Abstract
It is becoming apparent that the activity of many neural networks is shaped by effects of endogenous neuromodulators. Modulators exert second messenger-mediated actions that persist. We consider how this may impact network function and its potential role in the induction of repetition priming (increased performance when behavior is repeated). When effects of modulators persist and modulatory substances are repeatedly released, their effects will accumulate (summate) and become more pronounced. If this enhances the ability of a network to generate a particular output, performance will improve. We review data that support this model, and consider its implications for task switching. This model predicts that priming of one type of network activity will negatively impact the rapid transition to an incompatible type.
Introduction
Many neural networks are subject to modulation, which is essential for normal functioning. Recently there have been a number of comprehensive reviews that have addressed the question: Why is this modulatory input important? A consensus that has emerged is that it is essential for functional flexibility because it overcomes the inherent limitations of a hard-wired network [1–5]. How this can be achieved has been extensively reviewed at both cellular and molecular levels.
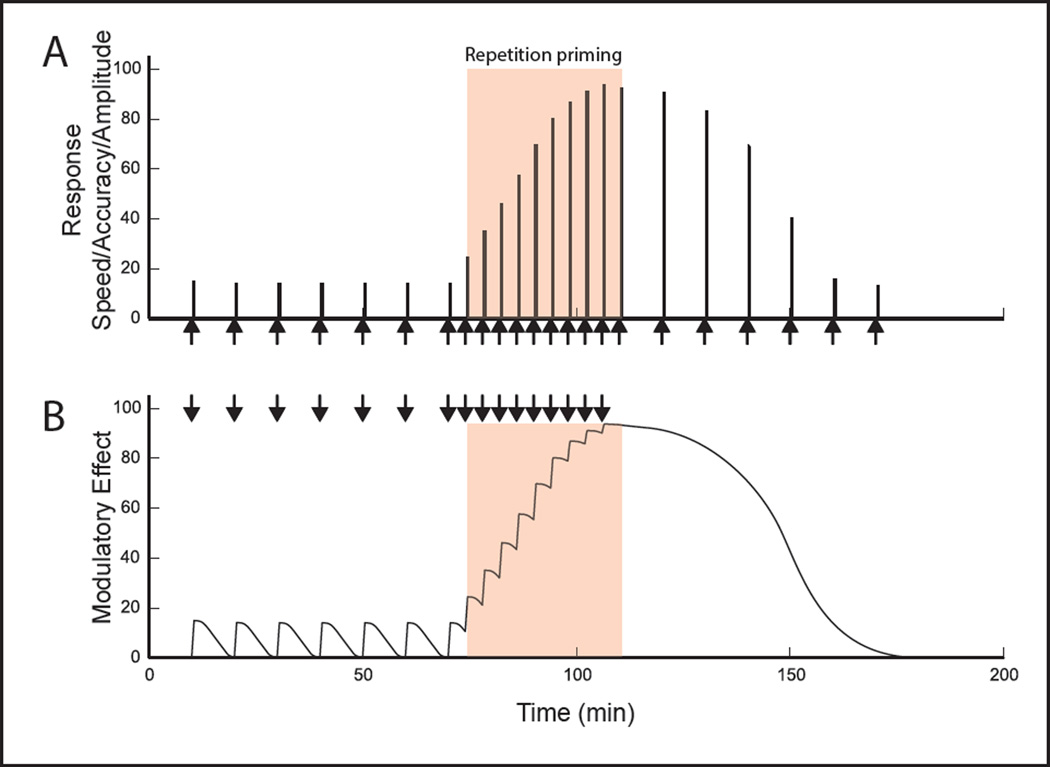
This review focuses on an important topic that has been less extensively discussed. Namely, we consider potential consequences of the persistence that is characteristic of neuromodulation. More specifically, we focus on persistence that lasts on the order of seconds and minutes. Further, we consider a particularly interesting situation-one in which modulator release is coupled to network activity, e.g., endogenous modulators are phasically released every time a cycle of activity is generated. This includes, but is not limited to the situation where modulators are intrinsic to the central pattern generator (CPG) itself. If modulatory actions persist, this is likely to lead to a situation where effects of modulators summate and cumulative effects become larger as activity progresses (Fig. 1B). We suggest that this type of mechanism is ideally suited for the induction of a well-known phenomenon-repetition priming (Fig. 1A). Repetition priming is defined as a progressive improvement in performance when behavior is repeated. Thus a very effective means of inducing an activity-dependent, progressive improvement in behavior is via cumulative effects of endogenously released modulatory substances.
Figure 1.
Schematic representation of the suggested role of endogenous modulation in the induction of repetition priming. (A) Repetition priming is induced when stimuli are applied with a short interstimulus interval (ISI) (red-orange panel). Repetition priming is manifested as progressive improvements in performance, which can be measured in a number of ways (e.g., as progressive decreases in latency (or increases in speed), as progressive increases in accuracy, or as progressive increases in response amplitude (magnitude)). Note that effects of repetition priming persist (right of red-orange panel). This is apparent when the ISI is lengthened, i.e., returned to the control value. (B) Our model postulates that priming can result from effects of modulators, which summate and become progressively larger with network activation with a short ISI (red-orange panel). Effects of repetition priming persist because it takes time for cumulative effects of modulators to dissipate (right of red panel).
That this occurs under physiologically relevant conditions is suggested by work in an experimentally advantageous model system-the feeding system of the mollusc Aplysia. Below we briefly review some of this work. Subsequently, we discuss similarities between the Aplysia feeding system and other systems.
The Aplysia feeding system
The Aplysia feeding network is multifunctional and generates both ingestive and egestive behaviors [6]. During ingestion the radula, the food-grasping organ, is open as it protracts (i.e., moves forward) and is closed as it retracts (i.e., moves backward). This pulls food into the buccal cavity. During egestion the radula is closed as it protracts and open as it retracts. This pushes food out. Repetition priming has been demonstrated at the behavioral level, e.g., progressive increases in the magnitude of biting responses are observed as animals begin to repetitively feed [7].
Repetition priming in Aplysia has also been extensively studied in vitro. These experiments take advantage of the fact that feeding motor programs can be triggered using inputs that drive ingestive or egestive responses. An ingestive input is a command-like projection neuron, CBI-2 [8]. CBI-2 receives excitatory input from sensory neurons activated when food contacts the lips [8]. Egestive activity is triggered by stimulating a branch of the esophageal nerve (EN) (e.g., [9, 10]. The EN contains processes of sensory neurons innervating the gut [11, 12].
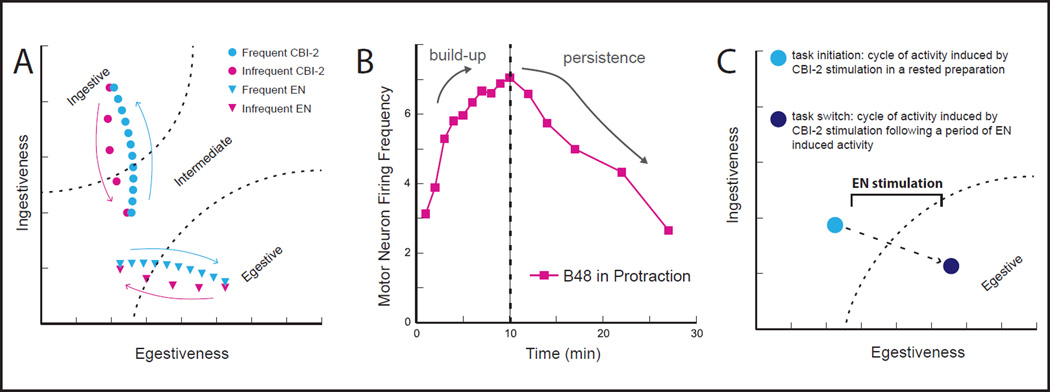
Interestingly, when a single cycle of a motor program is triggered in a quiescent preparation, evoked activity is neither ingestive nor egestive (Fig. 2A) [9]. Instead motor programs are poorly defined and are referred to as having ‘intermediate’ characteristics. This is the case both when activity is triggered by stimulating CBI-2, and when activity is triggered by stimulating the EN. In this situation, the network is in a default state [13]**.
Figure 2.
(A) Light blue, Repetition priming in the feeding network of Aplysia. When ingestive (CBI-2) or egestive (EN) inputs are stimulated in a rested preparation the first cycle of induced activity is poorly defined, i.e., it has intermediate characteristics. It is only with repetitive input activation that motor programs become either ingestive or egestive. Pink, With infrequent input activation programs lose their ingestive or egestive character. (B) Persistence of repetition priming, manifested as an increase in firing frequency of the radula opener motor neuron B48. Left of dashed line, Cycles of activity induced by stimulating CBI-2 with a relatively short ISI. This induces repetition priming (here manifested as a progressive increase (build-up) in the firing frequency of the ‘ingestive’ motor neuron B48). Right of dashed line, Cycles of activity induced by stimulating CBI-2 with a relatively long ISI, following repetition priming. Note that effects of repetition priming persist, i.e., it takes time for the B48 firing frequency to return to control levels. (C) Task-switch costs are observed in the Aplysia feeding network. For example, when a cycle of activity is induced by stimulating the ingestive input CBI-2 following a period of egestive activity, the cycle is egestive (dark blue).
When an input is repeatedly activated so that multiple cycles of motor programs are induced, however, program definition occurs (Fig. 2A) [13**–18]**. With repeated stimulation of CBI-2, activity becomes progressively more ingestive. With repeated stimulation of the EN, activity becomes progressively more egestive. Progressive changes in motor neuron activity that are observed under these conditions are sufficient to produce significant changes in feeding movements. For example, when CBI-2 is repeatedly stimulated there are progressive increases in the firing frequency of the radula opener motor neuron B48 that persist after CBI-2 stimulation ceases (Fig. 2B). Changes in motor neuron activity are sufficient to alter the magnitude of radula opening movements [17].
To determine how these persistent changes are mediated, experiments have focused on modulatory substances that are released by input activation. CBI-2 contains aceytcholine, which can exert muscarinic effects on the feeding circuitry [13, 19] and two modulatory neuropeptides CP-2 and FCAP [20–23]. EN afferents contain and release several neuropeptides including Aplysia Neuropeptide Y (aNPY) [24], the Small Cardioactive Peptides (SCPs) [25], and the FRFamide peptides [26].
To tie effects of modulators to repetition priming, analytical experiments have been performed at key sites in the feeding circuitry. This work has focused on motor neurons that open and close the radula since the phasing of this activity is altered when there are changes in the ingestive vs. egestive nature of feeding motor programs [27]. For simplicity this review will focus on one locus where effects of modulators are particularly well characterized-changes in the activity of a motor neuron that produces radula opening (B48).
As motor programs become ingestive, there are progressive increases in B48’s firing frequency that result from progressive increases in its excitability [17, 18]**. Excitability increases in B48 persist and outlast periods of CBI-2 stimulation [17]. They are observed when FCAP and CP-2, the two peptides present in CBI-2, are exogenously applied, and importantly, peptide application occludes effects of repetitive CBI-2 stimulation [18]**. Effects of FCAP/CP-2/CBI-2 on B48 excitability are cAMP mediated [18]**. For example, they are mimicked by application of the cAMP analog 8-Br-cAMP, which also produces increases in B48’s firing frequency during motor programs. Further, peptide effects are enhanced and prolonged in the presence of the phosphodiesterase inhibitor IBMX. Finally, Rp-cAMP blocks both peptide and CBI-2 induced increases in excitability.
To summarize, ingestive and egestive inputs to the feeding circuitry contain modulators that exert second messenger mediated effects that persist after input activation ceases. With repetitive input activation effects of modulators become cumulative and there are progressive alterations in motor neuron activity. This leads to progressive enhancements in feeding movements, i.e., repetition priming.
Endogenous modulation in other systems
In the Aplysia feeding system endogenous modulators are present in projection neurons and sensory neurons that trigger activity. This type of arrangement has been described. The best-characterized example is the crustacean stomatogastric ganglion (STG) (see Fig. 2 in [1] for a recent diagram that summarizes characterized modulatory input to the STG). Persistent effects of stimulation of STG projection neurons, or neurons that excite projection neurons have been noted (with differing time constants) [28, 29]. Differences in timing have also been noted in the Aplysia feeding system (e.g., effects of ingestive repetition priming often last longer than effects of egestive priming) [14]. In the feeding system these differences are likely to reflect, at least in part, differences in the second messenger systems utilized.
Interestingly not all of the modulatory input to the STG arises in this manner. Some modulatory input is referred to as ‘tonic’ and consists of substances that are released into the hemolymph. Tonic STG modulators appear to be important for maintaining basal circuit function [30–32]**. For example, dopaminergic tone regulates the conductance of a transient potassium current (IA) via a mechanism that is dependent on translation [32]**. Not all effects of dopamine on the STG are however tonic, i.e., there are also ‘phasic’ effects. Interestingly, tonic effects of dopamine differ from (actually oppose) phasic effects. For example, tonic dopamine produces an increase in the IA Gmax whereas phasic dopamine produces depolarizing shifts in the voltage dependence of IA. Thus the stomatogastric nervous system is a well-characterized system in which phasic modulatory actions can be contrasted with a second type of modulation (tonic).
In some systems modulators are intrinsic to the CPG itself. This clearly results in a situation where modulator release is closely tied to network activity. One of the first systems where this was described is the circuitry that mediates escape swimming in Tritonia [33–39]. Here serotonin is present in a CPG element, an identified neuron known as the dorsal swim interneuron (DSI). This cell exerts effects on a second interneuron, C2, which is also crucial for the behavior. Effects of 5-HT are in part mediated via interaction with metabotropic receptors [40, 41] and persist long enough so that they are likely to carry over from cycle to cycle [42].
An advantage of the invertebrate networks discussed above is that they are well characterized, e.g., individual neurons are re-identifiable and consequently are functionally characterized. It is therefore (relatively) simple to determine whether or not they are a part of the actual CPG. In the vertebrate networks these distinctions are not always as clear. Nevertheless it is apparent that vertebrate networks are heavily modulated by endogenous neural input. For example, the Pre-Botzinger (PreBotC) respiratory network receives aminergic and peptidergic input that is essential for maintaining normal activity (for review see [43]). When hypoxia alters its ability to respond to norepinephrine, activity becomes irregular [44]*.
It has long been apparent that the vertebrate locomotor CPG receives ‘extrinsic’ modulatory input from the brainstem [45]. Additionally, however it is becoming increasingly apparent that it is regulated by modulators that are intrinsic to the spinal cord. For example, ACh released from spinal VOc neurons binds to m2 receptors on motor neurons to increase motor neuron excitability and make motor neurons more responsive to input. VOc activity is tightly phase-locked to motor neuron activity during fictive locomotion [46]. Effects of nitric oxide (NO) on locomotor activity have also been demonstrated in the tadpole [47], lamprey [48, 49] and more recently in the mouse [50]. In the mouse and lamprey, nitric oxide effects are cGMP mediated [48, 50]. In the mouse persistent effects of relatively low NO concentrations induce long-lasting enhancements of the amplitude of locomotor network output [50].
An important theme that is emerging from work on both vertebrate and invertebrate networks is that small molecule transmitters can themselves exert modulatory effects via metabotropic glutamate receptors (e.g., [43, 51]), GABAB receptors [43, 52]**, and muscarinic ACh receptors [19, 46, 53]. These effects can be quite complex (e.g., see [52]** for a recently described situation where effects of GABA are exerted over three timescales). Some of these situations constitute clear cases of intrinsic modulation. Thus, the view that is emerging is that endogenous, often intrinsic modulation may be more of a rule than an exception.
Repetition priming is observed in many contexts
The phenomenon repetition priming is of considerable general interest since it is a tractable form of implicit memory. Consequently, it has been extensively studied in psychology experiments using human subjects. For example, an early use of the term was to describe effects of pre-exposing subjects to words with a common ‘morphology’ [54]. Related research has also demonstrated priming in situations where recognition of faces or objects is impacted by repeated presentation (for review see [55]). In some of these tasks there is a motor as well as sensory component. For example, one recent study (of the priming of Pop-out) specifically demonstrated a role of the motor response [56]*. In these experiments subjects searched for a color singleton target and either responded to its shape (go trial) or passively watched the display (no go). Repetition effects were larger after go trials.
Other studies have demonstrated repetition priming using sound stimuli (e.g., [57]) and in experiments that studied effects of anoxia on respiratory activity [58]. More recently priming has even been demonstrated during cognitive tasks (i.e., mathematical problem solving) [59]. In summary, repetition priming is observed in a number of contexts and is becoming increasingly popular as a tool for the study of implicit memory. Nevertheless, its underlying cellular/molecular mechanisms are currently not well understood. This is likely to change as tractable model systems are characterized where it can be studied.
General implications of these findings for future work
The suggested model, in which repetition priming is mediated via persistent effects of endogenous modulators, has interesting implications for task switching. Namely it predicts that a ‘cost’ may be observed if one behavior ceases and another begins shortly thereafter. This has in fact been demonstrated in ‘input switching’ experiments in the Aplysia feeding network (Fig. 2C) [14, 16, 17]. For example, if an egestive state is induced by repeated EN stimulation and a cycle of activity is then induced by stimulating CBI-2, this cycle will be egestive rather than intermediate (or ingestive). Task-switch costs are observed in other species (e.g., humans), and can significantly impact performance [60, 61]. In future work it will be of interest to determine how they arise on a cellular/molecular level, and whether endogenous modulators play a role in systems other than the Aplysia feeding network. Further, it will be of interest to determine whether repetition priming and task-switch costs commonly go hand in hand.
Highlights.
-
-
Many networks are persistently modulated by endogenous input
-
-
Modulator release is often ‘phasic’ and closely tied to network activity
-
-
Effects of modulators can become cumulative with repeated network activity
-
-
Cumulative effects of neuromodulation can lead to repetition priming
Acknowledgements
Supported by NIH grants NS066587, NS070583, MH051393, and National Natural Science Foundation of China grant 31371104.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth C. Cropper, Email: elizabeth.cropper@gmail.com.
Allyson K. Friedman, Email: Allyson.friedman@mssm.edu.
Jian Jing, Email: jingj01@gmail.com.
Matthew H. Perkins, Email: matthew.perkins@mssm.edu.
Klaudiusz R. Weiss, Email: klaudiusz.weiss@gmail.com.
References Cited
- 1.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucher D, Marder E. SnapShot: neuromodulation. Cell. 2013;155:482–482. doi: 10.1016/j.cell.2013.09.047. e481. [DOI] [PubMed] [Google Scholar]
- 3.Nusbaum MP, Blitz DM. Neuropeptide modulation of microcircuits. Curr Opin Neurobiol. 2012;22:592–601. doi: 10.1016/j.conb.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris-Warrick RM. Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol. 1974;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- 7.Susswein AJ, Weiss KR, Kupfermann I. Effects of food arousal on latency of biting in Aplysia. Journal of Comparative Physiology. 1978;123:31–41. [Google Scholar]
- 8.Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. Journal of Neuroscience. 1991;11:3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9447–9452. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhurov Y, Proekt A, Weiss KR, Brezina V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. Journal of Neuroscience. 2005;25:1268–1280. doi: 10.1523/JNEUROSCI.3361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Susswein AJ, Kupfermann I. Bulk as a stimulus for satiation in Aplysia. Behav Biol. 1975;13:203–209. doi: 10.1016/s0091-6773(75)91903-3. [DOI] [PubMed] [Google Scholar]
- 12.Kuslansky B, Weiss KR, Kupfermann I. Mechanisms underlying satiation of feeding behavior of the mollusc Aplysia. Behav Neural Biol. 1987;48:278–303. doi: 10.1016/s0163-1047(87)90836-3. [DOI] [PubMed] [Google Scholar]
- 13. Dacks AM, Siniscalchi MJ, Weiss KR. Removal of default state-associated inhibition during repetition priming improves response articulation. J Neurosci. 2012;32:17740–17752. doi: 10.1523/JNEUROSCI.4137-12.2012. Looks at mechanisms underlying repetition priming in the Aplysia feeding system. A particular emphasis of this study was to determine how the rested (default) state is altered as priming is induced. An important issue addressed was whether the default state is 'passive' in the sense that it does not promote or impede one behavior as opposed to another. The authors demonstrate that this is not the case, e.g., it includes active inhibition that prevents the expression of one type of behavior.
- 14.Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci U S A. 2004;101:9447–9452. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhurov Y, Proekt A, Weiss KR, Brezina V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. J Neurosci. 2005;25:1268–1280. doi: 10.1523/JNEUROSCI.3361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the Aplysia feeding network. J Neurophysiol. 2007;97:3046–3056. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- 17.Friedman AK, Zhurov Y, Ludwar B, Weiss KR. Motor outputs in a multitasking network: relative contributions of inputs and experience-dependent network states. J Neurophysiol. 2009;102:3711–3727. doi: 10.1152/jn.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci. 2010;30:8906–8919. doi: 10.1523/JNEUROSCI.1287-10.2010. Studies mechanisms underlying repetition priming during ingestive motor programs in the Aplysia feeding network. These experiments (together with those in [17] establish a role for two modulatory neuropeptides (FCAP and CP2) and demonstrate that effects are second messenger (i.e., cAMP) mediated.
- 19.Dembrow NC, Jing J, Brezina V, Weiss KR. A specific synaptic pathway activates a conditional plateau potential underlying protraction phase in the Aplysia feeding central pattern generator. J Neurosci. 2004;24:5230–5238. doi: 10.1523/JNEUROSCI.5649-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweedler JV, Li L, Rubakhin SS, Alexeeva V, Dembrow NC, Dowling O, Jing J, Weiss KR, Vilim FS. Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of Aplysia. J Neurosci. 2002;22:7797–7808. doi: 10.1523/JNEUROSCI.22-17-07797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilim FS, Alexeeva V, Moroz LL, Li L, Moroz TP, Sweedler JV, Weiss KR. Cloning, expression and processing of the CP2 neuropeptide precursor of Aplysia. Peptides. 2001;22:2027–2038. doi: 10.1016/s0196-9781(01)00561-7. [DOI] [PubMed] [Google Scholar]
- 22.Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol. 2000;84:1186–1193. doi: 10.1152/jn.2000.84.3.1186. [DOI] [PubMed] [Google Scholar]
- 23.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol. 2003;90:2074–2079. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 24.Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, et al. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JS, Vilim FS, Hatcher NG, Due MR, Sweedler JV, Weiss KR, Jing J. Composite modulatory feedforward loop contributes to the establishment of a network state. J Neurophysiol. 2010;103:2174–2184. doi: 10.1152/jn.01054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilim FS, Sasaki K, Rybak J, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, et al. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J Neurosci. 2010;30:131–147. doi: 10.1523/JNEUROSCI.3282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cropper EC, Evans CG, Hurwitz I, Jing J, Proekt A, Romero A, Rosen SC. Feeding neural networks in the mollusc Aplysia. Neurosignals. 2004;13:70–86. doi: 10.1159/000076159. [DOI] [PubMed] [Google Scholar]
- 28.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J Neurosci. 1989;9:1591–1599. doi: 10.1523/JNEUROSCI.09-05-01591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. Journal of Experimental Biology. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodgers EW, Fu JJ, Krenz WD, Baro DJ. Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron. J Neurosci. 2011;31:16387–16397. doi: 10.1523/JNEUROSCI.3770-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodgers EW, Krenz WD, Baro DJ. Tonic dopamine induces persistent changes in the transient potassium current through translational regulation. J Neurosci. 2011;31:13046–13056. doi: 10.1523/JNEUROSCI.2194-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodgers EW, Krenz WD, Jiang X, Li L, Baro DJ. Dopaminergic tone regulates transient potassium current maximal conductance through a translational mechanism requiring D1Rs, cAMP/PKA, Erk and mTOR. BMC Neurosci. 2013;14:143. doi: 10.1186/1471-2202-14-143. This, and the above two studies [30,31] present contrasts between 'phasic' and 'tonic' modulatory effects of dopamine. Phasic modulation consists of local transient release, which results in micromolar concentrations of dopamine. Tonic modulation occurs when dopamine is present in the extracellular space, commonly at lower concentrations (nM range). Interestingly tonic and phasic effects are clearly different (e.g., can oppose each other). This work therefore demonstrates that tonic and phasic modulatory input can have different functional roles.
- 33.Katz PS. Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia . Ann N Y Acad Sci. 1998;860:181–188. doi: 10.1111/j.1749-6632.1998.tb09048.x. [DOI] [PubMed] [Google Scholar]
- 34.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol. 1995;74:2281–2294. doi: 10.1152/jn.1995.74.6.2281. [DOI] [PubMed] [Google Scholar]
- 35.Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci. 1995;15:6035–6045. doi: 10.1523/JNEUROSCI.15-09-06035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakurai A, Katz PS. Spike timing-dependent serotonergic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Journal of Neuroscience. 2003;23:10745–10755. doi: 10.1523/JNEUROSCI.23-34-10745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai A, Darghouth NR, Butera RJ, Katz PS. Serotonergic enhancement of a 4-AP-sensitive current mediates the synaptic depression phase of spike timing-dependent neuromodulation. Journal of Neuroscience. 2006;26:2010–2021. doi: 10.1523/JNEUROSCI.2599-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai A, Calin-Jageman RJ, Katz PS. Potentiation phase of spike timing-dependent neuromodulation by a serotonergic interneuron involves an increase in the fraction of transmitter release. Journal of Neurophysiology. 2007;98:1975–1987. doi: 10.1152/jn.00702.2007. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai A, Katz PS. State-, timing-, and pattern-dependent neuromodulation of synaptic strength by a serotonergic interneuron. Journal of Neuroscience. 2009;29:268–279. doi: 10.1523/JNEUROSCI.4456-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemens S, Katz PS. Identified serotonergic neurons in the Tritonia swim CPG activate both ionotropic and metabotropic receptors. J Neurophysiol. 2001;85:476–479. doi: 10.1152/jn.2001.85.1.476. [DOI] [PubMed] [Google Scholar]
- 41.Clemens S, Katz PS. G protein signaling in a neuronal network is necessary for rhythmic motor pattern production. J Neurophysiol. 2003;89:762–772. doi: 10.1152/jn.00765.2002. [DOI] [PubMed] [Google Scholar]
- 42.Katz PS, Frost WN. Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator. J Neurosci. 1997;17:7703–7713. doi: 10.1523/JNEUROSCI.17-20-07703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramirez JM, Doi A, Garcia AJ, 3rd, Elsen FP, Koch H, Wei AD. The cellular building blocks of breathing. Compr Physiol. 2012;2:2683–2731. doi: 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zanella S, Doi A, Garcia AJ, 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci. 2014;34:36–50. doi: 10.1523/JNEUROSCI.3644-12.2014. Interesting study that demonstrates the importance of modulation by characterizing a situation where a pathological condition alters the network response to a modulatory substance.
- 45.Miles GB, Sillar KT. Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 2011;26:393–411. doi: 10.1152/physiol.00013.2011. [DOI] [PubMed] [Google Scholar]
- 46.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Kyriakatos A, Molinari M, Mahmood R, Grillner S, Sillar KT, El Manira A. Nitric oxide potentiation of locomotor activity in the spinal cord of the lamprey. J Neurosci. 2009;29:13283–13291. doi: 10.1523/JNEUROSCI.3069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song J, Kyriakatos A, El Manira A. Gating the polarity of endocannabinoid-mediated synaptic plasticity by nitric oxide in the spinal locomotor network. J Neurosci. 2012;32:5097–5105. doi: 10.1523/JNEUROSCI.5850-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster JD, Dunford C, Sillar KT, Miles GB. Nitric oxide-mediated modulation of the murine locomotor network. J Neurophysiol. 2014;111:659–674. doi: 10.1152/jn.00378.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- 52. Dacks AM, Weiss KR. Release of a single neurotransmitter from an identified interneuron coherently affects motor output on multiple time scales. J Neurophysiol. 2013;109:2327–2334. doi: 10.1152/jn.01079.2012. Studies a situation where an identified interneuron releases GABA which exerts three types of effects in a follower neuron: it induces a fast (millisecond) IPSP, a slow (seconds) EPSP, and a persistent (minutes) increase in excitability. Interestingly all three effects coherently affect motor output, i.e., they promote one type of motor program as opposed to another.
- 53.Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fowler CA, Napps SE, Feldman L. Relations among regular and irregular morphologically related words in the lexicon as revealed by repetition priming. Mem Cognit. 1985;13:241–255. doi: 10.3758/bf03197687. [DOI] [PubMed] [Google Scholar]
- 55.Kristjansson A, Campana G. Where perception meets memory: a review of repetition priming in visual search tasks. Atten Percept Psychophys. 2010;72:5–18. doi: 10.3758/APP.72.1.5. [DOI] [PubMed] [Google Scholar]
- 56. Yashar A, Makovski T, Lamy D. The role of motor response in implicit encoding: Evidence from intertrial priming in pop-out search. Vision Research. 2013;93:80–87. Studies of repetition priming in human subjects generally (and necessarily) utilize a task that requires a motor response. This study differs from others in that it demonstrates that repetition priming is impacted by motor activity.
- 57.Jackson A, Morton J. Facilitation of auditory word recognition. Mem Cognit. 1984;12:568–574. doi: 10.3758/bf03213345. [DOI] [PubMed] [Google Scholar]
- 58.Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87:2964–2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- 59.Salimpoor VN, Chang C, Menon V. Neural basis of repetition priming during mathematical cognition: repetition suppression or repetition enhancement? J Cogn Neurosci. 2010;22:790–805. doi: 10.1162/jocn.2009.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heuer H, Kleinsorge T, Klein W, Kohlisch O. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol (Amst) 2004;117:29–64. doi: 10.1016/j.actpsy.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Bratzke D, Rolke B, Steinborn MB, Ulrich R. The effect of 40 h constant wakefulness on task-switching efficiency. J Sleep Res. 2009;18:167–172. doi: 10.1111/j.1365-2869.2008.00729.x. [DOI] [PubMed] [Google Scholar]