Abstract
Purpose
To demonstrate dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) of the prostate with both high spatial and temporal resolution via a combination of golden-angle radial k-space sampling, compressed sensing, and parallel-imaging reconstruction (GRASP), and to compare image quality and lesion depiction between GRASP and conventional DCE in prostate cancer patients.
Materials and Methods
Twenty prostate cancer patients underwent two 3T prostate MRI examinations on separate dates, one using standard DCE (spatial resolution 3.0 × 1.9 × 1.9 mm, temporal resolution 5.5 sec) and the other using GRASP (spatial resolution 3.0 × 1.1 × 1.1 mm, temporal resolution 2.3 sec). Two radiologists assessed measures of image quality and dominant lesion size. The experienced reader recorded differences in contrast arrival times between the dominant lesion and benign prostate.
Results
Compared with standard DCE, GRASP demonstrated significantly better clarity of the capsule, peripheral/ transition zone boundary, urethra, and periprostatic vessels; image sharpness; and lesion conspicuity for both readers (P<0.001–0.020). GRASP showed improved interreader correlation for lesion size (GRASP: r=0.691–0.824, standard: r=0.495–0.542). In 8/20 cases, only GRASP showed earlier contrast arrival in tumor than benign; in no case did only standard DCE show earlier contrast arrival in tumor.
Conclusion
High spatiotemporal resolution prostate DCE is possible with GRASP, which has the potential to improve image quality and lesion depiction as compared with standard DCE.
Keywords: prostate, prostate cancer magnetic resonance imaging, imaging, perfusion
Dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) of the prostate comprises sequential T1-weighted imaging (T1WI) acquisitions following injection of gadolinium-based contrast agent and aims to depict abnormal pharmacokinetics within tumorous regions (1). DCE has become a routine component of multiparametric prostate MRI protocols and improves the detection, localization, and staging of prostate cancer (2,3). Findings on DCE have been incorporated into standardized reporting schemes for prostate MRI (2) and are useful for guiding prostate biopsy (4), planning treatment (5), and monitoring posttherapy recurrences (6).
One challenge in the implementation of DCE in the prostate is the inherent trade-off between spatial and temporal resolution in MRI (Table 1) (7–9). As the prostate is often highly vascular, differences in enhancement kinetics between benign and malignant regions can be subtle, such that high temporal resolution can assist in their differentiation (1). Indeed, recent expert guidelines advise a temporal resolution of at least 15 seconds (2). Nonetheless, many investigations report a considerably higher temporal resolution, in some instances under 3 seconds per acquisition (10). A higher temporal resolution is also essential for advanced pharmacokinetic modeling requiring an arterial input function (11). However, as prostate tumors are frequently small in size, potentially measuring less than 1 cm (12), their precise depiction can be critical for guiding a targeted biopsy or treatment, and a higher spatial resolution may be preferred. To this end, other studies have achieved higher spatial resolution by using a temporal resolution as low as 30 seconds (13,14). Also influencing this balance between spatial and temporal resolution is the impact of acquisition parameters on anatomic coverage, tissue contrast, motion robustness, and other artifacts (7). Given these confounding factors, there is currently a lack of technical standardization for prostate DCE in clinical practice (7).
Table 1.
Representative Combinations of Spatial and Temporal Resolutions Reported for DCE-MRI of the Prostate Within the Recent Peer-Reviewed Literaturea
| Authors | Year | Temporal resolution | Voxel volume | Number of slicesb |
|---|---|---|---|---|
| Chen et al (30) | 2012 | 2 sec | 4.0 × 2.8 × 2.8 mm | 15 |
| Hara et al (31) | 2012 | 21 sec | 4.9 × 1.4 × 1.4 mm | — |
| Isebaert et al (32) | 2012 | 9 sec | 4.0 × 2.1 × 1.4 mm | 14 |
| McClure et al (33) | 2012 | 15 sec | 1.5 × 0.9 × 1.2 mm | — |
| Punwani et al (34) | 2012 | 16 sec | 3.0 × 1.6 × 1.0 mm | — |
| Rouviere et al (35) | 2012 | 15 sec | 3.0 × 0.5 × 0.5 mm | 24 |
| Selnaes et al (36) | 2012 | 12.9 sec | 2.0 × 1.1 × 1.0 mm | 30 |
| Valentini et al (37) | 2012 | 7 sec | 6.0 × 0.8 × 1.6 | 12 |
| Rischke et al (38) | 2013 | 7 sec | 1.7 × 0.9 × 1.3 mm | — |
| Bratan et al (39) (3 DCE protocols reported) | 2013 | 15 sec | 3.0 × 0.9 × 1.8 mm | — |
| 7 sec | 3.0 × 1.3 × 1.2 mm | — | ||
| 5 sec | 3.0 × 1.8 × 1.8 mm | — | ||
| Costa et al (40) | 2013 | 15.8 sec | 2.6–3.0 × 0.5 × 0.6 mm | — |
| Roy et al (41) | 2013 | 6 sec | 3.5 × 0.7 × 0.9 mm | 30 |
| Schimmoller et al (42) | 2013 | 5 sec | 3.0 × 2.5 × 1.8 mm | — |
| Somford et al (43) | 2013 | 3.4 sec | 3.0 × 1.5 × 1.5 mm | — |
| Vos et al (44) | 2013 | 3 sec | 3.0–4.0 × 1.5–1.8 × 1.5–1.8 mm | — |
| Roethke et al (45) | 2014 | 9.9 sec | 1.5 × 1.6 × 1.6 mm | — |
| Current study | 2.3 sec | 3.0 × 1.1 × 1.1 mm | 21 |
Table includes studies with publication date in 2012 or 2013 that provide sufficient methodological details to compute temporal resolution and voxel volume. Only a single study is included in instances of multiple publications from the same group reporting similar DCE parameters.
Dash indicates that value not provided within study.
A number of recent advances in 3D gradient-echo T1WI may be useful for addressing these challenges. Compressed sensing (CS) exploits spatial correlations within images or spatiotemporal correlations among sequentially acquired images to substantially accelerate acquisitions (15). CS requires randomly under-sampled k-space data, which are preferably acquired using non-Cartesian k-space sampling schemes such as radial trajectories (16). Furthermore, advanced reconstruction techniques allow for the synergistic combination of CS and parallel imaging for processing of DCE data (17), which collectively offers simultaneous high spatial and high temporal resolution. The use of an underlying radial k-space sampling technique for this approach additionally increases robustness with respect to motion artifacts (18,19). A robust combination of CS and parallel imaging for rapid continuous acquisition with flexible spatiotemporal resolution using the golden-angle radial sampling scheme (20,21) (termed Golden-angle RAdial Sparse Parallel, or GRASP, imaging) has recently been applied to perform high-quality multiphase DCE of the liver during free-breathing (22).
The prostate may provide an ideal additional application of the GRASP technique. The high degree of spatiotemporal correlation of data over the course of a DCE acquisition facilitates the sparse data representations that form the basis of CS reconstruction. In addition, given the small size of prostate tumors, overlap in tumors’ enhancement characteristics with benign prostate, and presence of prostatic motion during an extended DCE acquisition, prostate DCE would stand to benefit greatly from the advantages offered by GRASP. Therefore, our aim in this study was to demonstrate the feasibility of performing high-spatiotemporal resolution DCE of the prostate by using GRASP and to compare image quality and lesion depiction between GRASP and conventional DCE in patients with biopsy-proven prostate cancer.
MATERIALS AND METHODS
Patients
Two authors (C.G. and C.G.) are employees of Siemens Medical Solutions; however, Siemens Medical Solutions provided no financial support for this study, and the remaining authors had control over all data. This retrospective study was Health Insurance Portability and Accountability Act (HIPAA)-compliant and approved by our Institutional Review Board with a waiver of the requirement for written informed consent. Following initial optimization and testing, GRASP was implemented as the routine sequence for DCE acquisition in all multiparametric prostate MRI examinations performed using one of the scanners at our institution. At the time of this study, we searched all patients who had undergone prostate MRI on this scanner using GRASP DCE to identify those who were on active surveillance for biopsy-proven prostate cancer and who had also undergone a prior MRI on the same scanner at our institution, although using a standard DCE sequence. Twenty-two patients were identified from this initial search. Of these patients, two were then excluded due to lack of any DCE abnormality in the region of the tumor on biopsy. This exclusion resulted in a final study cohort of 20 men (mean age 67±9 years), with mean prostate-specific antigen (PSA) of 5.4±3.6 ng/mL. The first MRI was performed prior to the pathologic diagnosis of prostate cancer in eight patients, and as part of an active surveillance protocol after establishing the diagnosis in 12 patients. The maximal Gleason score detected on the initial positive biopsy was 3+3 tumor in 19 cases and 3+4 tumor in one case. The mean interval between the two MRI examinations was 394±126 days (median 403 days; range 200–633 days). As of the time of this study, three patients had elected to receive therapy for their prostate cancer rather than remain on active surveillance.
MRI Technique
All subjects underwent MRI using a clinical 3T system (Magnetom Trio, Siemens Healthcare, Erlangen, Germany) using a combination of spine coil elements and a body matrix coil, resulting in a total of 12–15 receiver channels. Examinations included the following sequences in the prostate and seminal vesicles, which were not formally evaluated as part of this study: multiplanar turbo-spin echo (TSE) T2-weighted imaging (T2WI), axial TSE T1WI, and axial diffusion-weighted imaging (DWI) with reconstruction of the apparent diffusion coefficient (ADC) map. In addition, all examinations included DCE of the prostate and seminal vesicles, performed using an axial 3D gradient-echo T1WI acquisition with intravenous administration of 0.1 mmol/kg of gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals, Berlin, Germany), followed by a 20-cc saline flush. Both the contrast and saline flush were administered as an IV bolus at a rate of 3 cc/sec using a power injector (Spectris, Medrad, Pittsburgh, PA).
The standard DCE acquisition was performed using a conventional 3D fast low-angle shot (FLASH) technique with the following parameters: TR/TE 2.84/0.94, flip angle 16°, slice thickness 3 mm, 24 slices, field of view (FOV) 240 × 240 mm, matrix 128 × 128, receiver bandwidth 490 Hz/voxel, no parallel imaging, 6/8 partial Fourier in both phase and slice-encoding directions, one signal average. These parameters provided a voxel size of 3.0 × 1.9 × 1.9 mm and temporal resolution of 5.5 seconds. Following acquisition of a precontrast data volume, a total of 55 contrastenhanced data volumes were obtained over the course of 5 minutes 5 seconds, with the start of the first postcontrast acquisition corresponding with the start of the contrast injection.
The GRASP DCE acquisition was performed with a fat-suppressed 3D FLASH sequence using the "stack-of-stars" sampling scheme (Radial VIBE), which employs radial sampling in-plane and Cartesian sampling in the slice direction (19). A total of 3192 radial spokes were acquired continuously using the goldenangle scheme over the course of 5 minutes 38 seconds, which incorporated the precontrast portion of the acquisition; the contrast administration occurred following a 20-second injection delay. Additional parameters were as follows: TR/TE 4.10/1.89 msec, flip angle 12°, slice thickness 3 mm, 21 slices, FOV 240 × 240 mm, matrix 224 × 224, receiver bandwidth 500 Hz/voxel, 6/8 partial Fourier in slice encoding directions. These parameters provided a voxel size of 3.0 × 1.1 × 1.1 mm. Fat suppression was achieved using a scan-time-optimized saturation scheme that creates a single spectrally selective saturation pulse per stack of radial spokes, matched to yield optimal suppression for the central spokes along the slice direction for each angle. Given underlying fundamental differences between the two techniques, including the need for active fat suppression in radial imaging to avoid off-resonance artifacts, acquisition parameters were optimized independently for the standard DCE and GRASP DCE acquisitions based on empiric testing prior to initiation of this investigation.
Image Reconstruction
Standard DCE was reconstructed in-line by the MRI console using standard image reconstruction methodology.
GRASP was reconstructed using a radial version of the multicoil k-t SPARSE-SENSE method, which iteratively finds a solution that maintains consistency with the acquired k-space data while enforcing spatiotemporal sparsity of the images, as previously described in detail (21,22). In brief, the algorithm achieves this by combining parallel-imaging and compressed-sensing principles to synergistically take advantage of the spatial-encoding capabilities of the phased-array receiver coil and the redundancies contained in the acquired time series of data, thereby enabling reconstruction of dynamic frames with high temporal resolution (22). For this approach, 21 consecutive spokes were grouped into each dynamic frame, providing a total of 152 frames (145 frames after excluding pre-contrast reconstructed frames) with a temporal resolution of 2.3 seconds. The GRASP reconstruction was performed using a custom-developed implementation written in the C++ programming language, which reads k-space raw data exported from the scanner and generates images in the DICOM format. This processing was initiated by the MRI technicians after the patient was taken out of the scanner and typically took between 20 and 30 minutes on a Linux server with 64 CPU cores and 128 GB of memory. After the reconstruction was finished, the DICOM images were automatically sent to our Picture Archiving and Communications System (PACS), so that the dynamic images were available for reading together with other protocols within a time period of less than 1 hour.
Image Quality
Images were evaluated independently by two radiologists (A.R. and J.R., with 6 and 1 year of experience, respectively, in prostate MRI interpretation). Cases from the two acquisitions (GRASP and standard DCE) for each patient were reviewed in random order during two separate settings.
First, to assess image quality the two readers rated each sequence subjectively on a 1–5 scale (5=highest image quality) in terms of the following features: clarity of prostate capsule, clarity of peripheral zone (PZ) / transition zone (TZ) boundary, clarity of urethra, clarity of periprostatic vessels, image sharpness, and overall image quality.
Next, an assessment was performed to compare depiction of the dominant tumor between the two sequences. For this purpose, the dominant lesion was defined as the lesion having the highest grade on biopsy; for patients with multiple areas harboring the same grade of tumor on biopsy, the area with maximal tumor volume on biopsy (reported at our institution as percent tumor involvement within the core) was considered to represent the dominant lesion. Subsequently, the two readers jointly viewed images unblinded to pathology to identify a focus of visually increased enhancement on early postcontrast images within the sextant of the dominant tumor on biopsy, which was considered to represent the dominant tumor for each patient on MRI.
Following localization of the dominant lesions, the two readers independently scored the lesion’s visual conspicuity on a 1–5 scale (5=highest image quality) and measured the diameter of the dominant lesion in both anterior–posterior and transverse dimensions; early postcontrast images demonstrating the focus of increased enhancement were used for these assessments. Then the more experienced reader performed a quantitative assessment of lesion contrast on a single early postcontrast timepoint by placing a single region-of-interest (ROI) slightly within the outer margin of each lesion as well as within an area of normal-appearing PZ that was also benign on biopsy for both sequences. Based on these ROIs, tumor-to-PZ contrast was computed as (SItumor − SIPZ) / (SItumor + SIPZ), which provides a value between 0 and 1, with a higher value indicating greater relative contrast (23); given use of magnitude reconstruction, these SI values had a value of at least 1 in all cases. Finally, the more experienced reader recorded the mean contrast arrival time within the ROIs representing tumor and benign PZ for each case; this determination was performed using commercial software (Dynacad, v. 2.1.6, Invivo, Gainesville, FL). The difference in arrival time between tumor and benign PZ was then computed for each case; pharmacokinetic modeling was not performed.
Statistics
The subjective image quality scores were compared between GRASP and standard DCE using the paired Wilcoxon test. The correlation in measured lesion size between the two readers was computed separately for both GRASP and standard DCE using Pearson’s correlation coefficient and was categorized as follows: 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; 0.81–1.00, almost perfect agreement (24). Tumor-to-PZ contrast was compared between sequences using the paired t-test. The differences in arrival time between tumor and benign PZ were assessed for each sequence to identify instances with no difference in arrival time between the two tissue types. Finally, an assessment was performed for possible tumor progression between the two examinations in each patient. First, the maximal Gleason score and percent tumor involvement of an individual core were compared between each patient’s initial and any subsequent biopsies. In addition, the lesion size on DCE, determined as the lesion’s maximal diameter based on an average of the two reader’s measurements, was compared between the baseline and follow-up examinations using a paired t-test. Statistical analysis was performed using commercial software (MedCalc for Windows, v. 12.7; MedCalc Software, Ostend, Belgium).
RESULTS
GRASP images were successfully acquired and reconstructed in all 20 subjects. No restraints related to specific absorption rate limitations were encountered for either sequence for any patient. Representative cases of corresponding standard DCE and GRASP images in individual patients are shown in Figs. 1–3.
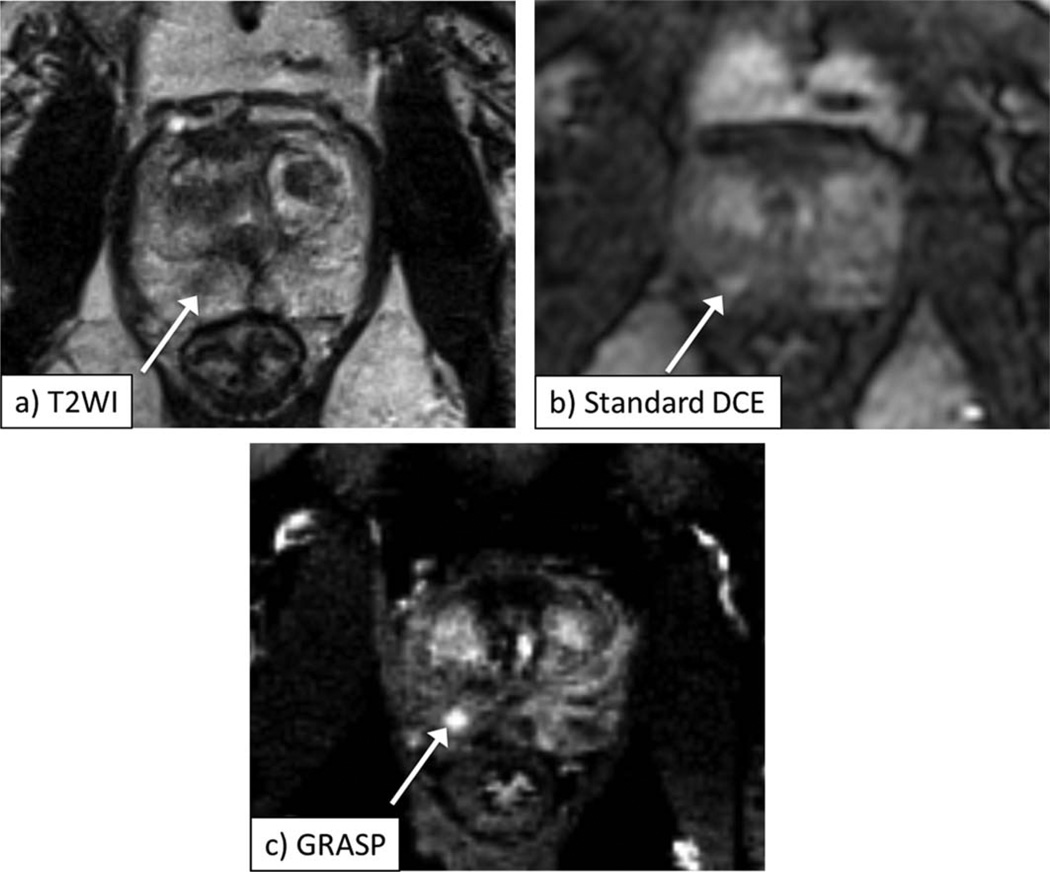
Figure 1.
A 62-year-old male with biopsy-proven Gleason 3+3 prostate cancer in the right apex of the prostate, as depicted by area of decreased signal on axial T2-weighted image (a, arrow). Early postcontrast images from standard DCE (b) and GRASP DCE (c) show corresponding focus of abnormal early enhancement in this sextant (arrow, b,c), which is better defined on GRASP image. Also note more distinct visualization of prostate capsule and transition zone boundary on GRASP image.
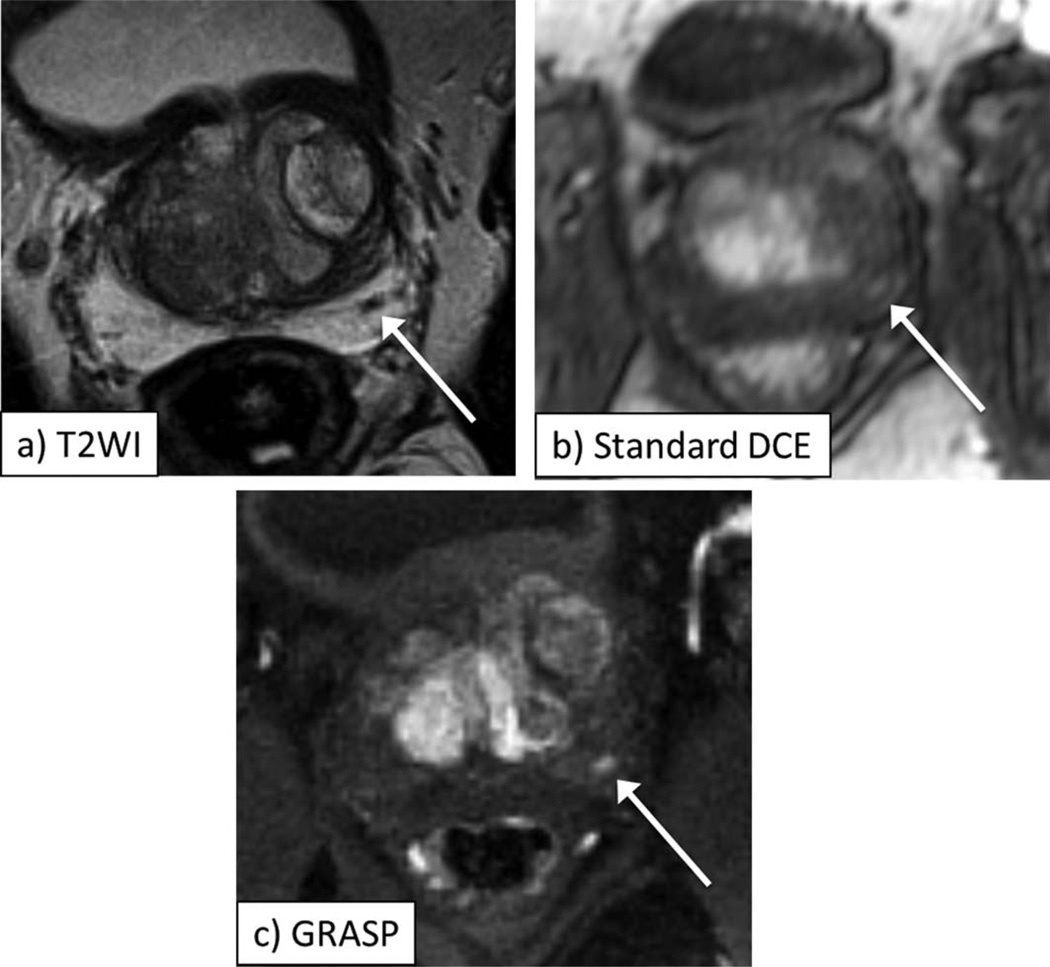
Figure 3.
A 64-year-old male with biopsy-proven Gleason 3+4 prostate cancer in the right apex of the prostate, as depicted by area of decreased signal on axial T2-weighted image (a, arrow). Early postcontrast images from standard DCE (b) and GRASP DCE (c) show corresponding focus of abnormal early enhancement in this sextant (arrow, b,c), which is better defined on GRASP image. On standard DCE at the earliest timepoint showing enhancement in right peripheral zone tumor, there is also avid enhancement in transition zone BPH nodules; on the other hand, on GRASP, contrast arrives in right apical tumor at an earlier timepoint than elsewhere in the prostate.
GRASP showed significantly better image quality than standard DCE in terms of clarity of the capsule, clarity of the PZ/TZ edge, clarity of the urethra, clarity of periprostatic vessels, image sharpness, and overall image quality for both readers (P≤0.007 for all comparisons for both readers) (Table 2).
Table 2.
Comparison of Subjective Parameters Between DCE Sequences
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
| Feature | Standard | GRASP | Pa | Standard | GRASP | Pa |
| Clarity of capsule | 4.0±0.9 | 4.7±0.6 | 0.007 | 3.9±0.7 | 4.7±0.5 | <0.001 |
| Clarity of PZ/TZ boundary | 3.1±0.8 | 4.6±0.7 | <0.001 | 3.6±0.7 | 4.5±0.7 | <0.001 |
| Clarity of urethra | 2.1±0.8 | 3.8±1.0 | <0.001 | 1.6±0.8 | 3.3±0.6 | <0.001 |
| Clarity of peri-prostatic vessels | 2.4±1.1 | 4.2±1.0 | <0.001 | 1.6±0.5 | 4.2±0.9 | <0.001 |
| Image sharpness | 3.1±0.4 | 4.8±0.4 | <0.001 | 3.3±0.6 | 4.8±0.4 | <0.001 |
| Overall image quality | 3.1±0.4 | 4.7±0.5 | <0.001 | 3.3±0.6 | 4.6±0.5 | <0.001 |
| Lesion conspicuity | 3.2±1.2 | 4.5±0.8 | <0.001 | 3.2±1.2 | 3.9±1.1 | 0.020 |
| Absence of streak artifact | — | 4.860.4 | — | — | 4.760.5 | — |
All features reported on a 1–5 scale.
Listed in bold when statistically significant at P<0.05.
GRASP showed significantly better subjective lesion conspicuity than standard DCE for both readers (reader 1: P<0.001; reader 2: P=0.020). In addition, the two readers’ bidirectional measurements of lesion size demonstrated moderate-to-substantial agreement using GRASP (r=0.691–0.824), compared with fair agreement using standard DCE (r=0.495–0.542). At quantitative assessment, there was no significant difference in lesion-to-PZ contrast between GRASP and standard DCE (P=0.581) (Table 3).
Table 3.
Comparison of Lesion Assessment Between DCE Sequences
| Feature | Standard | GRASP |
|---|---|---|
| Tumor-to-PZ contrasta | 0.19±0.13 | 0.21±0.12 |
| Subjective lesion contrast, reader 1b | 3.2±1.2 | 4.5±0.8 |
| Subjective lesion contrast, reader 2b | 3.2±1.2 | 3.9±1.1 |
| Inter-reader correlation, lesion AP diameter | 0.495 | 0.824 |
| Inter-reader correlation, lesion transverse diameter | 0.542 | 0.691 |
| Fraction of cases with earlier tumor enhancement detected only using given DCE technique | 0% (0/20) | 40% (8/20) |
Not statistically significant (P=0.581).
Reported on a 1–5 scale. Statistically significant for both readers (R1: P<0.001; R2: P=0.020).
In eight of the 20 patients, there was no observable difference in contrast arrival time between the lesion identified as tumor and benign PZ using standard DCE, yet an earlier arrival time in the tumor compared with benign PZ could be observed using GRASP (mean earlier arrival time of 3.5±1.7 sec, range 2.3 to 6.9 sec). In the remaining 12 patients, both sequences depicted earlier arrival of contrast in tumor compared with benign PZ. Thus, in no case was there an earlier arrival of contrast in tumor compared with benign PZ observed only with standard DCE.
Twelve of the 20 subjects underwent a follow-up biopsy during the study interval. In three subjects whose initial biopsy comprised only systematic non-targeted cores, the follow-up biopsy demonstrated an increase in tumor grade (n=2) or volume (n=1), although only on cores obtained using MRI/ultrasound fusion software, which was performed only at the time of follow-up biopsy; there was no increase in grade or volume when comparing the nontargeted cores between the two biopsy sessions in these patients. In one patient there was an increase in tumor volume when comparing nontargeted cores between the two biopsy sessions (from 5% to 50% maximal core involvement), although the same tumor grade (Gleason score 3+3). In the remaining patients, tumor grade, and volume were similar between the two biopsy sessions. In addition, there was no difference in maximal lesion size on DCE between the two MRI examinations (10.2±3.6 mm at baseline vs. 10.7±3.7 mm at follow-up; P=0.483).
DISCUSSION
In this study we demonstrated the feasibility of performing high-spatiotemporal resolution prostate DCE using a golden-angle radial k-space sampling technique with CS and parallel imaging reconstruction, termed GRASP. GRASP produced substantially better image quality than standard DCE in terms of a spectrum of features relating to clarity of key anatomic landmarks. Although quantitative tumor-to-PZ was similar, potentially related to the independent optimization of acquisition parameters for the two techniques, GRASP showed significantly improved subjective clarity of dominant lesions, as well as more reproducible measurements of size of such lesions. In addition, in 40% of cases only GRASP demonstrated differences in the tumor contrast arrival time in comparison with normal PZ. This more distinct definition of focal DCE abnormalities using GRASP has potential to assist the detection and localization of focal prostate tumors and raise reader confidence in identified lesions. The excellent performance of GRASP also has potential to benefit pharmacokinetic modeling and other advanced quantitative analyses.
Our preliminary data regarding the use of GRASP for prostate DCE are clinically relevant given ongoing challenges in the optimization and standardization of prostate DCE (7). This high performance was reached without an associated loss in tissue contrast. CS, which takes advantage of data sparsity resulting from spatiotemporal correlations within sequentially acquired images, was central in achieving the acceleration without a sacrifice of spatial resolution. The radial k-space scheme leads to an incoherent pattern of undersampling that is particularly well suited for CS reconstruction, especially due to the use of the golden-angle ordering of the acquisition that we employed to achieve incoherent sampling along time. As the readout direction is constantly rotating in a radial trajectory, aliasing and ghosting artifacts associated with phase-offset errors in traditional Cartesian acquisitions are essentially eliminated (22), thereby contributing to the sharper image appearance and increased clarity of anatomic structures using GRASP. While radial acquisition schemes have been described to be prone instead to radially oriented streak artifacts (25,26), streak artifact was reported as minimal by the two observers in our study. In abdominal exams, spurious streak artifacts often originate in the arms if placed next to the body, which is difficult to avoid in such exams. However, in prostate imaging the arms are placed outside of the FOV, which may explain the lower level of streak artifact as compared to these previous studies. Moreover, respiratory motion is generally less problematic in imaging of the pelvis in comparison with imaging of the upper abdomen.
An additional important advantage of the golden-angle sampling of GRASP is the ability to retrospectively reconstruct the image using any number of consecutive radial spokes, thereby giving flexibility in the attained temporal resolution (22). As the temporal resolution is more substantially increased by using a very small number of spokes in the reconstruction, the previously noted potential reconstruction-related artifacts will become more prominent. We selected the use of 21 consecutive radial spokes for reconstruction of temporal frames in our study based on initial optimization that aimed to maintain a temporal resolution below 3 seconds, in order to achieve a temporal resolution comparable to the higher resolutions reported for prostate DCE in the literature. The impact of using varying numbers of radial spokes for reconstruction of the individual frames from the continuous GRASP acquisition merits continued attention in further studies. While other approaches for accelerated k-space sampling such as advanced algorithms for combined phase and slice parallel imaging (27) as well as stochastic k-space sparse sampling methods (28) may achieve similar improvements in temporal-spatial sampling, the virtual elimination of motion-related ghosting artifacts and the ability for variable temporal sampling during retrospective reconstruction are unique advantages of the golden-angle radial scheme.
A number of limitations of this study warrant mention. A primary limitation is that, given the inability to assess dynamic postcontrast imaging using GRASP and standard DCE performed during the course of a single examination, we compared studies performed on different dates in patients on active surveillance, with an average interval between examinations of slightly over 1 year. Thus, we cannot fully exclude the possibility that observed differences in some cases may have been attributable in part to tumor progression between studies. However, given the slow rate of progression of prostate cancer and the highly consistent nature of the difference between patients, it is unlikely that this possibility accounts for the overall trend that we report. Perhaps more important, it would not be expected that true progression would impact a number of our assessed parameters, such as clarity of anatomic details and interreader concordance of lesion size. Furthermore, the sample size was small. Nonetheless, highly significant differences in image quality between GRASP and standard DCE were observed. In addition, as this was largely a study of the feasibility of a novel and previously unexplored technique in the prostate, the observers assessed tumor characteristics aware of tumor locations; future studies using a larger cohort could assess the diagnostic performance of GRASP DCE for detection of tumors by observers unaware of histologic findings at the time of image interpretation. Also, in patients on active surveillance for known prostate cancer undergoing prostate MRI, the radiologist would be aware of the location of tumor identified on prior biopsy. Moreover, GRASP includes fat-suppression, which is incorporated into the Radial VIBE sequence; in comparison, the conventional FLASH sequence used for standard DCE did not employ fat-suppression. While this may have contributed to the observed differences in quality between the two sequences, inclusion of fat-suppression in standard DCE would have further reduced this sequence’s temporal resolution compared with GRASP. An additional limitation is that more sophisticated pharmacokinetic parameters were not assessed; a past study showed greater performance of such parameters in differentiating benign and malignant prostate than achieved by simple heuristic kinetic measures such as time-to-arrival, as was assessed in the current study (29). Another limitation is the inexperience of one of the readers. Also, the assessed subjective image quality measures are prone to being influenced by reader bias. Finally, in view of the markedly different appearances of the GRASP and standard DCE images, it was not possible to blind the observers to which image set was being interpreted. Nonetheless, the two independent radiologists identified similar trends in terms of differences in image quality between the two image sets.
In conclusion, we have demonstrated the feasibility of performing DCE of the prostate with both high spatial and high temporal resolution using GRASP, which combines CS, parallel imaging, and golden-angle radial sampling. This technique achieved improvements in image quality, clarity of anatomic details, and spatiotemporal definition of focal lesions compared with a standard DCE acquisition. Future studies are warranted to assess the diagnostic performance of GRASP DCE for tumor detection in larger patient cohorts.
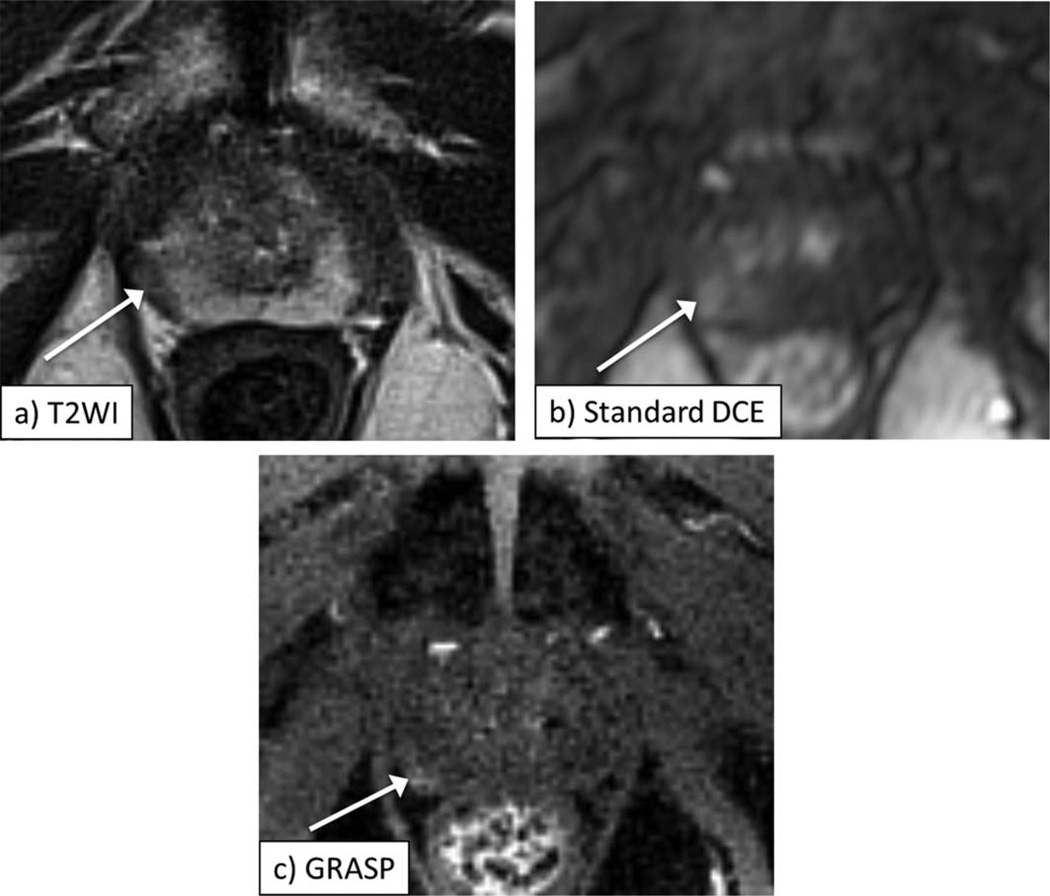
Figure 2.
A 63-year-old male with biopsy-proven Gleason 3+3 prostate cancer in the left midgland of the prostate, as depicted by area of decreased signal on axial T2-weighted image (a, arrow). Early postcontrast images from standard DCE (b) and GRASP DCE (c) show corresponding focus of abnormal early enhancement in this sextant (arrow, b,c), which is better defined on GRASP image. Also note more distinct visualization of anatomic details on GRASP image.
Acknowledgments
Sponsor: Joseph and Diane Steinberg Charitable Trust.
REFERENCES
- 1.Franiel T, Hamm B, Hricak H. Dynamic contrast-enhanced magnetic resonance imaging and pharmacokinetic models in prostate cancer. Eur Radiol. 2011;21:616–626. doi: 10.1007/s00330-010-2037-7. [DOI] [PubMed] [Google Scholar]
- 2.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding—multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 5.Ogura K, Maekawa S, Okubo K, et al. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: correlation with histopathologic results. Urology. 2001;57:721–726. doi: 10.1016/s0090-4295(00)01072-4. [DOI] [PubMed] [Google Scholar]
- 6.Akin O, Gultekin DH, Vargas HA, et al. Incremental value of diffusion weighted and dynamic contrast enhanced MRI in the detection of locally recurrent prostate cancer after radiation treatment: preliminary results. Eur Radiol. 2011;21:1970–1978. doi: 10.1007/s00330-011-2130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeks CM, Barentsz JO, Hambrock T, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 8.Margolis DJ, Bammer R, Chow LC. Parallel imaging of the abdomen. Top Magn Reson Imaging. 2004;15:197–206. doi: 10.1097/01.rmr.0000136557.27727.79. [DOI] [PubMed] [Google Scholar]
- 9.Barth MM, Smith MP, Pedrosa I, Lenkinski RE, Rofsky NM. Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiographics. 2007;27:1445–1462. doi: 10.1148/rg.275065204. discussion 62–64. [DOI] [PubMed] [Google Scholar]
- 10.Franiel T, Ludemann L, Rudolph B, et al. Evaluation of normal prostate tissue, chronic prostatitis, and prostate cancer by quantitative perfusion analysis using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Invest Radiol. 2008;43:481–487. doi: 10.1097/RLI.0b013e31816b2f63. [DOI] [PubMed] [Google Scholar]
- 11.Just N, Koh DM, D’Arcy J, Collins DJ, Leach MO. Assessment of the effect of haematocrit-dependent arterial input functions on the accuracy of pharmacokinetic parameters in dynamic contrast-enhanced MRI. NMR Biomed. 2011;24:902–915. doi: 10.1002/nbm.1648. [DOI] [PubMed] [Google Scholar]
- 12.Renshaw AA, Richie JP, Loughlin KR, Jiroutek M, Chung A, D’Amico AV. Maximum diameter of prostatic carcinoma is a simple, inexpensive, and independent predictor of prostatespecific antigen failure in radical prostatectomy specimens. Validation in a cohort of 434 patients. Am J Clin Pathol. 1999;111:641–644. doi: 10.1093/ajcp/111.5.641. [DOI] [PubMed] [Google Scholar]
- 13.Girouin N, Mege-Lechevallier F, Tonina Senes A, et al. Prostate dynamic contrast-enhanced MRI with simple visual diagnostic criteria: is it reasonable? Eur Radiol. 2007;17:1498–1509. doi: 10.1007/s00330-006-0478-9. [DOI] [PubMed] [Google Scholar]
- 14.Bloch BN, Furman-Haran E, Helbich TH, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging—initial results. Radiology. 2007;245:176–185. doi: 10.1148/radiol.2451061502. [DOI] [PubMed] [Google Scholar]
- 15.Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 16.Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn Reson Med. 2007;57:1086–1098. doi: 10.1002/mrm.21236. [DOI] [PubMed] [Google Scholar]
- 17.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated firstpass cardiac perfusion MRI. Magn Reson Med. 2010;64:767–776. doi: 10.1002/mrm.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin W, Guo J, Rosen MA, Song HK. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn Reson Med. 2008;60:1135–1146. doi: 10.1002/mrm.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandarana H, Block TK, Rosenkrantz AB, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011;46:648–653. doi: 10.1097/RLI.0b013e31821eea45. [DOI] [PubMed] [Google Scholar]
- 20.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Grimm R, Tobias Block K, et al. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2013 doi: 10.1002/mrm.24980. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013;48:10–16. doi: 10.1097/RLI.0b013e318271869c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornfeld DM, Israel G, McCarthy SM, Weinreb JC. Pelvic imaging using a T1W fat-suppressed three-dimensional dual echo Dixon technique at 3T. J Magn Reson Imaging. 2008;28:121–127. doi: 10.1002/jmri.21402. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 25.Azevedo RM, de Campos RO, Ramalho M, Heredia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650–657. doi: 10.2214/AJR.10.5881. [DOI] [PubMed] [Google Scholar]
- 26.Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med. 2004;52:815–824. doi: 10.1002/mrm.20237. [DOI] [PubMed] [Google Scholar]
- 27.Riffel P, Attenberger UI, Kannengiesser S, et al. Highly accelerated T1-weighted abdominal imaging using 2-dimensional controlled aliasing in parallel imaging results in higher acceleration: a comparison with generalized autocalibrating partially parallel acquisitions parallel imaging. Invest Radiol. 2013;48:554–561. doi: 10.1097/RLI.0b013e31828654ff. [DOI] [PubMed] [Google Scholar]
- 28.Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging. 2012;36:483–491. doi: 10.1002/jmri.23663. [DOI] [PubMed] [Google Scholar]
- 29.Sung YS, Kwon HJ, Park BW, et al. Prostate cancer detection on dynamic contrast-enhanced MRI: computer-aided diagnosis versus single perfusion parameter maps. AJR Am J Roentgenol. 2011;197:1122–1129. doi: 10.2214/AJR.10.6062. [DOI] [PubMed] [Google Scholar]
- 30.Chen YJ, Chu WC, Pu YS, Chueh SC, Shun CT, Tseng WY. Washout gradient in dynamic contrast-enhanced MRI is associated with tumor aggressiveness of prostate cancer. J Magn Reson Imaging. 2012;36:912–919. doi: 10.1002/jmri.23723. [DOI] [PubMed] [Google Scholar]
- 31.Hara T, Inoue Y, Satoh T, et al. Diffusion-weighted imaging of local recurrent prostate cancer after radiation therapy: comparison with 22-core three-dimensional prostate mapping biopsy. Magn Reson Imaging. 2012;30:1091–1098. doi: 10.1016/j.mri.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Isebaert S, De Keyzer F, Haustermans K, et al. Evaluation of semi-quantitative dynamic contrast-enhanced MRI parameters for prostate cancer in correlation to whole-mount histopathology. Eur J Radiol. 2012;81:e217–e222. doi: 10.1016/j.ejrad.2011.01.107. [DOI] [PubMed] [Google Scholar]
- 33.McClure TD, Margolis DJ, Reiter RE, et al. Use of MR imaging to determine preservation of the neurovascular bundles at roboticassisted laparoscopic prostatectomy. Radiology. 2012;262:874–883. doi: 10.1148/radiol.11103504. [DOI] [PubMed] [Google Scholar]
- 34.Punwani S, Emberton M, Walkden M, et al. Prostatic cancer surveillance following whole-gland high-intensity focused ultrasound: comparison of MRI and prostate-specific antigen for detection of residual or recurrent disease. Br J Radiol. 2012;85:720–728. doi: 10.1259/bjr/61380797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouviere O, Papillard M, Girouin N, et al. Is it possible to model the risk of malignancy of focal abnormalities found at prostate multiparametric MRI? Eur Radiol. 2012;22:1149–1157. doi: 10.1007/s00330-011-2343-8. [DOI] [PubMed] [Google Scholar]
- 36.Selnaes KM, Heerschap A, Jensen LR, et al. Peripheral zone prostate cancer localization by multiparametric magnetic resonance at 3 T: unbiased cancer identification by matching to histopathology. Invest Radiol. 2012;47:624–633. doi: 10.1097/RLI.0b013e318263f0fd. [DOI] [PubMed] [Google Scholar]
- 37.Valentini AL, Gui B, Cina A, et al. T2-weighted hypointense lesions within prostate gland: differential diagnosis using wash-in rate parameter on the basis of dynamic contrast-enhanced magnetic resonance imaging—hystopatology correlations. Eur J Radiol. 2012;81:3090–3095. doi: 10.1016/j.ejrad.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Rischke HC, Nestle U, Fechter T, et al. 3 Tesla multiparametric MRI for GTV-definition of dominant intraprostatic lesions in patients with prostate Cancer—an interobserver variability study. Radiat Oncol. 2013;8:183. doi: 10.1186/1748-717X-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratan F, Niaf E, Melodelima C, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol. 2013;23:2019–2029. doi: 10.1007/s00330-013-2795-0. [DOI] [PubMed] [Google Scholar]
- 40.Costa DN, Bloch BN, Yao DF, et al. Diagnosis of relevant prostate cancer using supplementary cores from magnetic resonance imaging-prompted areas following multiple failed biopsies. Magn Reson Imaging. 2013;31:947–952. doi: 10.1016/j.mri.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy C, Foudi F, Charton J, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013;200:W361–W368. doi: 10.2214/AJR.12.9106. [DOI] [PubMed] [Google Scholar]
- 42.Schimmoller L, Quentin M, Arsov C, et al. Inter-reader agreement of the ESUR score for prostate MRI using in-bore MRI-guided biopsies as the reference standard. Eur Radiol. 2013;23:3185–3190. doi: 10.1007/s00330-013-2922-y. [DOI] [PubMed] [Google Scholar]
- 43.Somford DM, Hoeks CM, Hulsbergen-van de Kaa CA, et al. Evaluation of diffusion-weighted MR imaging at inclusion in an active surveillance protocol for low-risk prostate cancer. Invest Radiol. 2013;48:152–157. doi: 10.1097/RLI.0b013e31827b711e. [DOI] [PubMed] [Google Scholar]
- 44.Vos EK, Litjens GJ, Kobus T, et al. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur Urol. 2013;64:448–455. doi: 10.1016/j.eururo.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 45.Roethke MC, Kuru TH, Schultze S, et al. Evaluation of the ESUR PI-RADS scoring system for multiparametric MRI of the prostate with targeted MR/TRUS fusion-guided biopsy at 3.0 Tesla. Eur Radiol. 2014;24:344–352. doi: 10.1007/s00330-013-3017-5. [DOI] [PubMed] [Google Scholar]