Abstract
This study investigated morning levels of pentraxin3 (PTX3) as a sensitive biomarker for acute inflammation in patients with obstructive sleep apnea (OSA). A total of 61 consecutive patients with OSA were divided into two groups: non-to-mild (n = 20) and moderate-to-severe (n = 41) OSA based on their apnea-hypopnea index (AHI) score. Those patients with moderate-to-severe OSA were further divided into continuous positive airway pressure (CPAP) treated (n = 21) and non-CPAP-treated (n = 20) groups. Morning and evening serum PTX3 and high-sensitivity (hs) C-reactive protein (CRP) levels were measured before and after 3 mo of CPAP therapy. The baseline hs-CRP and PTX3 levels were higher in patients with moderate-to-severe OSA than in those with non-to-mild OSA. Moreover, the serum PTX3 levels, but not the hs-CRP levels, were significantly higher after than before sleep in the moderate-to-severe OSA group (morning PTX3, 1.96 ± 0.52; evening PTX3, 1.71 ± 0.44 ng/ml). OSA severity as judged using the AHI was significantly correlated with serum PTX3 levels but not hs-CRP levels. The highest level of correlation was found between the AHI and morning PTX3 levels (r = 0.563, P < 0.001). CPAP therapy reduced evening and morning serum hs-CRP and PTX3 levels in patients with moderate-to-severe OSA; however, the reduction in PTX3 levels in the morning was greater than that in the evening (morning −29.8 ± 16.7% vs. evening −12.6 ± 26.8%, P = 0.029). Improvement in the AHI score following CPAP therapy was strongly correlated with reduced morning PTX3 levels(r = 0.727, P < 0.001). Based on these results, morning PTX3 levels reflect OSA-related acute inflammation and are a useful marker for improvement in OSA following CPAP therapy.
Keywords: CPAP therapy, pentraxin3, obstructive sleep apnea, inflammation
patients with obstructive sleep apnea (osa) are often diagnosed with acute coronary syndrome (21) and have a higher prevalence of midnight or early morning cardiovascular events than do patients without OSA (12). We previously reported that patients with OSA have abnormal blood coagulation and platelet profiles (24). Intermittent hypoxia during sleep, which is frequently observed in patients with OSA, may induce atherogenic stimuli such as oxidative stress (3). Moreover, OSA activates several inflammatory cascades mediated through multifactorial processes including hypoxia, increased sympathetic activation, and transient blood pressure surges, which lead to vascular damage (3). These findings suggest that vascular inflammation progresses rapidly during sleep in patients with OSA and may contribute to the initiation of acute coronary syndrome, resulting in midnight or early morning cardiovascular events.
An elevated serum C-reactive protein (CRP) level is a useful prognostic marker in patients with acute coronary syndrome (4). Pentraxin3 (PTX3) has recently emerged as a specific biomarker of vascular inflammation. Although PTX3 and CRP belong to the pentraxin superfamily, their respective origins and manner of induction differ. Serum CRP, a classical short pentraxin, is produced primarily in the liver in response to interleukin-6 stimulation (13). However, CRP does not immediately reflect inflammatory responses in patients with OSA. Mills et al. (18) reported that the mean serum CRP levels in patients with OSA were significantly higher during the day than during the night and that elevated daytime CRP levels occurred as a result of nighttime carryover arousal responses.
PTX3 is primarily produced in neutrophils, macrophages, smooth muscle cells, and endothelial cells stimulated by interleukin-1 or tumor necrosis factor-α (13). PTX3, but not CRP, is considered to be a rapid release, vascular-specific inflammatory biomarker because of its short half-life and rapid clearance rate (5). Furthermore, PTX3 is elevated in patients with OSA (9) and in patients with coronary artery disease (10). However, the difference between evening and morning levels of PTX3 in patients with OSA are unknown. Moreover, it was not fully evaluated whether PTX3 is a more sensitive marker of OSA-related acute inflammation than CRP.
Continuous positive airway pressure (CPAP) therapy is an effective therapeutic approach for hypoxia and cessation of breathing in patients with OSA. A previous observational study found that CPAP therapy was associated with a reduced incidence of vascular events in patients with OSA (16). Thus CPAP therapy is suitable for the investigation of biomarker sensitivity to OSA-related acute inflammation. We investigated the hypothesis that the morning PTX3 level is a useful biomarker for OSA-related acute vascular inflammation in patients with OSA.
METHODS
Study design.
We enrolled 61 consecutive patients who had OSA-related symptoms, including episodes of loud snoring, excessive daytime sleepiness, nocturnal dyspnea, and restless sleep, between April of 2008 and March of 2012. The patients were divided into two groups according to the results of polysomnography (PSG): moderate-to-severe OSA [apnea-hypopnea index (AHI) score ≥15/h; n = 41] and non-to-mild OSA (AHI score <15/h; n = 20). Patients with moderate-to-severe OSA were fitted with a CPAP device during the initial 7 days of treatment to determine whether they would be able to continue CPAP therapy. Some patients refused CPAP therapy because of the discomfort of wearing a mask or because of the positive airway pressure. Thus the patients with moderate-to-severe sleep-disordered breathing (SDB) were further divided into CPAP-treated (n = 21) and non-CPAP-treated (n = 20) groups. Figure 1 shows a flow chart of the study protocol. Patients were excluded from the study if they had any of the following conditions: acute myocardial infarction, acute pneumonia, severe chronic obstructive pulmonary disease, psychotic disorders, pharyngeal disease, poor drug compliance, current smoking status, atrial fibrillation, a history of coronary artery treatment, heart failure, or a history of severe cerebral infarction. Physical characteristics, cardiovascular risk factors, medical histories, and medication data were compared among the groups. PSG data and blood samples for the measurement of high-sensitivity (hs) CRP and PTX3 were collected prior to and after 3 mo of CPAP therapy. Prescriptions, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-II receptor blockers (ARBs), statins, β-blockers, and diuretics were not changed during the follow-up period. All participants provided informed consent prior to enrollment in the study. This study was approved by the clinical research and ethics committee of the University of Akita.
Fig. 1.
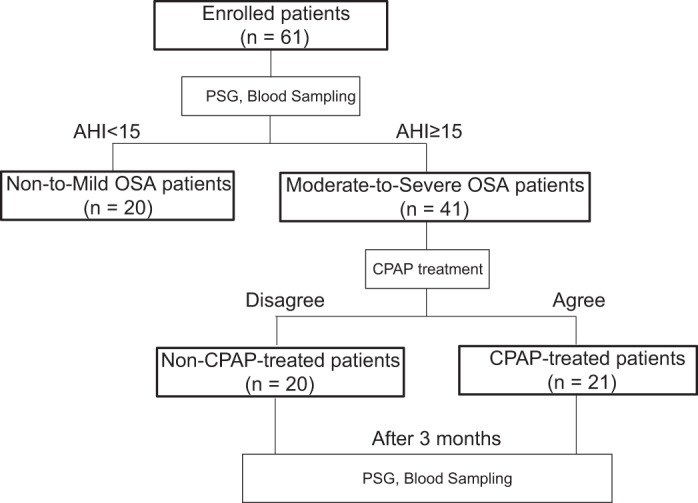
Flow chart of the study protocol. The 61 enrolled patients were divided into two groups according to their polysomnographic results. Patients with moderate-to-severe obstructive sleep apnea (OSA) were fitted with continuous positive airway pressure (CPAP) devices for the initial 7 days of treatment to determine whether they would be able to continue CPAP therapy for the remainder of the study. Some patients refused CPAP treatment because of discomfort; thus the patients with moderate-to-severe OSA were further divided into CPAP-treated and non-CPAP-treated groups. Polysomnographic data and blood samples were obtained prior to and after the 3-mo treatment period. AHI, apnea-hypopnea index.
PSG analyses.
All patients underwent complete overnight PSG using the ProFusion PSG Sleep Diagnostic system (Compumedics, Abbotsford, VIC, Australia), which continuously monitored variables during sleep, including an electroencephalogram, electro-oculogram (used in sleep staging), oxygen saturation (SaO2), airflow, snoring, and thoracoabdominal motion. Apnea was defined as an absence of breathing with no ribcage or abdominal motion for ≥10 s; hypopnea was defined as a ≥30% reduction in monitored airflow accompanied by a ≥4% decrease in SaO2. Arousal responses were defined according to the recommendations of the American Sleep Disorders Association (2). The AHI score was defined as the number of episodes of apnea and hypopnea per hour of sleep. The oxygen desaturation index (ODI) was calculated as the number of SaO2 decreases of ≥4% per hour of sleep. Patients with an AHI score <5/h were diagnosed as non-OSA and those with an AHI score ≥5/h but <15/h were diagnosed with mild OSA. An AHI score ≥15/h was diagnosed as moderate-to-severe OSA. OSA was defined as an AHI score ≥5/h, with ≥50% of the events labeled as obstructive rather than central.
Acquisition of inflammatory biomarkers.
Serum PTX3 levels were measured using an enzyme-linked immunesorbent assay kit (Human PTX3/TSG-14, Perseus Proteomics, Tokyo, Japan) and an automatic analyzer (Kyowa Medics, Tokyo, Japan). Serum hs-CRP levels were measured using an ultra-sensitive latex-enhanced immunoassay (CRP-Latex, Denka Seiken, Tokyo, Japan) with an automatic analyzer (LABOSPECT008, Hitachi-Medical, Tokyo, Japan). Fasting blood samples were acquired at 7:00 AM and 7:00 PM before meals, centrifuged immediately, and stored at −80°C.
CPAP therapy.
CPAP therapy was initiated in patients with moderate-to-severe OSA following a manual titration study to set the appropriate pressure. Compliance data were downloaded from the CPAP device and checked monthly. Patients who could not tolerate CPAP therapy or could not use the CPAP device for >4 h/day during the 3-mo follow-up period were placed in the non-CPAP-treated group.
Statistical analyses.
Continuous variables are expressed as the mean ± SD. Between-group comparisons of continuous and normally distributed data were made using Student's t-test, and the Mann-Whitney U test was used for non-normally distributed data. Categorical variables were compared using the chi-squared test and Yate's correction, as necessary. Correlations were analyzed using Pearson's correlation coefficient; P values <0.05 were deemed statistically significant. A receiver-operator characteristic curve analysis was used to determine the ability of changes in the PTX3 level to predict the improvement of AHI score by CPAP therapy. All statistical tests were conducted using Statistical Package for the Social Sciences version 16 for Windows (SPSS, Chicago, IL).
RESULTS
Baseline characteristics and sleep study data.
The baseline characteristics of the patients with non-to-mild and moderate-to-severe OSA are summarized in Table 1. No patients experienced complications or refused to participate in the sleep study. No significant differences were observed in the mean age, sex ratio, pharmacological treatments (ACEIs, ARBs, β-blockers, diuretics, calcium antagonists, or statins), medical history (history of hypertension, diabetes mellitus, or hyperlipidemia), body mass index (BMI), or total sleep time between the groups. The AHI value, obstructive apnea index, central apnea index, hypopnea index, arousal index, and sleep stage 1 were significantly higher in the moderate-to-severe compared with the non-to-mild OSA group, whereas the mean SaO2 value and sleep stages 3 and 4 were lower in the moderate-to-severe than in the non-to-mild OSA group (non-to-mild 96.1 ± 1.5% vs. moderate-to-severe 94.9 ± 1.5%, P = 0.001).
Table 1.
Baseline characteristics of the enrolled patients (non-to-mild OSA patients versus moderate-to-severe OSA patients)
| Non-to-Mild (n = 20) | Moderate-to-Severe (n = 41) | P value | |
|---|---|---|---|
| Age, yr | 64.6 ± 5.9 | 65.2 ± 7.3 | 0.649 |
| Male sex, n (%) | 17 (85.0) | 37 (90.2) | 0.546 |
| BMI, kg/m2 | 26.5 ± 1.8 | 27.0 ± 0.6 | 0.590 |
| Hypertension, n (%) | 14 (70.0) | 34 (82.9) | 0.247 |
| Dyslipidemia, n (%) | 8 (40.0) | 18 (43.9) | 0.772 |
| Diabetes mellitus, n (%) | 3 (15.0) | 8 (19.5) | 0.667 |
| Smoker, n (%) | 3 (15.0) | 6 (14.6) | 0.969 |
| Medication, n (%) | |||
| ACEIs/ARBs | 10 (50.0) | 26 (63.4) | 0.317 |
| Ca antagonists | 6 (30.0) | 20 (48.8) | 0.164 |
| ß-blockers | 4 (20.0) | 13 (31.7) | 0.338 |
| Statins | 4 (20.0) | 7 (17.1) | 0.780 |
| Polysomnography data | |||
| AHI, n/h | 9.2 ± 3.0 | 41.1 ± 18.0 | <0.001 |
| AI, n/h | 4.8 ± 2.9 | 27.9 ± 20.2 | <0.001 |
| Obstructive, n/h | 3.9 ± 2.1 | 21.3 ± 16.4 | <0.001 |
| Central, n/h | 0.6 ± 0.6 | 3.6 ± 2.9 | <0.001 |
| HI, n/h | 4.4 ± 4.1 | 13.4 ± 8.7 | <0.001 |
| Arousal index, n/h | 16.9 ± 5.2 | 41.4 ± 15.5 | <0.001 |
| Nocturnal SaO2 (%) | |||
| Mean SaO2 (%) | 96.1 ± 1.5 | 94.9 ± 1.5 | 0.001 |
| Minimum SaO2 (%) | 87.8 ± 5.9 | 79.6 ± 9.8 | <0.001 |
| 4% ODI, n/h | 6.0 ± 3.9 | 34.1 ± 20.2 | <0.001 |
| Total sleep time, h | 6.4 ± 1.3 | 6.1 ± 1.0 | 0.255 |
| Sleep stage (%) | |||
| NREM stage 1 | 17.9 ± 9.9 | 32.0 ± 11.8 | <0.001 |
| NREM stage 2 | 57.2 ± 8.8 | 52.6 ± 10.1 | 0.179 |
| NREM stage 3 | 6.9 ± 7.6 | 1.9 ± 2.7 | 0.029 |
| NREM stage 4 | 2.0 ± 3.3 | 0.3 ± 0.8 | 0.038 |
| REM | 15.9 ± 5.0 | 14.5 ± 9.9 | 0.100 |
The values are reported as the means ± SD.
BMI, body mass index; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; AHI, Apnea hypopnea index; AI, apnea index; HI, hypopnea index; ODI, oxygen desaturation index; NREM, nonrapid eye movement sleep; REM, rapid eye movement sleep.
The baseline characteristics of the CPAP-treated and non-CPAP-treated groups are shown in Table 2. The patients in the CPAP-treated group were able to wear the CPAP device for >4 h/day during the 3-mo follow-up period. No significant differences were observed between the groups. Table 3 shows the PSG and BMI data before and after therapy in the CPAP-treated group and the non-CPAP-treated group. The AHI score, obstructive apnea index, central apnea index, hypopnea index, arousal index, and sleep stage 1 were significantly lower, whereas the mean SaO2 and sleep stages 2 and 3 were significantly higher 3 mo after the initiation of CPAP therapy compared with baseline values. We observed no change in BMI following CPAP therapy. In the non-CPAP-treated group, the PSG parameters and BMI data did not change during the 3-mo follow-up period.
Table 2.
Baseline characteristics of the enrolled patients (non-CPAP-treated patients versus CPAP-treated patients)
| Non-CPAP-Treated Patients (n = 20) | CPAP-Treated Patients (n = 21) | P Value | |
|---|---|---|---|
| Age, yr | 65.1 ± 6.7 | 65.3 ± 8.0 | 0.237 |
| Male sex, n (%) | 18 (90.0) | 19 (90.5) | 0.959 |
| BMI, kg/m2 | 27.2 ± 2.3 | 26.7 ± 3.1 | 0.705 |
| Hypertension, n (%) | 18 (90.0) | 16 (76.2) | 0.240 |
| Dyslipidemia, n (%) | 8 (40.0) | 10 (47.6) | 0.623 |
| Diabetes mellitus, n (%) | 4 (20.0) | 4 (19.1) | 0.939 |
| Smoker, n (%) | 3 (15.0) | 3 (14.3) | 0.948 |
| Medication, n (%) | |||
| ACEIs/ARBs | 13 (65.0) | 13 (61.9) | 0.837 |
| Ca antagonists | 10 (50.0) | 10 (47.6) | 0.879 |
| β-blockers | 6 (30.0) | 7 (33.3) | 0.819 |
| Statins | 3 (15.0) | 4 (19.1) | 0.736 |
| Polysomnography data | |||
| AHI, n/h | 41.2 ± 16.5 | 40.9 ± 19.6 | 0.834 |
| AI, n/h | 28.1 ± 20.7 | 27.7 ± 20.2 | 0.418 |
| Obstructive, n/h | 20.8 ± 14.7 | 21.9 ± 18.4 | 0.750 |
| Central, n/h | 3.4 ± 3.5 | 3.7 ± 2.4 | 0.773 |
| HI, n/h | 13.5 ± 8.7 | 13.3 ± 9.1 | 0.725 |
| Arousal index, n/h | 40.7 ± 10.3 | 42.1 ± 19.4 | 0.667 |
| Nocturnal SaO2 (%) | |||
| Mean SaO2 (%) | 94.6 ± 1.7 | 95.1 ± 1.3 | 0.462 |
| Minimum SaO2 (%) | 78.3 ± 11.1 | 80.9 ± 8.5 | 0.539 |
| 4% ODI, n/h | 32.0 ± 16.8 | 36.2 ± 23.3 | 0.784 |
| Total sleep time, h | 6.1 ± 1.1 | 6.0 ± 0.9 | 0.938 |
| Sleep stage (%) | |||
| NREM stage 1 | 33.1 ± 11.2 | 31.0 ± 12.6 | 0.465 |
| NREM stage 2 | 53.2 ± 8.5 | 51.9 ± 11.6 | 0.658 |
| NREM stage 3 | 1.8 ± 2.9 | 2.0 ± 2.7 | 0.432 |
| NREM stage 4 | 0.1 ± 0.5 | 0.4 ± 0.9 | 0.112 |
| REM | 14.1 ± 11.8 | 14.8 ± 8.0 | 0.389 |
The values are reported as the mean ± SD.
Table 3.
Results of the polysomnography and BMI data in the CPAP- and non-CPAP-treated patients
| CPAP-Treated Patients |
Non-CPAP-Treated Patients |
|||||
|---|---|---|---|---|---|---|
| Baseline | After 3 mo | P value | Baseline | After 3 mo | P value | |
| AHI, n/h | 40.9 ± 19.6 | 4.6 ± 4.1 | <0.001 | 41.2 ± 16.6 | 39.0 ± 16.3 | 0.532 |
| AI, n/h | 27.6 ± 20.2 | 2.2 ± 3.6 | <0.001 | 28.1 ± 20.7 | 22.8 ± 14.6 | 0.257 |
| Obstructive, n/h | 21.8 ± 18.4 | 2.1 ± 3.5 | <0.001 | 20.8 ± 14.7 | 15.8 ± 9.1 | 0.111 |
| Central, n/h | 3.7 ± 2.4 | 0.1 ± 0.1 | 0.003 | 3.4 ± 3.5 | 3.7 ± 4.2 | 0.605 |
| HI, n/h | 13.3 ± 9.1 | 2.3 ± 2.4 | <0.001 | 13.6 ± 8.7 | 12.8 ± 6.5 | 0.646 |
| Nocturnal SaO2, (%) | ||||||
| Mean SaO2 (%) | 95.1 ± 1.3 | 97.6 ± 0.9 | <0.001 | 94.6 ± 1.7 | 94.8 ± 2.1 | 0.449 |
| Minimum SaO2 (%) | 80.9 ± 8.5 | 91.4 ± 3.9 | <0.001 | 78.3 ± 11.2 | 77.5 ± 10.7 | 0.214 |
| 4% ODI, n/h | 36.2 ± 23.3 | 6.7 ± 12.2 | <0.001 | 32.0 ± 16.8 | 28.4 ± 13.1 | 0.119 |
| Arousal index, n/h | 42.1 ± 19.4 | 22.3 ± 10.8 | <0.001 | 40.7 ± 10.3 | 37.6 ± 10.1 | 0.264 |
| Sleep stage (%) | ||||||
| NREM stage 1 | 31.0 ± 12.6 | 18.9 ± 8.2 | <0.001 | 33.1 ± 11.2 | 29.5 ± 13.8 | 0.126 |
| NREM stage 2 | 51.9 ± 11.6 | 61.8 ± 12.1 | <0.001 | 53.2 ± 8.5 | 54.1 ± 12.5 | 0.682 |
| NREM stage 3 | 2.0 ± 2.7 | 5.0 ± 6.5 | 0.049 | 1.8 ± 2.9 | 2.8 ± 3.9 | 0.281 |
| NREM stage 4 | 0.4 ± 0.9 | 0.8 ± 3.0 | 0.485 | 0.1 ± 0.5 | 0.2 ± 0.7 | 0.403 |
| REM | 14.8 ± 8.0 | 13.5 ± 4.0 | 0.467 | 14.1 ± 11.8 | 15.7 ± 9.0 | 0.199 |
| BMI, kg/m2 | 26.7 ± 3.1 | 26.5 ± 3.1 | 0.125 | 27.2 ± 2.2 | 27.1 ± 2.6 | 0.641 |
The values are reported as the means ± SD.
Baseline inflammatory biomarker levels.
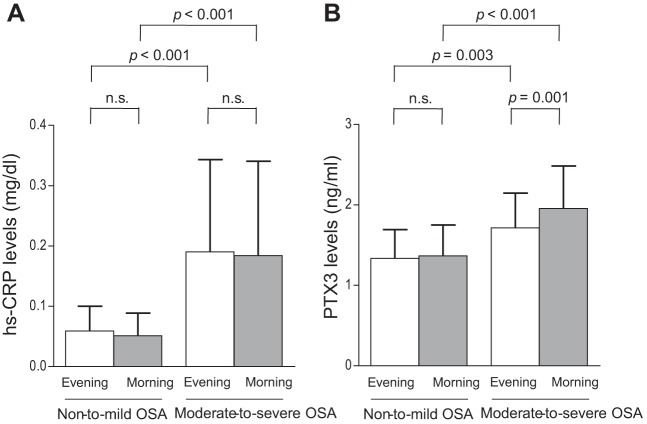
The baseline morning and evening serum hs-CRP levels were significantly higher in patients with moderate-to-severe OSA than in those with non-to-mild OSA (evening moderate-to-severe OSA 0.19 ± 0.15 vs. non-to-mild OSA 0.06 ± 0.04 mg/dl, P < 0.001; morning moderate-to-severe OSA 0.18 ± 0.16 vs. non-to-mild OSA 0.05 ± 0.04 mg/dl, P < 0.001). However, the hs-CRP levels before and after sleep were not significantly different in either patient group (non-to-mild OSA evening 0.06 ± 0.04 vs. morning 0.05 ± 0.04 mg/dl, P = 0.280; moderate-to-severe OSA evening 0.19 ± 0.15 vs. morning 0.18 ± 0.16 mg/dl, P = 0.300; Fig. 2A).
Fig. 2.
Baseline high-sensitivity C-reactive protein (hs-CRP) and pentraxin3 (PTX3) levels in patients with non-to-mild and moderate-to-severe OSA. A: Between- and within-group comparisons of evening and morning serum hs-CRP levels. B: Between- and within-group comparisons of evening and morning serum PTX3 levels.
The baseline evening and morning serum PTX3 levels were higher in patients with moderate-to-severe than in those with non-to-mild OSA (evening moderate-to-severe OSA 1.71 ± 0.44 vs. non-to-mild OSA 1.33 ± 0.36 ng/ml, P = 0.003; morning moderate-to-severe OSA 1.96 ± 0.52 vs. non-to-mild OSA 1.36 ± 0.38 ng/ml, P < 0.001). The serum PTX3 levels before and after sleep did not differ in patients with non-to-mild OSA. However, the morning levels of the biomarker were significantly higher than those in the evening in patients with moderate-to-severe OSA (morning 1.96 ± 0.52 vs. evening 1.71 ± 0.44 ng/ml, P = 0.001; Fig. 2B).
Correlations between PSG data and inflammatory biomarker levels.
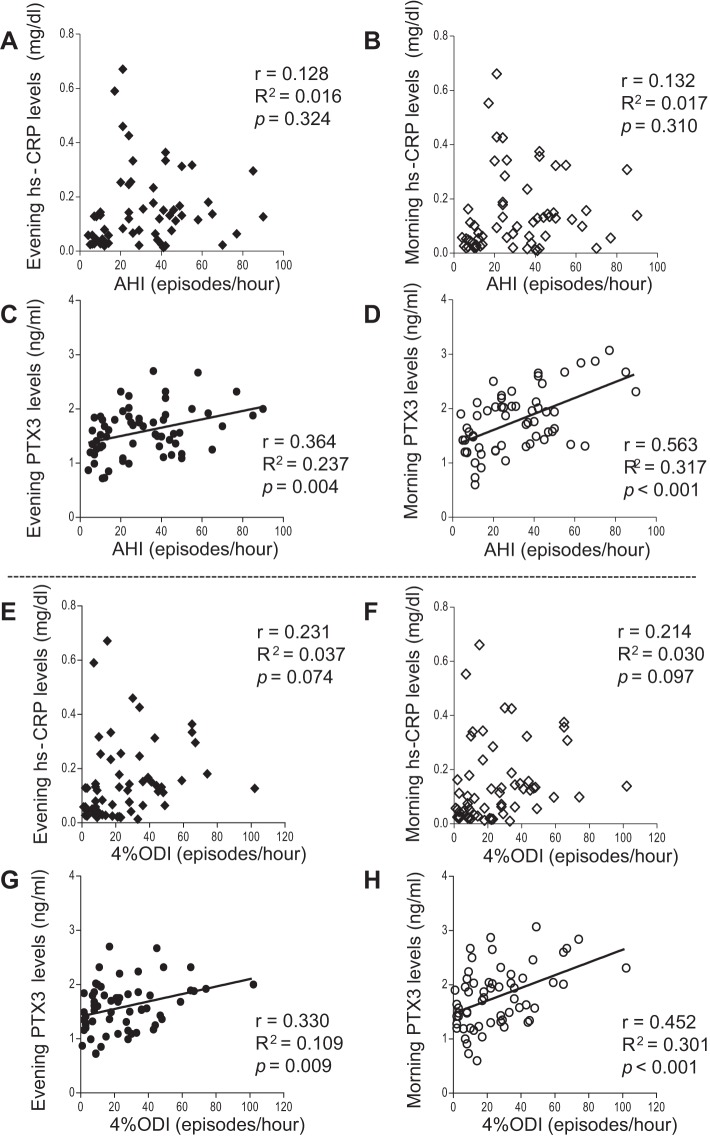
No significant correlation was found between the AHI scores and evening or morning hs-CPR levels (evening r = 0.128, R2 = 0.016, P = 0.324; Fig. 3A; morning r = 0.132, R2 = 0.017, p = 0.310; Fig. 3B). In contrast, a weak correlation between PTX3 in the evening and AHI score was found (evening, r = 0.364, R2 = 0.237, P = 0.004; Fig. 3C). Of great interest was the strong correlation between the AHI score and PTX3 level in the morning (r = 0.563, R2 = 0.317, P < 0.001; Fig. 3D). We also evaluated the relationship between inflammatory biomarkers and 4% ODIs (Fig. 3, E–H). The morning PTX3 levels were significantly correlated to the 4% ODIs (r = 0.452, R2 = 0.301, P < 0.001; Fig. 3H).
Fig. 3.
Correlations between inflammatory biomarker levels and polysomnography data. A: Correlation between evening hs-CRP levels and apnea-hypopnea index (AHI) severity. B: Correlation between morning hs-CRP levels and AHI severity. C: Correlation between evening PTX3 levels and AHI severity. D: Correlation between morning PTX3 levels and AHI severity. E: Correlation between evening hs-CRP levels and the 4% oxygen desaturation index (ODI). F: Correlation between morning hs-CRP levels and the 4% ODI. G: Correlation between evening PTX3 levels and the 4% ODI. H: Correlation between morning PTX3 levels and the 4% ODI.
Changes in inflammatory biomarker levels in the CPAP-treated and non-CPAP-treated groups.
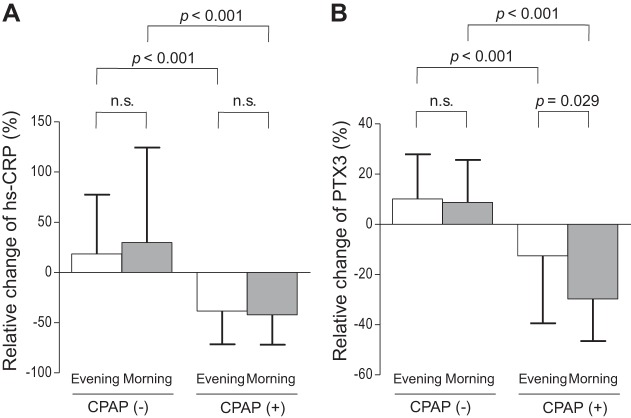
Figure 4A shows the relative changes in serum hs-CRP level for those patients in the CPAP therapy and nontherapy groups before and after 3 mo of treatment. The evening and morning serum hs-CRP levels were significantly reduced in the CPAP-treated group compared with those in the non-CPAP-treated group (evening CPAP −38.3 ± 33.1% vs. non-CPAP 18.3 ± 59.0%, P < 0.001; morning CPAP −42.1 ± 29.8% vs. non-CPAP 29.6 ± 94.5%, P < 0.001). However, the relative changes of the morning and evening hs-CRP serum levels were not significantly different in the CPAP-treated group (morning −42.1 ± 29.8% vs. evening −38.3 ± 33.1%, P = 0.697). Figure 4B shows the changes in the evening and morning PTX3 serum levels before and after 3 mo of treatment. The evening levels of PTX3 were significantly lower in the CPAP group compared with the non-CPAP-treated group (CPAP −12.6 ± 26.8% vs. non-CPAP 10.1 ± 17.9%, P < 0.001). Similarly, the morning PTX3 levels were significantly lower in the CPAP than in the non-CPAP-treated group (CPAP −29.8 ± 16.7% vs. non-CPAP 8.6 ± 16.9%, P < 0.001). Although the relative change in evening PTX3 was significant, the morning level of the protein was more responsive to CPAP therapy (morning −29.8 ± 16.7% vs. evening −12.6 ± 26.8%, P = 0.029).
Fig. 4.
Changes in hs-CRP and PTX3 levels in the CPAP-treated and non-CPAP-treated groups. A: Changes in morning and evening hs-CRP levels before and after 3 mo of CPAP therapy. B: Changes in morning and evening PTX3 levels before and after 3 mo of CPAP therapy.
Correlations between inflammatory biomarker levels and PSG data.
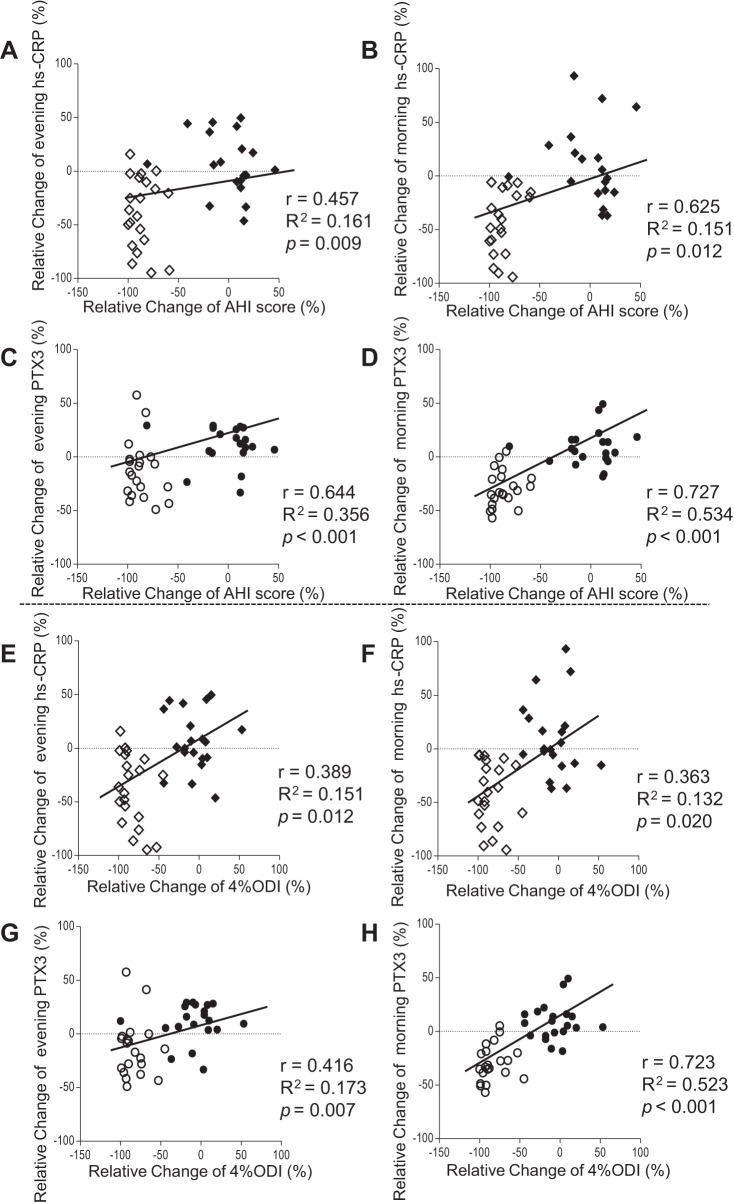
The correlations between the relative changes in inflammatory biomarkers and AHI scores of patients in the CPAP therapy and non-CPAP therapy groups are shown in Fig. 5, A–D. The changes in evening and morning hs-CRP levels had only low correlations with changes in the AHI score (evening, r = 0.457, R2 = 0.161, P = 0.009; morning, r = 0.625, R2 = 0.151, P = 0.012; Fig. 5, A and B). We found a significant correlation between the relative changes in morning and evening PTX3 levels and AHI scores (evening, r = 0.644, R2 = 0.356, P < 0.001; morning, r = 0.727, R2 = 0.534, P < 0.001; Fig. 5, C and D). The correlation coefficient between changes in the morning PTX3 level and the AHI score was greater than that found between evening PTX3 levels and the AHI score (evening r = 0.644 vs. morning r = 0.727). The correlation and coefficient of determinations between the change in morning PTX3 levels and AHI score were higher than those found for the change in morning levels of hs-CRP and AHI score (PTX3 r = 0.727, R2 = 0.534; hs-CRP r = 0.625, R2 = 0.151). Additionally, the correlation coefficient between changes in the morning PTX3 level and the 4% ODI was greater than that found between evening PTX3 levels and the 4% ODI (evening r = 0.416 vs. morning r = 0.723; Fig. 5, G and H). The correlation coefficient between changes in the morning PTX3 level and 4% ODI was significantly higher compared with that found between morning CRP levels and the 4% ODI (PTX3 r = 0.723, R2 = 0.523; hs-CRP r = 0.363, R2 = 0.132; Fig. 5, F and H). Upon a receiver-operator characteristic analysis, the change of morning PTX3 levels of −18.5% was the threshold value for predicting the improvement of AHI scores with 58.6% sensitivity and 98.0% specificity (area under the curve: 0.836, P < 0.001).
Fig. 5.
Correlations between the changes in the levels of inflammatory biomarkers and polysomnography data. A: Correlation between changes in the evening hs-CRP level and AHI score. B: Correlation between changes in the morning hs-CRP level and AHI score. C: Correlation between changes in the evening PTX3 level and AHI score. D: Correlation between changes in the morning PTX3 level and AHI score. E: Correlation between changes in the evening hs-CRP level and the 4% ODI. F: Correlation between changes in the morning hs-CRP level and the 4% ODI. G: Correlation between changes in the evening PTX3 level and the 4% ODI. H: Correlation between changes in the morning PTX3 level and the 4% ODI. ◇, hs-CRP levels in a CPAP-treated patients; ⧫, hs-CRP levels in a non-CPAP-treated patients; ○, PTX3 level in a CPAP-treated patients; ●, PTX3 levels in a non-CPAP-treated patients.
DISCUSSION
Our findings show that the baseline levels of hs-CRP and PTX3 were higher in patients with moderate-to-severe OSA than in those with non-to-mild OSA, and that the serum PTX3 levels, but not hs-CRP levels, were significantly higher after than before sleep in patients with moderate-to-severe OSA. Furthermore, we found that OSA severity as indicated by the AHI and 4% ODIs was significantly correlated with serum PTX3 but not hs-CRP levels. The highest level of correlations was found between the OSA severity and morning PTX3 levels. CPAP therapy reduced serum hs-CRP and PTX3 levels in the evening and morning; however, the greatest reduction was observed in morning PTX3 levels.
OSA is associated with cardiovascular disease (25), and acute coronary syndrome tends to occur during sleep in patients with OSA (12). The induction of vascular inflammation by various pathological events including repetitive hypoxic episodes may underlie the association between OSA and the acute coronary syndrome during sleep (7). However, intraday fluctuations in the vascular inflammatory responses of patients with OSA have not been fully evaluated because of a lack of appropriate vascular-specific and acute phase inflammatory biomarkers. Although serum hs-CRP is a useful biomarker for systemic inflammation, including vascular injuries (21), its long half-life makes hs-CRP an unsuitable choice for the evaluation of intraday differences in inflammation. A previous study found that daytime hs-CRP levels were significantly higher than nighttime levels in patients with OSA (18). Furthermore, we did not find a significant difference between pre- and postsleep levels of serum hs-CRP (Fig. 2A), possibly due to a carryover effect (18). Acute vascular inflammation caused by OSA is postulated to be closely linked to vascular injury, which may lead to plaque rupture and the development of acute coronary syndrome during sleep in patients with OSA (6). Thus the identification of a rapid-response inflammatory biomarker is necessary to assess the inflammation caused by OSA.
PTX3 is an emerging biomarker for vascular inflammation. Kotooka et al. showed that PTX3 reflected the vascular injury induced by coronary stenting in association with an acute inflammatory response (10). Kume et al. showed that PTX3 was a sensitive and specific biomarker for the diagnosis of acute coronary syndrome (11). As PTX3 is released rapidly from various inflammatory cells and endotherial cells (14), the elevation of PTX3 is associated with high-risk plaque components. Therefore, PTX3 measurement is useful for diagnosis of acute vascular inflammation and may be a valuable predictive measure for acute coronary syndrome. In this study, its half-life of ∼1 h (5) allowed us to assess OSA-related acute vascular inflammation during sleep by measuring serum levels before and after sleep in patients with OSA. We found that the baseline serum PTX3 levels were significantly higher in the morning than in the evening in patients with moderate-to-severe OSA (Fig. 2B), whereas the serum PTX3 levels were not significantly different at either time point in patients with non-to-mild OSA (Fig. 2B). Evening and morning PTX3 levels were significantly correlated with OSA severity. In particular, morning PTX3 levels were more highly correlated with the AHI score than evening levels (Fig. 3, C and D), suggesting that PTX3 is an appropriate acute response marker for inflammation caused by OSA. Furthermore, the good correlations between 4% ODIs and morning PTX3 levels, and between the relative changes in 4% ODIs and that of morning PTX3 levels support our hypothesis that the vascular inflammation caused by OSA is mediated in part by hypoxic stress (Fig. 3H). A recent study has shown the hypoxic induction of oxidized low-density lipoprotein and its receptor (15). Vascular endothelial and smooth muscle cells produce copious amounts of PTX3 in response to inflammatory signals including the induction of oxidized low-density lipoproteins under hypoxic conditions (14). Indeed, previous studies showed the increased concentration or enhanced gene expression of PTX3 under hypoxic conditions (20, 23). Moreover, many clinical studies have shown the causative relationship between organ ischemia and increase in PTX3 (11, 13, 17). Therefore, the induction of vascular inflammation by repetitive hypoxic stress caused by upper airway obstructive episodes may increase PTX3.
We found that despite its short half-life, evening PTX3 levels were significantly higher in patients with moderate-to-severe OSA than in those with non-to-mild OSA (Fig. 2B). These results suggest that the vascular inflammation during sleep had a carryover effect and was thus present in the daytime.
CPAP therapy is an effective treatment for patients with OSA and is widely used in clinical practice (16). Previous studies have shown that CPAP therapy reduces sudden cardiac death, decreases the risk of myocardial infarction, and improves the prognosis of patients with cardiovascular disease (1). The various biological reactions caused by upper airway obstruction may also be relieved by CPAP therapy. CPAP therapy has been shown to reduce serum hs-CRP levels (19). Moreover, Kasai et al. (9) showed that CPAP therapy for 1 mo significantly reduced serum PTX3 levels but did not change hs-CRP levels. Our study, in which morning and evening levels of inflammatory biomarkers were measured, found that CPAP therapy for 3 mo reduced evening and morning serum hs-CRP and PTX3 levels and improved nocturnal SaO2 (Fig. 4, A and B, and Table 2). Interestingly, the improvement in morning PTX3 levels was more evident than in evening PTX3 levels in the CPAP-treated group (Fig. 4B), indicating that CPAP therapy could prevent vascular injury and atherosclerotic responses in patients with OSA (8). In contrast to hs-CRP, the correlation between the change in AHI score and morning PTX3 levels was higher than that for the evening levels (Fig. 5, C and D). Furthermore, the correlation between the change in the 4% ODI and the morning PTX3 levels was greater than that between the 4% ODI and the evening PTX3 levels (Fig. 5, G and H). These results suggest that morning levels of PTX3 are a more sensitive marker for OSA-related acute inflammation in patients with OSA than evening levels.
Our study has some limitations. The pathogenesis of vascular inflammation in OSA patients seems to be a multifactorial process. Increase in sympathetic nerve activity, activated RAS, large intrathoracic pressure swings, transient blood pressure surges, and arousal responses as well as hypoxic stress are involved in the elevation of serum PTX3 levels. Moreover, obesity could be associated with the increase of PTX3 levels. Thus we could not clarify the detailed mechanism underlying the OSA-related acute inflammation. The observational nature of the study design did not allow us to draw conclusions about causal relationships, and our sample size was small. Further study is needed to investigate other inflammatory biomarkers, including cytokines, to clarify the mechanisms underlying acute vascular inflammation in patients with OSA.
In conclusion, morning PTX3 levels reflect OSA-related acute inflammation and are a useful marker for improvement in OSA following CPAP therapy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.K., T.K., and H.W. conceived and designed the research; Y.K. and T.K. performed experiments; Y.K., T.K., and H.W. analyzed data; Y.K., T.K., and H.W. interpreted results of experiments; Y.K. and T.K. prepared figures; Y.K. and T.K. drafted manuscript; T.K., H.W., and H.I. edited and revised manuscript; H.W. and H.I. approved final version of manuscript.
REFERENCES
- 1.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M., Martínez-Alonso M., Carmona C., Barceló A., Chiner E., Masa J.F, Gonzalez M, Marín JM, Garcia-Rio F, Diaz de Atauri J, Terán J, Mayos M, de la Peña M, Monasterio C, del Campo F, Montserrat JM. Spanish sleep and breathing network effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307(20):2161–2168, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet M, Carley D, Carskadon M, Easton P, Guilleminault C, Harper R, Hayes B, Hirshkowitz M, Ktonas P, Keenan S, Pressman M, Roehrs T, Smith J, Walsh J, Weber S, Westbrook P. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 174–184, 1992. [PubMed] [Google Scholar]
- 3.Butt M, Dwivedi G, Khair O, Lip GY. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol 139: 7–16, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Caixeta A, Stone GW, Mehran R, Lee EA, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Ohman EM, Palmerini T, Syros G, Kittas C, Fahy M, Hooper WC, Lansky AJ, Dangas GD. Predictive value of C-reactive protein on 30-day and 1-year mortality in acutecoronary syndromes: an analysis from the ACUITY trial. J Thromb Thrombolysis 31: 154–164, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A. Regulation of leukocyterecruitment by the long pentrax in PTX3. Nat Immunol 11: 328–334, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 140: 534–542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH. Vascular inflammation in obesity and sleep apnea. Circulation 121: 1014–1021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117: 2270–2278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasai T, Inoue K, Kumagai T, Kato M, Kawana F, Sagara M, Ishiwata S, Ohno M, Yamaguchi T, Momomura S, Narui K. Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens 24: 401–407, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Kotooka N, Inoue T, Fujimatsu D, Morooka T, Hashimoto S, Hikichi Y, Uchida T, Sugiyama A, Node K. Pentraxin3 is a novel marker for stent-induced inflammation and neointimal thickening. Atherosclerosis 197: 368–374, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Kume N, Mitsuoka H, Hayashida K, Tanaka M. Pentraxin 3 as a biomarker for acute coronary syndrome: comparison with biomarkers for cardiac damage. J Cardiol 58: 38–45, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am CollCardiol 52: 343–346, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A, Lipid Assessment Trial Italian Network (LATIN) Investigators. Prognostic significance of the long pentraxinPTX3 in acute myocardial infarction. Circulation 110: 2349–2354, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez de la Torre Y, Latini R. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol 45: 326–330, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Mao X, Xie L, Greenberg DA. LOX-1 expression and oxidized LDL uptake and toxicity in the HN33 neuronal cell line. Neurosci Lett 580: 182–185, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Matsui S, Ishii J, Kitagawa F, Kuno A, Hattori K, Ishikawa M, Okumura M, Kan S, Nakano T, Naruse H, Tanaka I, Nomura M, Hishida H, Ozaki Y. Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis 210: 220–225, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Mills PJ, Natarajan L, von Känel R, Ancoli-Israel S, Dimsdale JE. Diurnal variability of C-reactive protein in obstructive sleep apnea. Sleep Breath 13: 415–420, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patruno V, Aiolfi S, Costantino G, Murgia R, Selmi C, Malliani A, Montano N. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest 131: 1393–1399, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Sciacca P, Betta P, Mattia C, Li Volti G, Frigiola A, Curreri S, Amato M, Distefano G. Pentraxin-3 in late-preterm newborns with hypoxic respiratory failure. Front Biosci(Elite Ed) 2: 805–809, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163: 19–25, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105: 2462–2464, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Tafani M, Russo A, Di Vito M, Sale P, Pellegrini L, Schito L, Gentileschi S, Bracaglia R, Marandino F, Garaci E, Russo MA. Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment. Cancer Sci 101: 1014–1023, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terada S, Koyama T, Watanabe H, Makabe S, Igarashi G, Seki K, Ito H. Abnormal coagulation and platelet profile in patients with obstructive sleep apnea syndrome. Int J Cardiol 146: 423–425, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Trombetta IC, Somers VK, Maki-Nunes C, Drager LF, Toschi-Dias E, Alves MJ, Fraga RF, Rondon MU, Bechara MG, Lorenzi-Filho G, Negrão CE. Consequences of comorbid sleep apnea in the metabolic syndrome—implications for cardiovascular risk. Sleep 33: 1193–1199, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]