Abstract
The first-line treatment for cervical dystonia (CD) is botulinum toxin type A (BoNT-A), which has been established as a highly effective and well-tolerated therapy. However, this treatment is also complex and challenging to apply in clinical practice. Approximately 20% of patients discontinue therapy due to treatment failure, adverse effects, and other reasons. In addition, expert consensus recommendations are lacking to guide physicians in the optimal use of BoNT-A for CD. Among the issues still to be clarified is the optimal dosing frequency. The generally accepted standard for intervals between BoNT-A injections is ≥12 weeks; however, this standard is based primarily on the methodology of pivotal trials for the BoNT-A products, rather than on evidence that it is optimal in comparison to other intervals. While some retrospective, observational studies of BoNT-A used in clinical practice appear to support the use of ≥12-week dosing intervals, it is often unclear in these studies how the need for reinjection was determined. In contrast, a prospective dose-ranging trial in which patients were allowed to request reinjection as early as 8 weeks showed that about half of patients receiving abobotulinumtoxinA, at the currently recommended initial dose of 500 U, requested reinjection at 8 weeks. Moreover, results from an open-label, 68-week extension phase of the pivotal trial of incobotulinumtoxinA showed that 47.1% of patients had received reinjection at ≤12 weeks. Ongoing studies, such as the Cervical Dystonia Patient Registry for Observation of BOTOX® Efficacy (CD PROBE), may help clarify this question of optimal dosing intervals for BoNT-A in CD.
Keywords: AbobotulinumtoxinA, incobotulinumtoxinA, onabotulinumtoxinA, rimabotulinumtoxinB, cervical dystonia
Introduction
Cervical dystonia (CD) is a movement disorder clinically characterized by involuntary contractions of cervical muscles, which cause abnormal head movements and postures, often associated with head tremor and chronic pain.1,2 Classifications of CD include torticollis (turning or rotation of the head towards one side); laterocollis (tilting of the head towards one side); anterocollis (head and neck flexion), and retrocollis (head and neck extension) or a combination of these movements.1,3 CD is the most common of the focal dystonias, which include blepharospasm and writer's cramp.4 Prevalence estimates for CD in the general population have varied widely, from 0.006% from a clinic-based study in eight European countries,4 to 0.4% in the USA, based on a consumer database survey.5 The symptoms and burden of CD may severely impair quality of life (QOL) and lead to social and occupational disability.6–9 Although the etiology of CD remains unknown, it is physiologically characterized by a deficiency of cortical motor inhibition, which is associated with abnormalities in the motor circuit involving the sensorimotor cortex, basal ganglia, and cerebellum.10–13
Injection of botulinum toxin (BoNT) is the recommended first-line treatment for CD, based primarily on data from seven randomized, controlled, Class I clinical trials in which it was shown to be highly effective and well tolerated.14–16 Use of BoNT type A (BoNT-A) is the preferred treatment while BoNT type B (BoNT-B) is recommended if there is resistance to BoNT-A.15 BoNT formulations approved by the US Food and Drug Administration for CD have been available to clinicians in the USA since 2000.17 These agents include three types of BoNT-A—onabotulinumtoxinA (BOTOX®), abobotulinumtoxinA (Dysport®), and incobotulinumtoxinA (Xeomin®)—and one BoNT-B, rimabotulinumtoxinB (Myobloc/Neurobloc).15 These four agents differ significantly with regard to manufacturing, including complexity and purity, potency, and dosing; the potency units of these products are specific to each and considered non-interchangeable.15,16
BoNT relieves CD symptoms by inhibiting the presynaptic release of acetylcholine from peripheral terminals of motor neurons, causing temporary denervation and muscle weakness lasting typically about 3 months.17 The clinical application of BoNT injection is both a science and an art, requiring highly individualized treatment.3 There is often a delicate balance to be found between achieving optimal efficacy and avoiding adverse events (AEs), such as dysphagia, and occurrence of primary or secondary non-response.3,16,18,19 Major factors to consider in BoNT treatment include the number and selection of neck and adjacent muscles to inject, the amount (dose) of toxin to use, and the length of intervals of dosing (reinjection).20,21
However, few data on optimal use of BoNT for CD or expert consensus recommendations are available to guide physicians in consideration of these factors. Although this treatment for CD has been used in clinical practice and studied for more than 25 years, the variability of CD symptomatology and other factors such as comorbidities and concomitant medications make it difficult to draw general treatment schemas from clinical trial data.21 Moreover, many technical questions regarding administration of BoNT treatment, such as optimal dosing, dilution ratios, number of injection sites, dosing intervals, and targeting procedure are inadequately studied to support clear and detailed recommendations.21,22 Outcomes measurement in CD has also been controversial and continues to evolve.21,23,24 Therefore, important questions remain regarding various aspects of the optimal clinical application of BoNT for CD.21,24 This review will focus on the question of optimal dosing intervals for repeat injections using BoNT-A formulations.
Challenges in BoNT treatment for CD
Reviews of BoNT treatment for CD suggest that 70–90% of patients with CD derive symptomatic benefit from BoNT with at least one injection.16,21,24 However, approximately 20% of patients who receive at least one injection discontinue long-term BoNT treatment, most commonly because of treatment failure.22 BoNT treatment failure can be described as any situation in which the patient, the physician, or both are dissatisfied with the treatment outcome, such as primary or secondary non-response (i.e., lack of efficacy), or intolerable adverse events (AEs).25 Primary non-response has been defined as no response following the first or any subsequent injection.25 Secondary non-response may be described as at least two successful injections characterized by clinical improvement and/or atrophy of injected muscles, and/or typical AEs followed by at least two unsuccessful injections in a row without patient improvement, no typical AEs, and with or without evaluation for immunoresistance.25 The most common BoNT treatment-related AEs leading to discontinuation are dysphagia and neck weakness.22,26,27 In addition, discontinuations may occur because of remission and significant improvement, or simply inconvenience and other non-medical issues.26,28,29 Some patients may also develop unacceptable weakness without significant benefits even if all identifiable factors are optimal (i.e., they cannot be adequately treated with BoNT).
An 8-year study (October 1988 to December 1995) in 616 clinic CD patients who had received at least one injection of abobotulinumtoxinA found that 126 (20.5%) patients had discontinued for various reasons, including primary non-response in 33 (5.4%) patients, and secondary non-response in 17 (4.8% of 357 patients who received at least six injections); 27 (4.4%) patients in the total cohort discontinued because of AEs, the most frequently named being dysphagia (Table 1).26 Among the 17 secondary non-responders, at least one antibody test detected neutralizing serum antibodies in nine patients with an average age of onset of symptoms at 30 years. The non-responders to BoNT-A had received significantly higher units per injection, a greater rate of booster injections (defined as injections administered within 6 weeks following the previous injection), and shorter intervals of repeat injection intervals than responders to treatment. This may reflect increased dose after non-response first appears. In this study, only a few characteristics were statistically significantly different and non-responders with antibodies were treated at a younger age than non-responders without antibodies. The difference in detection of antibody between non-responders may be due to younger patients being more likely to develop neutralizing antibodies than older patients (Table 2).26 A similar 10-year retrospective analysis (January 1990 to December 1999) of 106 clinic patients with CD treated with onabotulinumtoxinA found that 63% of patients at 5 years were experiencing sustained benefit from the treatment, while 20 (18.9%) discontinued because of either primary non-response (11; 10.4%) or secondary non-response (nine; 8.5%) (Table 1).28 Only three (2.8%) patients had discontinued because of AEs. Although reported to occur in 5.4%26 and 10.4%28 of CD patients, primary non-response is very rare and is usually thought to be due to inadequate dose, injection of inappropriate muscles, inappropriate technique, or prior immunization against BoNT. One of the most common reasons for failure to return for treatment is discontinuation due to relocation or a change to another treatment center.
Table 1. Withdrawal Data from Selected Retrospective Studies of Long-Term BoNT-A Treatment for CD in Clinical Practice.
| Study Citation | N | Study Period (years) | Withdrawals/Total, Reasons n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Primary Non-response | Secondary Non-response | AEs | Remission/Improvement | Other1 | |||
| Kessler et al.,262 | 616 | 7 | 126 (20.5) | 33 (5.4) | 17 (2.8) | 27 (4.4) | 26 (4.2) | 34 (5.5) |
| Hsiung et al.28 | 106 | 10 | 49 (46.2) | 11 (10.4) | 9 (8.5) | 3 (2.8) | 4 (3.8) | 22 (20.8) |
| Haussermann et al.29 | 90 | 12 | 33 (36.7) | 1 (1.1) | 3 (3.3) | 11 (12.2) | 6 (6.7) | 12 (13.3) |
Other reasons included inconvenience (costs, travel), moved away, discontinued by physician, lost to follow up/unknown.
Some patients gave more than one reason; BoNT-A, botulinum toxin type A; CD, cervical dystonia.
Table 2. Characteristics of Secondary Non-responders and Responders from Retrospective Clinical Practice Study of BoNT-A Treatment for CD (total N = 3571).
| Characteristic, median (range) | Secondary Non-responders (n = 17) | Responders (n = 303) | p-Value2 | |
|---|---|---|---|---|
| Ab+ (n = 9) | Ab− (n = 8) | |||
| Age at onset of symptoms, years | 30 (13–51) | 39 (28–55) | 41 (8–50) | 0.0073 |
| Duration of symptoms, months | ||||
| Before treatment | 38 (19–121) | 47 (12–192) | 58 (2–426) | n.d. |
| Before non-response | 36 (20–53) | 40 (29–72) | – | n.d. |
| MU per session | 875 (400–1750) | 820 (400–2000) | 750 (150–2250) | 0.00013 |
| Cumulative dose (MU) | 9000 (6455–12405) | 9675 (8475–18050) | 7430 (2700–22475) | 0.083 |
| Interval, days | 91 (11–271) | 91 (3–273) | 105 (1–874) | 0.00013 |
| No. of injections | 11 (7–14) | 14 (9–22) | 10.2±3.2 | n.d. |
| Booster4 | 4/96 injections (4.2%) | 7/117 injections (5.9%) | 41/3089 injections (1.3%) | 0.053,5 |
Ab, Neutralizing Antibodies; BoNT-A, Botulinum Toxin Type A; CD, Cervical Dystonia; MU, Mouse Units.
Non-parametric statistical comparisons (two-tailed Mann–Whitney U tests: χ2 only for the booster injections) were performed between the Ab+ group and the responders group.
Population included all clinic patients with cervical dystonia over a 7-year period who had received six or more botulinum toxin type A injections (54 patients discontinued; secondary non-response was never seen before the sixth injection).
For comparisons between Ab+ secondary non-responding and responding patients.
Mann–Whitney U test (two-tailed).
Booster injections defined as injections within 6 weeks following the previous injection.
Fisher's exact test for 2×2 tables.
Data were previously published by Kessler et al.26
Another trial followed 100 consecutive clinic patients with CD over a 10–12-year period after they had been initially treated with abobotulinumtoxinA.29 Of the 90 evaluable patients (six were lost to follow-up and four had died), 57 (63.3%) were still being treated with BoNT-A and 36.7% had discontinued, more than half of whom had dropped out after only one injection. Discontinuation because of non-response occurred in four (4.4%) patients, including 1 (1.1%) with primary non-response and three (3.3%) with secondary non-response, while 11 (12.2%) discontinued because of AEs (Table 1); 18 (18.9%) patients stopped treatment because of significant improvement or inconvenience (costs, travel) of the treatment. In addition, a retrospective survey trial of 133 clinic patients with primary CD treated with onabotulinumtoxinA over a 6-year period found that 104 (78.2%) patients were continuing to receive the treatment, while 29 (21.8%) had discontinued.27 Major patient-stated reasons for discontinuation included “injections did not help symptoms” in 9.8% of patients, and AEs in 7.5%, including 3.0% for “swallowing problems,” and 4.5% for excessive neck weakness. Of the patients who discontinued, 37.9% had received only one or two injections.
Risk factors for BoNT-A discontinuation
Based on these and other studies, as well as clinical observation, a number of possible risk factors for discontinuation because of treatment failure and AEs have been identified.25,26 Factors that could be associated with primary non-response include pseudodystonia (incorrect diagnosis), wrong muscles injected or inaccessibility of the implicated muscles, inadequate dosing, and unrealistic patient expectations.25,26 In one study, for example, five patients who were unresponsive to an initial injection of abobotulinumtoxinA became responsive to a second injection at the same dose (500 U) given 6 weeks later, which appeared to suggest that the initial response was associated with suboptimal administration technique rather than patient factors.30 Secondary non-response may also be associated with injection of the wrong muscles or inadequate dosing.31,32 Other potential risk factors for secondary non-response, or patient perception of non-response, include immunoresistance; patient depression or stress; changes in the pattern of neck muscle activity, and too high patient expectation (as the patient may have noticed the greatest improvement with the initial injection compared to baseline).25,27,32
Reviews have indicated that AEs associated with BoNT treatment, which typically include dysphagia, neck weakness, dry mouth, dysphonia, and injection site pain, are generally transient and either mild to moderate or intermittent.16,22,33,34 However, severe treatment-related AEs may develop on rare occasions.16 Patients with smaller neck muscle mass and those requiring bilateral injections into the sternocleidomastoid muscles may be at increased risk of dysphagia. Particularly, injections in the lower two-thirds of the sternocleidomastoid muscles may increase the risk of dysphagia compared to injections in the middle and superior portions of said muscle. Injections in the posterior triangle of the neck (levator scapulae, scalenus medius) and deep injections in the splenius capitus also may increase the risk of dysphagia (unpublished data).35
The risk of immunoresistance leading to secondary non-response has been a continuing concern associated with BoNT treatment for CD since its inception.25,36 Reported frequency of immunoresistance with BoNT-A treatment of CD patients, as documented by formation of blocking antibodies, have varied, with reported rates of up to 17%, particularly in the older studies that had utilized the older formulation of onabotulinumtoxinA containing a much higher protein load of 25 ng of protein/100 U. In contrast, the currently used formulation of onabotulinumtoxinA, which was introduced in 1999, only contains 5 ng of protein/100 U and has approximately a sixfold reduced potential for immunogenicity compared to the original formulation.32,37,38 This finding suggested that higher protein load of the BoNT agent is a risk factor for antibody formation.37 The long-term treatment risk of antibody formation in CD patients with current BoNT-A formulations is reported to be generally in the range of 1–3%.32,38–40
IncobotulinumtoxinA, the only BoNT product free of any complexing proteins, has the lowest molecular weight (150 kDA) of the BoNT agents.41,42 The absence of complexing proteins in incobotulinumtoxinA may reduce the rate of antibody formation, as complexing proteins have been reported to contribute to the risk of formation of non-neutralizing antibodies against the BoNT-A.43,44 A report investigating the administration of the BoNT-B, rimabotulinumtoxinB, in four separate clinical trials for the treatment of CD showed no correlation between antibodies against the toxin and outcome of the treatments. The study evaluated the efficacy, safety and immunogenicity of rimabotulinumtoxinB using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score, Subject Global Assessment, monitoring of AEs, and mouse neutralizing antibody (MNA) assay. The toxin was administered every 3 months, for periods up to 6 years. Results from the study showed rimabotulinumtoxinB was effective for the long-term treatment of CD, and there was no correlation between results from the MNA assay and the outcome of the treatments. The lack of correlation between results from the MNA assay may originate from the nature of the investigation in which results from four separate clinical trials were pooled. Alternatively, it is possible the assays were not performed properly or there was a marked placebo effect.45 While immunoresistance is not the cause of all secondary non-response to BoNT-A treatment, formation of antibodies has been observed in approximately 50% of non-responders with CD.26,32
Other postulated risk factors for immunoresistance include higher doses per treatment cycle, use of “booster injections” 2 to 3 weeks after an initial injection, more frequent injections, and younger age at onset of CD.26,32,36,37,46–48 The risk of immunoresistance has been cited in expert reviews as one reason to avoid frequent reinjections, although recommended minimal intervals between injections have ranged from 8 to 12 weeks.16,20 In addition, data indicate that immunoresistance tends to occur between 1 and 4 years after initial treatment, with declining incidence after such period.36,48 However, the identification of risk factors for immunoresistance remains uncertain and complex due to the inconsistency of data and the retrospective designs of studies of non-response and immunoresistance.25 For example, it is sometimes unclear whether greater frequency of injections occurred initially because of non-response, or led to immunoresistance that caused the non-response.
Current standards and practice of dosing frequency
In general, expert reviews of BoNT treatment for CD recommend using the lowest effective dose at the longest dosing interval that effectiveness can be maintained, which is widely considered to be about 12 weeks for most patients.16,22,49,50 Although the product labeling for BoNT products supports this general guideline, the data on which these recommendations are based is limited.
The labeling for onabotulinumtoxinA recommends a total dose not to exceed 360 U administered every 12–16 weeks or at longer intervals, with dosing tailored to the individual patient based on head and neck position, localization of pain, muscle hypertrophy, patient response, and AE history.51 This recommendation is based primarily on the protocol of a pivotal, randomized, double-blind, placebo-controlled phase 3 clinical trial.52 In this trial, conducted in the USA and Canada (May 1995 to October 1997), 170 patients who had previously been responsive to one open-label injection of onabotulinumtoxin-A for a 10-week period, but continued to exhibit a 20% or greater deviation of head position from normal, were subsequently administered one injection of onabotulinumtoxinA or placebo and followed for another 10 weeks. There was up to 6 weeks between the first and second injections. Subjects received a maximum dose of BoNT-A of 360 U. This study used onabotulinumtoxinA containing the original bulk of BoNT-A (formulation contained 25 ng of toxin per 100 U).52 OnabotulinumtoxinA treatment significantly improved Cervical Dystonia Severity Scale scores, compared with placebo, at weeks 2, 4, 6, 8, and 10; however, most patients had returned to pretreatment status by 3 months after treatment.51
The product labeling for abobotulinumtoxinA recommends an initial dose of 500 U, with repeat doses of 250 U to 1000 U as needed, at intervals of 12–16 weeks or longer as necessary, based on return of clinical symptoms.40 The abobotulinumtoxinA package insert (PI) document specifies that retreatment should not occur at intervals of less than 12 weeks.40 The recommendations for abobotulinumtoxinA are primarily based on two phase 3, randomized, placebo-controlled studies in CD patients.53,54 In both trials, the TWSTRS total score at week 4, the primary endpoint, was significantly improved from baseline in patients treated with abobotulinumtoxinA compared with placebo. In the first trial (n = 80), about 75% of patients had been previously treated with BoNT for CD, and these patients were required to have had their last injection ≥16 weeks before study entry.53 The mean (SD) duration of efficacy in responders to abobotulinumtoxinA treatment, defined as the time until recurrence of symptoms to within 10% of the baseline TWSTRS total score, was 22.8 (12.5) weeks, and the median (range) time was 18.5 (9–46) weeks.53 The second trial (n = 116) included an open-label extension phase of up to 94 weeks, and including up to four repeat injections; the mean follow-up was 51.9 weeks.54 In this trial, more than 80% of patients had been previously treated with BoNT, with a minimum interval of 16 weeks before study entry as in the first trial. Among responders to abobotulinumtoxinA, the mean (range) interval between the initial injection of the randomized, controlled trial and the first open label reinjection was 14.4 (3.9–29.9) weeks and the mean (SD) interval between treatments during the open-label phase ranged from 15.0 (5.6) to 17.1 (8.0) weeks.54
The PI document for incobotulinumtoxinA recommends an initial dose of 120 U, with frequency of subsequent injections to be determined by clinical response “but should generally be no more frequent than every 12 weeks”.35 This recommendation is primarily based on a phase 3, randomized, double-blind, placebo-controlled, 20-week, multicenter trial in 233 patients with CD in which the TWSTRS severity score at week 4, the primary endpoint, was significantly improved in patients administered either incobotulinumtoxinA 120 U or 240 U, compared with placebo.35,55 Most of the patients in this trial (61%) had been previously treated with onabotulinumtoxinA, and their last injection was required to be ≥10 weeks prior to study entry.55 Following initial injection, reinjection was allowed at a minimum of 8 weeks, based on clinical need and/or a return to baseline of TWSTRS total score to ≥20; however, the percentage of patients requiring reinjection before the maximum 20-week follow up was not reported.
In summary, the generally recommended minimum interval between repeat BoNT-A injections provided in product PIs is about 12 weeks. The onabotulinumtoxinA PI recommends administration every 12–16 weeks or longer intervals, although noting that in the pivotal clinical trial, most patients had “returned to pretreatment status by 3 months post-treatment”.51 The abobotulinumtoxinA PI also recommends reinjection at intervals of 12–16 weeks or longer, but further states that “retreatment should not occur in intervals of less than 12 weeks”.40 The incobotulinumtoxinA PI provides more flexible guidance, stating that frequency of repeat treatments should be determined by clinical response, although it should generally be no more frequent than every 12 weeks.35 It is reasonable to speculate that the differences in recommendations for frequency of dosing given by the product labeling documents are based on differences in the designs of the pivotal trials for each agent.
Studies of variable BoNT-A administration patterns
Apart from the BoNT-A pivotal trials, a number of studies in CD patients have evaluated the duration of efficacy and treatment intervals with BoNT-A agents. In one such study, 75 toxin-naïve patients were randomly assigned to double-blind treatment with placebo or total doses of abobotulinumtoxinA 250 U, 500 U, or 1000 U for and assessed at 2, 4, and 8 weeks.18 At week 8, need for reinjection was assessed and treatment was unblinded, allowing for open-label follow-up for determining duration of effect. Efficacy outcomes included the modified Tsui scale, pain rating, and global patient and investigator assessments for efficacy. This study found that duration of effect was dose dependent, with an insignificantly greater mean improvement in the modified Tsui score in the 1000 U dose group, compared to the lower dose groups. In addition, about half of the patients in the 250 U and 500 U dose groups, and 39% of patients in the 1000 U groups, requested reinjection at 8 weeks. This study demonstrated dose dependency of both duration of efficacy and risk of AEs.18 Moreover, the high percentages of patients requesting reinjection with both the 500 U (currently recommended) and 1000 U doses would seem to indicate that the recommendation of ≥12 weeks for dosing intervals given in the abobotulinumtoxinA PI, and other BoNT-A products, may not be optimal for all patients. In fact, a multi-national survey of botulinum toxin injectors and patients who were receiving onabotulinumtoxinA or abobotulinumtoxinA for CD indicated that 55% of physicians and 70% of patients prefer shorter injection intervals than those intervals actually received.56,57
A retrospective chart review study in 102 patients with CD who had been under continuous care for 1 year and 10 months (January 1, 1998, to August 31, 1999) found that the mean duration of efficacy, inferred to be the time between repeat injections, was a mean (range) of 15.5 (12.2–24.3) weeks.58 Long-term patterns of onabotulinumtoxinA treatment for CD were also evaluated in a retrospective survey study in 133 patients over 6 years (also discussed above).58 In this study, among patients who were continuing with the treatment after 6 years (n = 104; 78.2%), the mean interval (SD) between treatments was 137.32 days or 19.62 weeks (90.43). Among those who had stopped the treatment for a variety of reasons (n = 29; 21.8%), the mean interval (SD) between treatments was significantly longer at 144.9 days or 20.7 weeks (192.73), p = 0.01. Most patients in this study also achieved a stable dose and injection frequency in their BoNT treatment regimen.58 A prospective, open label study assessed dosing patterns in 326 patients with CD who had been treated with onabotulinumtoxinA for a mean (range) 2.5 years (3.2 months to 4.2 years), primarily to assess the incidence of immunogenicity.38 Patients received a median (maximum) of nine (12) injections. The median treatment interval from the initial injection to first repeat treatment was 92 days (or 13.1 weeks); over the maximum of 12 injections, the range of median intervals was 86–96 days (or 12.2–13.7 weeks). Only four (1.2%) of the 326 patients developed antibodies as determined by mouse protection assay, three of whom stopped responding clinically to the BoNT-A treatment.
These studies appear to support the general recommendation that BoNT-A injection intervals should be in the range of 12–16 weeks. However, the retrospective studies do not clarify whether such a regimen is optimal clinical practice because varying intervals are not correlated with efficacy and tolerability outcomes, and it is unclear in such studies whether the injection intervals chosen were determined by the physician or patient, and whether they were arbitrary or specifically determined by clinical need, i.e., return of symptoms. Indeed, the randomized, double-blind, prospective, dose-finding study of abobotulinumtoxinA, which allowed patients to request retreatment based on their symptoms starting at 8 weeks, found that about half of patients given the now-recommended dose of 500 U of abobotulinumtoxinA had requested retreatment at 8 weeks.18
Few studies have prospectively assessed dosing intervals and other BoNT-A treatment factors in correlation with efficacy, tolerability, and safety outcomes. Such an assessment was performed in an ad hoc analysis of the extension phase of the pivotal, 20-week, randomized, placebo-controlled study of incobotulinumtoxinA treatment, dosed at 120 U or 240 U, in 233 patients with CD (see above).55,59 This long-term extension phase of open-label treatment continued for more than 1 year, including 48 weeks of treatment and 20 weeks of follow up.59 The extension phase protocol allowed for ≥6-week intervals between injections, based on physician and patient discretion, but with the requirement of a TWSTRS total score of ≥20 for retreatment, as in the double-blind phase of the study. Of the patients participating in the randomized, placebo-controlled trial, 214 completed the double-blind phase and entered the extension phase.59 In a post hoc analysis of dosing intervals, each patient who received two or more extension phase injections was classified into one of three interval groups according to their median injection interval: ≤10 weeks; >10 to ≤12 weeks; >12 to ≤14 weeks; or >14 weeks. Mean change in TWSTRS total score across all injection sessions and incidence of AEs were then analyzed by injection interval group.
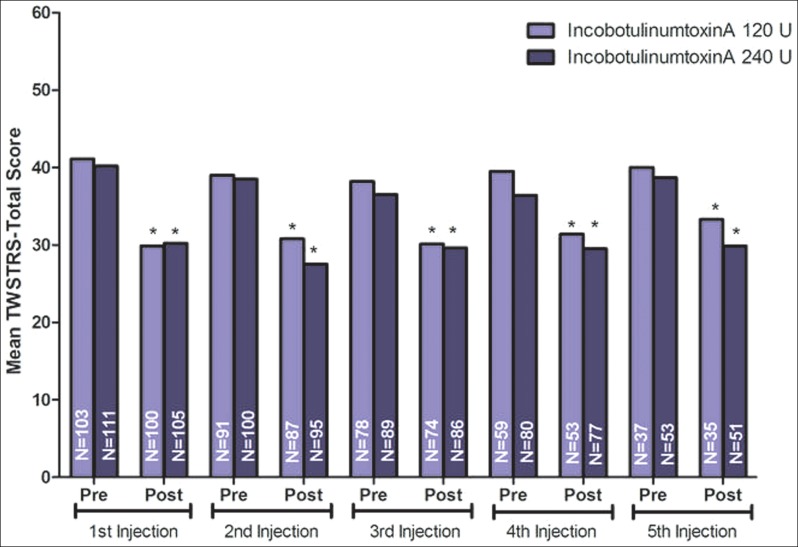
During the extension phase, 191 (89%) patients received two or more injections.59 One-third of patients participating in the extension phase received re-injections at >14 weeks; the rest of the patients were roughly equally divided between the intervals of ≤10 weeks (22.5%), >10 to ≤12 weeks (24.6%); and >12 weeks to ≤14 weeks (19.4%).59 Notably, this showed that 47.1% of patients had received reinjections at median intervals of ≤12 weeks, which is shorter than the standard recommended interval for BoNT-A therapy for CD patients. Mean doses received for both dose groups (120 U and 240 U) were similar across all injection sections and interval groups.59 The mean changes from baseline in TWSTRS total scores in each interval group showed statistically significant improvements 4 weeks after each injection (p<0.0001) (Figure 1).59 In addition, there were no statistically significant differences in the overall occurrence of AEs between interval groups (Table 3).59 Increased antibody production occurred in three (1.3%) patients and had no correlation with dosing frequency during the extension phase.
Figure 1. Mean (SD) Toronto Western Spasmodic Torticollis Rating Scale Total Score.
Results at 4 weeks after each of five injection sessions. Error bars represent SD. *p<0.001; p-value is a one-sample t-test of change in TWSTRS total score from the injection visit to the visit 4 weeks later (with no replacement of missing data).
Table 3. Incidence of TEAEs by Interval Group (Pooled Data of Both Treatment Groups) in CD Patients Who Received ≥2 BoNT-A Injections (incobotulinumtoxinA 120 U or 240 U) During the Open Label Extension Phase of a Randomized, Placebo-Controlled Trial.
| Interval Group | Number Patients | Incidence of TEAEs | Comparison of Frequency of TEAEs Between Interval Groups |
|---|---|---|---|
| Total N = 191 | Total N = 110 | ||
| n (%) | n (%) | ||
| ≤10 weeks | 43 (22.5) | 31 (72.1) | NS |
| >10 to ≤12 weeks | 47 (24.6) | 22 (46.8) | NS |
| >12 to ≤14 weeks | 37 (19.4) | 21 (56.8) | NS |
| >14 weeks | 64 (33.5) | 36 (56.3) | NS |
NS, Not Significant; TEAE, Treatment Emergent Adverse Event.
Data were previously published by Evidente et al.59
A prospective clinical study aimed at investigating the injection of fixed doses of incobotulinumtoxinA at flexible intervals (6–20 weeks) was recently conducted in patients with CD. In this study, patients received up to six injections of incobotulinumtoxinA in two treatments; a main period with treatment of 120 U, 240 U incobotulinumtoxinA or placebo (214 patients), followed by subsequent randomization to 120 U or 240 U during the extension period (169 patients). Doses and intervals of incobotulinumtoxinA injections were determined by physician assessment, using the TWSTRS total score, upon patient request. As reported by Evidente et al.,60 44.9% of patients received incobotulinumtoxinA injections at intervals less than 12 weeks. Results from the study showed treatment intervals of 6–7 weeks with incobotulinumtoxinA were well tolerated and AE frequency was similar for injection intervals <12 weeks and ≥12 weeks with repeated injections of incobotulinumtoxinA. The most frequent AEs were dysphagia and neck pain.60 Brin et al.38 and Evidente et al.59 reported 1.2% and 1.3% of patients treated for CD with onabotulinumtoxinA or incobotulinumtoxinA developed antibodies over treatment periods of 4.2 years and over a year, respectively. Evidente et al.60 reported incobotulinumtoxinA treatments were well tolerated for injection intervals <12 weeks and ≥12 weeks with repeated injections of incobotulinumtoxinA. Hence, the likelihood of developing antibodies for injection intervals whether <12 weeks or ≥12 weeks with repeated injections of incobotulinumtoxinA or BoNT-A should remain low.
Future studies
Other ongoing studies promise to provide further clinical data on facets of optimal BoNT-A treatment practice for CD, including injection intervals. Among them, the Cervical Dystonia Patient Registry for Observation of BOTOX® Efficacy (CD PROBE) is a prospective, multicenter, clinical registry in the US that is enrolling patients with CD who are toxin-naïve and/or new to physicians' practices or had been in a clinical trial and received their last injection ≥16 weeks prior to enrollment.21 Patients are followed over three injection cycles of onabotulinumtoxinA with assessments at time of injection and 4–6 weeks afterward. Data on patient demographics and CD disease history, treatment, including dosing intervals, and efficacy and safety/tolerability outcomes will be gathered and assessed. In this open-label study, the frequency of injection is up to the physician. Therefore, it is unlikely the study will provide useful data about injection frequency. An interim report of physician-reported outcomes for CD PROBE, including 499 enrolled patients, showed that the mean (SD) interval between the first and second injections was 100.4 (22.9) days and 100.0 (22.3) days between the second and third injections; however, the percentages of patients receiving injections at <12 weeks or ≥12 weeks (∼84 days) was not reported.61 The interim data also showed that 96 (19.2%) patients had discontinued treatment, while nine patients withdrew for lack of efficacy and nine because of AEs, 55 of the patients were either lost to follow up or withdrew consent. Hence, discontinuations continue to be a problem affecting approximately 20% of patients receiving BoNT-A therapy.
Conclusions
Although the application of BoNT-A treatment for CD is a complex and challenging procedure, there are few expert recommendations and supporting clinical data to guide clinicians in optimizing this therapy. With regard to dosing frequency, there is a generally accepted standard of ≥12 weeks for BoNT-A injection intervals. However, this standard is based primarily on previous methodology of pivotal clinical trials for BoNT-A products, rather than clear evidence of its optimal efficacy, safety, and tolerability. In addition, this standard has not been assessed in comparison with alternative intervals. Some study data suggest a subgroup of patients would prefer dosing more frequently than every 12 weeks or longer; however, the risks and benefits of shorter dosing intervals are not well studied. Ongoing studies such as CD PROBE may help answer some of these questions.
Acknowledgments
Technical writing contributions by Linnea Elliott of The Curry Rockefeller Group and Starr L. Grundy, B.Sc. Pharm of SD Scientific, Inc., funded by Merz North America, Inc.
Footnotes
Funding: Supported by Merz North America, Inc.
Financial Disclosures: Dr. Evidente has received consulting compensation and research support from Merz North America, Inc.
Conflict of Interests: V.G.H.E. has received consulting compensation and research support from Merz North America, Inc. E.J.P. was an employee of Merz North America, Inc.
References
- 1.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: Clinical characteristics. Mov Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: Clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/WNL.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 3.Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics. 2009;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247:787–792. doi: 10.1007/s004150070094. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism Relat Disord. 2007;13:411–416. doi: 10.1016/j.parkreldis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Camfield L, Ben-Shlomo Y, Warner TT. Impact of cervical dystonia on quality of life. Mov Disord. 2002;17:838–841. doi: 10.1002/mds.10127. [DOI] [PubMed] [Google Scholar]
- 7.Slawek J, Friedman A, Potulska A, et al. Factors affecting the health-related quality of life of patients with cervical dystonia and the impact of botulinum toxin type A injections. Funct Neurol. 2007;22:95–100. [PubMed] [Google Scholar]
- 8.Muller J, Kemmler G, Wissel J, et al. The impact of blepharospasm and cervical dystonia on health-related quality of life and depression. J Neurol. 2002;249:842–846. doi: 10.1007/s00415-002-0773-6. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Camfield L, Warner T. What are the determinants of quality of life in people with cervical dystonia? J Neurol Neurosurg Psychiatry. 2002;72:608–614. doi: 10.1136/jnnp.72.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–829. doi: 10.1056/NEJMra055549. [DOI] [PubMed] [Google Scholar]
- 11.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia–a review. Neuroimage. 2011;56:1011–1020. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia. Curr Opin Neurol. 2009;22:406–413. doi: 10.1097/WCO.0b013e32832d9ef3. [DOI] [PubMed] [Google Scholar]
- 14.Simpson DM, Blitzer A, Brashear A, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 16.Swope D, Barbano R. Treatment recommendations and practical applications of botulinum toxin treatment of cervical dystonia. Neurol Clin. 2008;26(Suppl 1):54–65. doi: 10.1016/S0733-8619(08)80005-9. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poewe W, Deuschl G, Nebe A, et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. J Neurol Neurosurg Psychiatry. 1998;64:13–17. doi: 10.1136/jnnp.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hefter H, Kupsch A, Mungersdorf M, et al. A botulinum toxin A treatment algorithm for de novo management of torticollis and laterocollis. BMJ Open. 2011;1:e000196. doi: 10.1136/bmjopen-2011-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benecke R, Dressler D. Botulinum toxin treatment of axial and cervical dystonia. Disabil Rehabil. 2007;29:1769–1777. doi: 10.1080/01421590701568262. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic J, Adler CH, Charles PD, et al. Rationale and design of a prospective study: Cervical Dystonia Patient Registry for Observation of OnaBotulinumtoxinA Efficacy (CD PROBE) BMC Neurol. 2011;11:140. doi: 10.1186/1471-2377-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comella CL, Thompson PD. Treatment of cervical dystonia with botulinum toxins. Eur J Neurol. 2006;13(Suppl 1):16–20. doi: 10.1111/j.1468-1331.2006.01440.x. [DOI] [PubMed] [Google Scholar]
- 23.Cano SJ, Hobart JC, Fitzpatrick R, Bhatia K, Thompson AJ, Warner TT. Patient-based outcomes of cervical dystonia: A review of rating scales. Mov Disord. 2004;19:1054–1059. doi: 10.1002/mds.20055. [DOI] [PubMed] [Google Scholar]
- 24.Zoons E, Dijkgraaf MG, Dijk JM, van Schaik IN, Tijssen MA. Botulinum toxin as treatment for focal dystonia: A systematic review of the pharmaco-therapeutic and pharmaco-economic value. J Neurol. 2012;259(12):2519–26. doi: 10.1007/s00415-012-6510-x. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord. 2004;19(Suppl 8):S92–100. doi: 10.1002/mds.20022. [DOI] [PubMed] [Google Scholar]
- 26.Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: Efficacy, safety, and antibody frequency. German Dystonia Study Group. J Neurol. 1999;246:265–274. doi: 10.1007/s004150050345. [DOI] [PubMed] [Google Scholar]
- 27.Brashear A, Bergan K, Wojcieszek J, Siemers ER, Ambrosius W. Patients' perception of stopping or continuing treatment of cervical dystonia with botulinum toxin type A. Mov Disord. 2000;15:150–153. doi: 10.1002/1531-8257(200001)15:1<150::AID-MDS1024>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Hsiung GY, Das SK, Ranawaya R, Lafontaine AL, Suchowersky O. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17:1288–1293. doi: 10.1002/mds.10252. [DOI] [PubMed] [Google Scholar]
- 29.Haussermann P, Marczoch S, Klinger C, Landgrebe M, Conrad B, Ceballos-Baumann A. Long-term follow-up of cervical dystonia patients treated with botulinum toxin A. Mov Disord. 2004;19:303–308. doi: 10.1002/mds.10659. [DOI] [PubMed] [Google Scholar]
- 30.Wissel J, Kanovsky P, Ruzicka E, et al. Efficacy and safety of a standardised 500 unit dose of Dysport (clostridium botulinum toxin type A haemaglutinin complex) in a heterogeneous cervical dystonia population: Results of a prospective, multicentre, randomised, double-blind, placebo-controlled, parallel group study. J Neurol. 2001;248:1073–1078. doi: 10.1007/s004150170028. [DOI] [PubMed] [Google Scholar]
- 31.Gelb DJ, Yoshimura DM, Olney RK, Lowenstein DH, Aminoff MJ. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Ann Neurol. 1991;29:370–376. doi: 10.1002/ana.410290407. [DOI] [PubMed] [Google Scholar]
- 32.Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: Much ado about nothing? Clin Neuropharmacol. 2009;32:213–218. doi: 10.1097/WNF.0b013e3181914d0a. [DOI] [PubMed] [Google Scholar]
- 33.Costa J, Espirito-Santo C, Borges A, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2005:CD003633. doi: 10.1002/14651858.CD003633.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Costa J, Espirito-Santo C, Borges A, et al. Botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev. 2005:CD004315. doi: 10.1002/14651858.CD004315.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Merz Pharmaceuticals, LLC XEOMIN® (incobotulinumtoxinA) for injection US Prescribing Information. 2011. Version 07/2011.
- 36.Mejia NI, Vuong KD, Jankovic J. Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov Disord. 2005;20:592–597. doi: 10.1002/mds.20376. [DOI] [PubMed] [Google Scholar]
- 37.Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology. 2003;60:1186–1188. doi: 10.1212/01.WNL.0000055087.96356.BB. [DOI] [PubMed] [Google Scholar]
- 38.Brin MF, Comella CL, Jankovic J, Lai F, Naumann M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–1360. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- 39.Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX(R)) across multiple indications. Mov Disord. 2010;25(13):2211–8. doi: 10.1002/mds.23254. Oct 15. [DOI] [PubMed] [Google Scholar]
- 40.Ipsen Biopharm, Ltd Dysport® (abobotulinumtoxinA) for injection Prescribing Information. 2010. Version 04/2010.
- 41.Benecke R. Xeomin in the treatment of cervical dystonia. Eur J Neurol. 2009;16(Suppl 2):6–10. doi: 10.1111/j.1468-1331.2009.02878.x. [DOI] [PubMed] [Google Scholar]
- 42.Frevert J. Xeomin is free from complexing proteins. Toxicon. 2009;54:697–701. doi: 10.1016/j.toxicon.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Goschel H, Wohlfarth K, Frevert J, et al. Botulinum A toxin therapy: Neutralizing and nonneutralizing antibodies-therapeutic consequences. Exp Neurol. 1997;147:96–102. doi: 10.1006/exnr.1997.6580. [DOI] [PubMed] [Google Scholar]
- 44.Joshi SG, Elias M, Singh A, et al. Modulation of botulinum toxin-induced changes in neuromuscular function with antibodies directed against recombinant polypeptides or fragments. Neuroscience. 2011;179:208–222. doi: 10.1016/j.neuroscience.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Chinnapongse RB, Lew MF, Ferreira JJ, et al. Immunogenicity and long-term efficacy of botulinum toxin type B in the treatment of cervical dystonia: Report of 4 prospective, multicenter trials. Clin Neuropharmacol. 2012;35:215–223. doi: 10.1097/WNF.0b013e318263163c. [DOI] [PubMed] [Google Scholar]
- 46.Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213–217. doi: 10.1002/mds.870090216. [DOI] [PubMed] [Google Scholar]
- 47.Jankovic J, Schwartz K. Response and immunoresistance to botulinum toxin injections. Neurology. 1995;45:1743–1746. doi: 10.1212/WNL.45.9.1743. [DOI] [PubMed] [Google Scholar]
- 48.Dressler D. Clinical features of antibody-induced complete secondary failure of botulinum toxin therapy. Eur Neurol. 2002;48:26–29. doi: 10.1159/000064953. [DOI] [PubMed] [Google Scholar]
- 49.Comella CL, Jankovic J, Brin MF. Use of botulinum toxin type A in the treatment of cervical dystonia. Neurology. 2000;55:S15–21. [PubMed] [Google Scholar]
- 50.Jankovic J. Botulinum toxin therapy for cervical dystonia. Neurotox Res. 2006;9:145–148. doi: 10.1007/BF03033933. [DOI] [PubMed] [Google Scholar]
- 51.Allergan, Inc Botox® (onabotulinumtoxinA) for injection, for intramuscular, intradetruser, or intradermal use US Prescribing Information. 2011. Version 08/2011.
- 52.Charles D, Brashear A, Hauser RA, et al. Efficacy, tolerability, and immunogenicity of onabotulinumtoxina in a randomized, double-blind, placebo-controlled trial for cervical dystonia. Clin Neuropharmacol. 2012;35:208–214. doi: 10.1097/WNF.0b013e31826538c7. [DOI] [PubMed] [Google Scholar]
- 53.Truong D, Duane DD, Jankovic J, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: Results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20:783–791. doi: 10.1002/mds.20403. [DOI] [PubMed] [Google Scholar]
- 54.Truong D, Brodsky M, Lew M, et al. Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord. 2010;14:331–335. doi: 10.1016/j.parkreldis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Comella CL, Jankovic J, Truong DD, Hanschmann A, Grafe S. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN(R), botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci. 2011;308:103–109. doi: 10.1016/j.jns.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 56.Fargel M, Psaila E. Results of a multi-national patient and physician survey on treatment satisfaction with current botulinum toxin treatment in focal dystonia (Abstract #980) Mov Disord. 2010;25:S511. doi: 10.1002/mds.22923. [DOI] [Google Scholar]
- 57.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: A cross-sectional survey of patients with cervical dystonia. J Med Econ. 2012;15:419–423. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 58.Brashear A, Watts MW, Marchetti A, Magar R, Lau H, Wang L. Duration of effect of botulinum toxin type A in adult patients with cervical dystonia: A retrospective chart review. Clin Ther. 2000;22:1516–1524. doi: 10.1016/S0149-2918(00)83049-0. [DOI] [PubMed] [Google Scholar]
- 59.Evidente VG, Fernandez HH, LeDoux MS, et al. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin(®)) in cervical dystonia. J Neural Transm. 2013;120:1699–1707. doi: 10.1007/s00702-013-1048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evidente VG, Truong D, Jankovic J, et al. IncobotulinumtoxinA (Xeomin®) injected for blepharospasm or cervical dystonia according to patient needs is well tolerated. J Neurol Sci. 2014 doi: 10.1016/j.jns.2014.08.004. Aug 10. pii: S0022-510X(14)00517-6. [DOI] [PubMed] [Google Scholar]
- 61.Jankovic J, Adler CH, Charles PD, et al. Cervical dystonia patient registry for observation of Botox efficacy (CD PROBE): Interim results of physician-reported outcomes. Poster presented at 63rd Annual Meeting of the American Academy of Neurology. Accessed October 9, 2012 2011 April 9–16 (Honolulu, HI) http://www.bcm.edu/neurology/pdf/poster_pdcmdc_CD-PROBE5.pdf. [Google Scholar]