Abstract
Background
Aggressive fibromatosis is a rare but invasive tumor infiltrating widely between fascia and muscle fibers. It has a high tendency to be locally recurrent despite complete resection. Effectiveness of adjuvant treatment for aggressive fibromatosis including radiotherapy, pharmacological agents, hormonal treatments, and chemotherapy have been previously reported. The purpose of this article was to collect and analyze all information regarding the effectiveness and side effects of oral methotrexate in aggressive fibromatosis.
Methods
From 2005 to 2011, eleven patients with aggressive fibromatosis treated with oral methotrexate at our institution were analyzed in this study. Oral methotrexate was administered once per week at 10 mg per week. Authors collected information about effectiveness concerning cases of local recurrence and metastasis.
Results
Eleven patients had remission, two patients had local recurrence. Fatal complications or toxicity were not observed.
Conclusions
Oral methotrexate given at this dose and schedule was considered as a useful treatment in primary inoperable fibromatosis and recurrent fibromatosis.
Keywords: Aggressive fibromatosis, Oral methotrexate
Aggressive fibromatosis (AF) is a rare but invasive fibroblastic musculoaponeurotic tumor of intermediate malignancy with a high rate of recurrence. Annual incidence of AF is 2-5 per 1,000,000 and more often in young adults. There is a 2- to 3-fold female predominance.1,2) Histologically, AF consists of fibroblastic proliferation arising from musculoaponeurotic tissue. AF does not have metastatic potential, but its aggressive and invasive features make local remission difficult. There is a recurrence rate of 24%-77% after any therapy.1,2)
Surgery is the mainstay treatment for AF, but local recurrence is common after surgical resection. Additional therapies may be needed, such as radiotherapy, hormonal therapy, chemotherapy or noncytotoxic agents.1,2,3,4,5,6,7) Here we report 11 cases of primary or recurrent AF treated with oral methotrexate.
METHODS
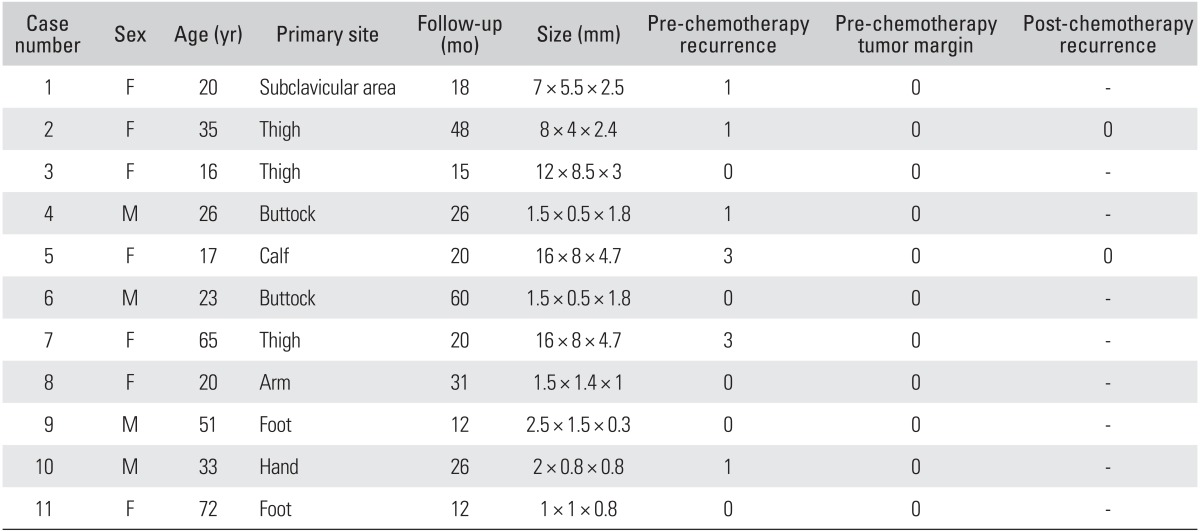
Between 2005 and 2011, 11 patients with primary or recurrent AF received oral methotrexate at a dose of 10 mg per week. All patients received clinical and radiological follow-up examinations every month for a minimum of 12 months. All data were analyzed retrospectively. The median age was 37 years (range, 11 to 72 years) with a female predominance of 65%. The primary tumor locations included thigh (n = 3), hand (n = 2), foot (n = 2), buttock (n = 2), calf (n = 1), and subclavian area (n = 1). Six patients presented with primary disease without having been previously treated by any therapy, whereas five patients had a recurrent tumor following one or more surgical resections. Operations were performed with wide excisional margin.
All 11 patients received oral methotrexate once per week at a dose of 10 mg per week and continued chemotherapy until the most recent follow-up date. Follow-up was performed in outpatient clinical setting with clinical and radiological assessment. Recurrence was diagnosed radiologically and confirmed by biopsy after surgical resection. Median follow-up period was 27 months (range, 12 to 54 months) (Table 1).
Table 1.
Patient Characteristics
RESULTS
No deaths were caused by AF, chemotherapy, or complications from treatment. An objective response was achieved in 9 of 11 patients (81%). In two cases (18%), recurrence was diagnosed during chemotherapy and the tumor was then widely resected. Recurrence was diagnosed at an average of 20.1 months after the first operation. Primary tumor size averaged 5.6 cm in diameter. Recurrent tumors averaged 2 cm with an 8-cm primary tumor. No recurrence (0%) was found in primary 5 cases, but two cases (33.3%) had a recurrence in recurrent 6 cases. The most common toxicity observed was gastrointestinal complications, such as nausea, vomiting, and dyspepsia. General weakness, itching, rash, and hepatotoxicity were observed but not severe. They were all managed with medication in an outpatient clinical setting.
DISCUSSION
AF is a rare tumor representing 3% of all soft tissue tumors. It features a broad group of benign, fibrous proliferations that are biologically intermediate between benign fibrous tissues and fibrosarcomas. AF has a low mitotic activity but a highly infiltrative growth pattern along tissue planes with an ability to invade adjacent tissues such as fascia, muscle, neurovascular tissue, and periosteum.1,2,3,4)
Many issues regarding the optimal treatment of patients with AF remain controversial. Surgical resection with negative margins is the treatment of choice, except when surgery may result in functional loss or major morbidity. In cases with positive margins, postoperative treatment is indicated to reduce the local recurrence rate. For inoperable cases, primary nonsurgical treatment is necessary.5,6,7,8,9,10) Surgery is the treatment of choice. Negative margins are also important because of AF's infiltrative pathology. The rate of positive margins after surgery is high, ranging from 44% to 61%.11) However, Nuyttens et al.12) reported that positive margins do not necessarily imply a recurrence. Furthermore, cases with negative margins after surgery may have recurrence rates ranging from 0% to 28%.5,6,9,13,14) Although there is no consensus, many studies agree that resected tumors with positive margins will have a higher recurrence rate and a worse prognosis than those with negative margins.
Radiation is an effective treatment option alone or in combination with surgery. Ballo et al.15) reported that radiation and surgery were equally effective in uncontaminated cases of AF. In positive cases, he observed that radiation combined with surgery was more effective, with a lower recurrence rate than surgery alone (25% vs. 52%). Radiation should be used only in a selective group of patients due to related morbidity and complications, such as fibrosis and urogenital injury.12,15)
Cytotoxic chemotherapy in AF was reported as early as 1982, but there are limited reports of single-agent chemotherapy for AF. Combination chemotherapy regimens have been reported more frequently with information on the effectiveness and complications. Doxorubicin- or methotrexate-based combination chemotherapy has been commonly used. Okuno and Edmonson16) reported a doxorubicin-based chemotherapy had overall response rates ranging from 17% to 100%. However, doxorubicin's cardiotoxicity has led some investigators to discontinue treatment in order to manage life-threatening complications.16,17) Methotrexate combination chemotherapy has lower toxicity than doxorubicin, so this regimen is indicated for younger patients who will require longer treatment periods.18,19) van der Hul et al.19) reported that only 20% of tumors progressed after administration of methotrexate combination chemotherapy. However, 90% of patients had severe complications that prevented them from receiving the projected treatment.19) Weiss and Lackman18) reported that 77% of patients had partial or complete remission with methotrexate combination therapy. However, drug regimen may have to be modified depending on life-threatening complications, such as interstitial pneumonitis, severe hepatic failure, and myelosuppression.18,19) van der Hul et al.19) reported that the use of single-agent chemotherapy with pegylated liposomal doxorubicin for unresectable AF had a 36% partial remission rate. Complications associated with doxorubicin included erythema and mucositis, but the drug was more acceptable and safer for patients than other intravenous chemotherapies.19)
Oral methotrexate is widely used in systemic rheumatic disorders where adequate balance of efficacy and toxicity favors mono-therapy over combination with other drugs. The oral methotrexate regimens use a starting dose of 10-15 mg/week, with escalation of 5 mg every 2-4 weeks up to 20-30 mg/week, depending on clinical response and tolerability. Oral methotrexate can be prescribed in an outpatient clinical setting if alanine aminotransferase/aspartate aminotransferase and blood urea nitrogen/creatinine are checked every 1-1.5 months with clinical assessment for side effects at each visit.
We attempted to treat AF with the same oral methotrexate regimen as is used for rheumatoid arthritis. In this study, oral methotrexate was shown to have activity against AF without causing serious complications. Objective response was documented in 83.4% of cases. Since all cases were confirmed of marginal positive after surgery, authors have considered the effectiveness of oral methotrexate to prevent a recurrence on marginal positive cases. Dose reduction due to toxicity was not required in any cases. Gastrointestinal trouble and hepatotoxicity were the most severe side effects but were conservatively and appropriately managed. This study sample was small, and cases had markedly heterogeneous features of disease extent, size, location and recurrence. Follow-up was relatively short, and optimum dose and duration of treatment have not been resolved.
This study suggests that oral methotrexate is effective for the management of primary or recurrent AF. Oral methotrexate at the studied dose and schedule has led to acceptable toxicity and the possibility of local control.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Biermann JS. Desmoid tumors. Curr Treat Options Oncol. 2000;1(3):262–266. doi: 10.1007/s11864-000-0038-5. [DOI] [PubMed] [Google Scholar]
- 2.Reitamo JJ, Hayry P, Nykyri E, Saxen E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77(6):665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- 3.Mendez-Fernandez MA, Gard DA. The desmoid tumor: "benign" neoplasm, not a benign disease. Plast Reconstr Surg. 1991;87(5):956–960. doi: 10.1097/00006534-199105000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AJ, Lackman RD. Low-dose chemotherapy of desmoid tumors. Cancer. 1989;64(6):1192–1194. doi: 10.1002/1097-0142(19890915)64:6<1192::aid-cncr2820640605>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Easter DW, Halasz NA. Recent trends in the management of desmoid tumors: summary of 19 cases and review of the literature. Ann Surg. 1989;210(6):765–769. doi: 10.1097/00000658-198912000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt RT, Morgan HC, Ackerman LV. Principles in the management of extra-abdominal desmoids. Cancer. 1960;13(4):825–836. doi: 10.1002/1097-0142(196007/08)13:4<825::aid-cncr2820130427>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis CP, Mayordomo J, Zografos GC, Driscoll DL. Desmoid tumors of the trunk and extremity. Cancer. 1993;72(5):1637–1641. doi: 10.1002/1097-0142(19930901)72:5<1637::aid-cncr2820720524>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Khorsand J, Karakousis CP. Desmoid tumors and their management. Am J Surg. 1985;149(2):215–218. doi: 10.1016/s0002-9610(85)80067-2. [DOI] [PubMed] [Google Scholar]
- 9.Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome: new aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151(2):230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 10.Reitamo JJ. The desmoid tumor. IV. Choice of treatment, results, and complications. Arch Surg. 1983;118(11):1318–1322. doi: 10.1001/archsurg.1983.01390110066014. [DOI] [PubMed] [Google Scholar]
- 11.Mehrotra AK, Sheikh S, Aaron AD, Montgomery E, Goldblum JR. Fibromatoses of the extremities: clinicopathologic study of 36 cases. J Surg Oncol. 2000;74(4):291–296. doi: 10.1002/1096-9098(200008)74:4<291::aid-jso10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Nuyttens JJ, Rust PF, Thomas CR, Jr, Turrisi AT., 3rd Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88(7):1517–1523. [PubMed] [Google Scholar]
- 13.Enzinger FM, Shiraki M. Musculo-aponeurotic fibromatosis of the shoulder girdle (extra-abdominal desmoid): analysis of thirty cases followed up for ten or more years. Cancer. 1967;20(7):1131–1140. doi: 10.1002/1097-0142(196707)20:7<1131::aid-cncr2820200716>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100(3):612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 15.Ballo MT, Zagars GK, Pollack A. Radiation therapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1998;42(5):1007–1014. doi: 10.1016/s0360-3016(98)00285-5. [DOI] [PubMed] [Google Scholar]
- 16.Okuno SH, Edmonson JH. Combination chemotherapy for desmoid tumors. Cancer. 2003;97(4):1134–1135. doi: 10.1002/cncr.11189. [DOI] [PubMed] [Google Scholar]
- 17.Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I. Advanced aggressive fibromatosis: effective palliation with chemotherapy. Acta Oncol. 2011;50(3):455–461. doi: 10.3109/0284186X.2010.509105. [DOI] [PubMed] [Google Scholar]
- 18.Weiss A, Lackman R. Therapy of desmoid tumors, fibromatosis, and related neoplasms. Int J Oncol. 1995;7(4):773–776. doi: 10.3892/ijo.7.4.773. [DOI] [PubMed] [Google Scholar]
- 19.van der Hul RL, Seynaeve C, van Geel BN, Verweij J. Low Dose methotrexate and vinblastine, given weekly to patients With desmoid tumours, is associated with major toxicity. Sarcoma. 2003;7(3-4):153–157. doi: 10.1080/13577140310001644779. [DOI] [PMC free article] [PubMed] [Google Scholar]


