer cells without affecting non-tumor cells [8,28,25,1,29]. In a recent study, diet supplementation of tocotrienols to AIN93G-based diet for 13 weeks did not show any side effects in Fisher 344/slc rats [42]. In this study, we did not observe any cell-death with Tocomin® in non-cancerous MCF-10A cells (Figure 1) suggesting a cancer-cell specific response. Here we demonstrate that Tocomin® inhibits cell proliferation and induces apoptosis in breast cancer cells regardless of their ER and p53 status. ER status independent apoptotic response in breast cancer cells by Vitamin E compounds have also been demonstrated in other studies [8,43,44]. Next we studied autophagic response of Tocomin®. In triple negative MDA-MB 231 cells we noticed a profound induction of autophagy as evident by MDC staining and LC3B immunoblotting (Figure 3). Interestingly, we did not observe any autophagy in MCF-7 cells when treated with Tocomin®. Bioavailability of vitamin E compounds vary for its different isoforms and mode of delivery [45]. Bioavailability of tocotrienols, tocotrienol rich fraction (TRF) alone and as inclusion with γ-cyclodextrins and other carriers has been studied [45–47]. Miyoshi eta al., reported the Cmax and AUC values in mice receiving TRF has ranged from 7.9±3.3 μM and 47.7±14.6 μM*h respectively [45]. The concentrations were modestly increase by 1.4 folds when TRF was delivered with γ-cyclodextrins (Cmax=11.5±4.5 μM and AUC=68.1±18.3 μM*h). The TRF exposure as reported corresponds to the Tocomin® concentrations used in this study emphasizing its physiological relevance. Chemopreventive and chemotherapeutic agents (including those that induce ER-stress and UPR) are known to induce autophagy in cancer cells, probably, initially as a protective response maintaining cellular homeostasis and eventually as promoter of cell death. In case of ER-stress inducing compounds such as Tocotrienols, autophagy can help in cope with ER-stress by eliminating the unfolded protein aggregates or ER-stress-induced cell death [32,36,37]. Although, our earlier studies indicated ER stress response in both MCF-7 and MDA-MB 231 cells by purified γ-T3 [29], we did not observe any autophagy in MCF-7 cells that may be explained by difference between the two compounds (Tocomin® vs. purified γ-T3) or other genetic differences between the two cell lines that are important in regulating autophagy. To eliminate the possibility of batch specific effect, 3 independent batches of Tocomin® were tested for similar results. To gain insights into the mechanisms involved in inducing autophagy in MDA-MB 231 cells, we analyzed the expression of multiple signaling proteins known to regulate apoptosis and autophagy. Class I phosphoinositide 3-kinase (PI3K) pathway plays important role in the regulation of apoptosis and autophagy. PI3K is activated by growth receptor signaling at the plasma membrane and activates AKT activating mTOR pathway that inhibits autophagy [34,30,31]. The PI3K enzyme is composed of a regulatory p85 subunit and a catalytic p110 subunit. P85 binds, stabilizes and inhibits the p110 subunit. As shown in Figure 4, we observed downregulation of PI3 kinase subunits and mTOR components in cells treated with Tocomin®. Inhibition of PI3K (p85 and p110) along with mTOR and its binding partner Raptor by Tocomin® may contribute to its apoptotic and autophagic properties. The interaction of p85 and p110 is a complex process. Inhibition of p85 stabilizes p110 and contributes to cell survival. However, we also observed a downregulation of p110 that suggest and over all negative modulation of PI3K pathway by Tocomin® and may be responsible for inhibiting mTOR resulting in apoptosis as well as induction of autophagy. Autophagy induced by anticancer agents can be cytoprotective and the inhibition of such autophagic response has been shown to enhance cell death inducing properties of chemotherapeutic agents [37,36]. If autophagic response to Tocomin® is a cell survival response as it is in case of several anti-cancer therapies, we asked if MDA-MB 231 cells can be sensitized to Tocomin® by inhibiting autophagy. As shown in figure 5, treatment with a well-known autophagy inhibitor 3-MA resulted in increased apoptosis in Tocomin® treated cells. 3-MA is a widely used inhibitor of autophagy that blocks the formation of autophagosome by modulating class III PI3K which is involved in the sequestration of cytoplasmic material during autophagy. In conclusion, although, further studies are warranted to further elucidate the mechanisms of action of Tocomin®, we demonstrate that Tocomin® exhibits anticancer effects in breast cancer cells and like several chemopreventive and chemotherapeutic agents induce cytoprotective autophagic response.
Acknowledgments
We thank Carotech for the gift of Tocomin®. HK is an MARC U*STAR Honors Fellow (GM087172). We thank Dr. Anvesha Srivastava for assisting with submission of the manuscript.
Grant Support: CA141935, CA162264 from the National Cancer Institute to DK
Abbreviations
- T3s
Tocotrienols
- Ts
Tocopherols
- 3-MA
3-Methyladenine
- ER
Estrogen receptor
- TRF
Tocotrienol rich fraction of palm oil
- γ-T3
Gamma tocotrienol
- ER-Stress
Endoplasmic reticulum-stress
- UPR
Unfolded protein response
- MDC
Monodansylcadaverine
- PI3K
Phosphoinositide 3-kinase
- mTOR
Mammalian Target of Rapamycin
- LC3β
microtubule-associated protein 1light chain 3 beta
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest
References
- 1.Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Canali R, Virgili F. Tocotrienol-rich fraction from palm oil and gene expression in human breast cancer cells. Ann NY Acad Sci. 2004;1031:143–157. doi: 10.1196/annals.1331.014. [DOI] [PubMed] [Google Scholar]
- 2.Komiyama K, Iizuka K, Yamaoka M, Watanabe H, Tsuchiya N, Umezawa I. Studies on the biological activity of tocotrienols. Chem Pharm Bull (Tokyo) 1989;37 (5):1369–1371. doi: 10.1248/cpb.37.1369. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–261. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noguchi N, Hanyu R, Nonaka A, Okimoto Y, Kodama T. Inhibition of THP-1 cell adhesion to endothelial cells by alpha-tocopherol and alpha-tocotrienol is dependent on intracellular concentration of the antioxidants. Free Radic Biol Med. 2003;34 (12):1614–1620. doi: 10.1016/s0891-5849(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346 (2):447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- 6.Sakai M, Okabe M, Yamasaki M, Tachibana H, Yamada K. Induction of apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anticancer Res. 2004;24 (3a):1683–1688. [PubMed] [Google Scholar]
- 7.Ling MT, Luk SU, Al-Ejeh F, Khanna KK. Tocotrienol as a potential anticancer agent. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr261. [DOI] [PubMed] [Google Scholar]
- 8.Nesaretnam K, Meganathan P, Veerasenan SD, Selvaduray KR. Tocotrienols and breast cancer: the evidence to date. Genes & nutrition. 2012;7 (1):3–9. doi: 10.1007/s12263-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylvester PW, Wali VB, Bachawal SV, Shirode AB, Ayoub NM, Akl MR. Tocotrienol combination therapy results in synergistic anticancer response. Frontiers in bioscience: a journal and virtual library. 2012;17:3183–3195. doi: 10.2741/3905. [DOI] [PubMed] [Google Scholar]
- 10.Kannappan R, Gupta SC, Kim JH, Aggarwal BB. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes & nutrition. 2012;7 (1):43–52. doi: 10.1007/s12263-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Mesquita ML, Araujo RM, Bezerra DP, Filho RB, de Paula JE, Silveira ER, Pessoa C, de Moraes MO, Costa Lotufo LV, Espindola LS. Cytotoxicity of delta-tocotrienols from Kielmeyera coriacea against cancer cell lines. Bioorganic & medicinal chemistry. 2011;19 (1):623–630. doi: 10.1016/j.bmc.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Luk SU, Yap WN, Chiu YT, Lee DT, Ma S, Lee TK, Vasireddy RS, Wong YC, Ching YP, Nelson C, Yap YL, Ling MT. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. International journal of cancer Journal international du cancer. 2011;128 (9):2182–2191. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 13.Taridi NM, Yahaya MF, Teoh SL, Latiff AA, Ngah WZ, Das S, Mazlan M. Tocotrienol rich fraction (TRF) supplementation protects against oxidative DNA damage and improves cognitive functions in Wistar rats. La Clinica terapeutica. 2011;162 (2):93–98. [PubMed] [Google Scholar]
- 14.Ren Z, Pae M, Dao MC, Smith D, Meydani SN, Wu D. Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. The Journal of nutrition. 2010;140 (7):1335–1341. doi: 10.3945/jn.110.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafid SR, Radhakrishnan AK, Nesaretnam K. Tocotrienols are good adjuvants for developing cancer vaccines. BMC cancer. 2010;10:5. doi: 10.1186/1471-2407-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SJ, Sylvester PW. Gamma-tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp Biol Med(Maywood) 2005;230 (4):235–241. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 17.Shah S, Sylvester PW. Tocotrienol-induced caspase-8 activation is unrelated to death receptor apoptotic signaling in neoplastic mammary epithelial cells. Exp Biol Med(Maywood) 2004;229 (8):745–755. doi: 10.1177/153537020422900806. [DOI] [PubMed] [Google Scholar]
- 18.Shin-Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, Reddy SA, Krishnan K. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free radical biology & medicine. 2011;51 (6):1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Katuru R, Fernandes NV, Elfakhani M, Dutta D, Mills N, Hynds DL, King C, Mo H. Mevalonate depletion mediates the suppressive impact of geranylgeraniol on murine B16 melanoma cells. Experimental biology and medicine. 2011;236 (5):604–613. doi: 10.1258/ebm.2011.010379. [DOI] [PubMed] [Google Scholar]
- 20.Elangovan S, Hsieh TC, Wu JM. Growth inhibition of human MDA-mB-231 breast cancer cells by delta-tocotrienol is associated with loss of cyclin D1/CDK4 expression and accompanying changes in the state of phosphorylation of the retinoblastoma tumor suppressor gene product. Anticancer Res. 2008;28 (5A):2641–2647. [PubMed] [Google Scholar]
- 21.Loganathan R, Selvaduray KR, Nesaretnam K, Radhakrishnan AK. Tocotrienols promote apoptosis in human breast cancer cells by inducing poly(ADP-ribose) polymerase cleavage and inhibiting nuclear factor kappa-B activity. Cell proliferation. 2013;46 (2):203–213. doi: 10.1111/cpr.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopalan A, Yu W, Jiang Q, Jang Y, Sanders BG, Kline K. Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells. Molecular nutrition & food research. 2012;56 (12):1803–1811. doi: 10.1002/mnfr.201200350. [DOI] [PubMed] [Google Scholar]
- 23.Liu HK, Wang Q, Li Y, Sun WG, Liu JR, Yang YM, Xu WL, Sun XR, Chen BQ. Inhibitory effects of gamma-tocotrienol on invasion and metastasis of human gastric adenocarcinoma SGC-7901 cells. The Journal of nutritional biochemistry. 2010;21 (3):206–213. doi: 10.1016/j.jnutbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Xu YW, Wang B, Ding CH, Li T, Gu F, Zhou C. Differentially expressed micoRNAs in human ooc. 2011 doi: 10.1007/s10815-011-9590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Reimann K, Razak G, Virgili F. Tocotrienol-rich fraction from palm oil affects gene expression in tumors resulting from MCF-7 cell inoculation in athymic mice. Lipids. 2004;39 (5):459–467. doi: 10.1007/s11745-004-1251-1. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Loo G. Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem Pharmacol. 2004;67 (2):315–324. doi: 10.1016/j.bcp.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Constantinou C, Neophytou CM, Vraka P, Hyatt JA, Papas KA, Constantinou AI. Induction of DNA Damage and Caspase-Independent Programmed Cell Death by Vitamin E. Nutrition and cancer. 2011 doi: 10.1080/01635581.2012.630167. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal MK, Agarwal ML, Athar M, Gupta S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle. 2004;3 (2):205–211. doi: 10.4161/cc.3.2.654. [DOI] [PubMed] [Google Scholar]
- 29.Patacsil D, Tran AT, Cho YS, Suy S, Saenz F, Malyukova I, Ressom H, Collins SP, Clarke R, Kumar D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. The Journal of nutritional biochemistry. 2012;23 (1):93–100. doi: 10.1016/j.jnutbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell death and differentiation. 2007;14 (2):230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 31.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and cellular biology. 2006;26 (24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. The Journal of clinical investigation. 2010;120 (1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, Noh JY, Oh Y, Kim Y, Chang JW, Chung CW, Lee ST, Kim M, Ryu H, Jung YK. IRE1 plays an essential role in ER stress-mediated aggregation of mutant huntingtin via the inhibition of autophagy flux. Human molecular genetics. 2012;21 (1):101–114. doi: 10.1093/hmg/ddr445. [DOI] [PubMed] [Google Scholar]
- 34.Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, Qiu SJ, Fan J. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7 (10):1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa T, Tsuno NH, Okaji Y, Shuno Y, Sasaki K, Hongo K, Sunami E, Kitayama J, Takahashi K, Nagawa H. Inhibition of autophagy potentiates sulforaphane-induced apoptosis in human colon cancer cells. Annals of surgical oncology. 2010;17 (2):592–602. doi: 10.1245/s10434-009-0696-x. [DOI] [PubMed] [Google Scholar]
- 36.Carew JS, Medina EC, Esquivel JA, 2nd, Mahalingam D, Swords R, Kelly K, Zhang H, Huang P, Mita AC, Mita MM, Giles FJ, Nawrocki ST. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. Journal of cellular and molecular medicine. 2010;14 (10):2448–2459. doi: 10.1111/j.1582-4934.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livesey KM, Tang D, Zeh HJ, Lotze MT. Autophagy inhibition in combination cancer treatment. Current opinion in investigational drugs. 2009;10 (12):1269–1279. [PubMed] [Google Scholar]
- 38.Ren Y, Huang F, Liu Y, Yang Y, Jiang Q, Xu C. Autophagy inhibition through PI3K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB reports. 2009;42 (9):599–604. doi: 10.5483/bmbrep.2009.42.9.599. [DOI] [PubMed] [Google Scholar]
- 39.Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, Tang L, Wang M, Wang Q, Wang HY. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Laboratory investigation; a journal of technical methods and pathology. 2011;91 (8):1146–1157. doi: 10.1038/labinvest.2011.97. [DOI] [PubMed] [Google Scholar]
- 40.Xie BS, Zhao HC, Yao SK, Zhuo DX, Jin B, Lv DC, Wu CL, Ma DL, Gao C, Shu XM, Ai ZL. Autophagy inhibition enhances etoposide-induced cell death in human hepatoma G2 cells. International journal of molecular medicine. 2011;27 (4):599–606. doi: 10.3892/ijmm.2011.607. [DOI] [PubMed] [Google Scholar]
- 41.Chen LH, Loong CC, Su TL, Lee YJ, Chu PM, Tsai ML, Tsai PH, Tu PH, Chi CW, Lee HC, Chiou SH. Autophagy inhibition enhances apoptosis triggered by BO-1051, an N-mustard derivative, and involves the ATM signaling pathway. Biochemical pharmacology. 2011;81 (5):594–605. doi: 10.1016/j.bcp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Shibata A, Nakagawa K, Shirakawa H, Kobayashi T, Kawakami Y, Takashima R, Ohashi A, Sato S, Ohsaki Y, Kimura F, Kimura T, Tsuduki T, Komai M, Miyazawa T. Physiological effects and tissue distribution from large doses of tocotrienol in rats. Bioscience, biotechnology, and biochemistry. 2012;76 (9):1805–1808. doi: 10.1271/bbb.120387. [DOI] [PubMed] [Google Scholar]
- 43.Nesaretnam K, Dorasamy S, Darbre PD. Tocotrienols inhibit growth of ZR-75-1 breast cancer cells. IntJ Food Sci Nutr. 2000;51(Suppl):S95–103. [PubMed] [Google Scholar]
- 44.Nesaretnam K, Stephen R, Dils R, Darbre P. Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids. 1998;33 (5):461–469. doi: 10.1007/s11745-998-0229-3. [DOI] [PubMed] [Google Scholar]
- 45.Miyoshi N, Wakao Y, Tomono S, Tatemichi M, Yano T, Ohshima H. The enhancement of the oral bioavailability of gamma-tocotrienol in mice by gamma-cyclodextrin inclusion. The Journal of nutritional biochemistry. 2011;22 (12):1121–1126. doi: 10.1016/j.jnutbio.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Fu JY, Che HL, Tan DM, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutrition & metabolism. 2014;11 (1):5. doi: 10.1186/1743-7075-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. The Journal of pharmacy and pharmacology. 2001;53 (1):67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]