Abstract
The present study compared peak muscle deoxygenation ([HHb]peak) responses at three quadriceps sites during occlusion (OCC), ramp incremental (RI), severe- (SVR) and moderate-intensity (MOD) exercise. Seven healthy men (25 ± 4 yr) each completed a stationary cycling RI (20 W/min) test to determine [HHb]peak [at distal and proximal vastus lateralis (VLD and VLP) and rectus femoris (RF)], peak V̇o2 (V̇o2peak), gas exchange threshold (GET), and peak work rate (WRpeak). Subjects also completed MOD (WR = 80% GET) and SVR exercise (WR corresponding to 120% V̇o2peak) with absolute [HHb] (quantified by multichannel, time-resolved near-infrared spectroscopy) and pulmonary VO2 (V̇o2p) monitored continuously. Additionally, [HHb] and total hemoglobin ([Hb]tot) were monitored at rest and during subsequent OCC (250 mmHg). Site-specific adipose tissue thickness was assessed (B-mode ultrasound), and its relationship with resting [Hb]tot was used to correct absolute [HHb]. For VLD and RF, [HHb]peak was higher (P < 0.05) during OCC (VLD = 111 ± 38, RF = 114 ± 26 μM) than RI (VLD 64 ± 14, RF = 85 ± 20) and SVR (VLD = 63 ± 13, RF = 81 ± 18). [HHb]peak was similar (P > 0.05) across these conditions at the VLP (OCC = 67 ± 17, RI = 69 ± 17, SVR = 63 ± 17 μM). [HHb] peaked and then decreased prior to exercise cessation during SVR at all three muscle sites. [HHb]peak during MOD was consistently lower than other conditions at all sites. A “[HHb] reserve” exists during intense cycling at the VLD and RF, likely implying either sufficient blood flow to meet oxidative demands or insufficient diffusion time for complete equilibration. In VLP this [HHb] reserve was absent, suggesting that a critical Po2 may be challenged during intense cycling.
Keywords: near-infrared spectroscopy, muscle deoxygenation, incremental exercise
increases in exercise intensity are generally accompanied by predictable increases in the rate of O2 consumption (V̇o2) until the point of maximal VO2 (V̇o2 max) has been reached (22); at this point, a failure of either the O2 delivery system or the muscles' inability to utilize available O2 prevents further increases in V̇o2 and eventually, fatigue ensues. As such, the balance between local muscle O2 availability and O2 utilization may be a key determinant of exercise capacity. This O2 availability-utilization balance can be assessed noninvasively at rest and during exercise by considering changes in near-infrared spectroscopy (NIRS)-derived muscle deoxygenation ([HHb]). To this end, several studies have examined the kinetic profile of [HHb] during incremental cycling exercise (7, 8, 14, 17, 19, 21, 29–31, 35), and a near-plateau (30, 31, 35) has been described during the final moments of very intense exercise. Importantly, however, it has previously been shown that the peak [HHb] value (reflecting the peak fractional O2 extraction) observed during dynamic, rhythmic exercise (i.e., cycling) is significantly less than that elicited during ischemic occlusion (OCC) at rest in healthy individuals (20). Resting OCC has been used to elicit a plateau in the [HHb] response (14, 26), which, presumably, suggests that maximal [HHb] (and O2 extraction) had been achieved. Based on these findings, the peak [HHb] from an incremental cycling test should not necessarily be considered a true maximal value, and an untapped “O2 extraction reserve” is suggested; that is, the venous O2 content during OCC will be lower than that observed at peak intensity during incremental cycling exercise.
Several studies have compared both the dynamic (i.e., kinetic) and peak V̇o2 (V̇o2peak) responses from incremental tests with values obtained during constant load exercise at so-called “supramaximal” intensities [i.e., severe intensity (SVR)], usually ranging from 105 to 120% of the peak work rate (WR) attained during incremental tests (1, 3, 4, 23, 33). Whereas the V̇o2peak is often similar under these conditions, it remains unclear from these studies whether the peak [HHb] during SVR exceeds that witnessed during “maximal” work (i.e., highest WR attained during incremental testing); that is, to date no study has examined whether a proposed O2 extraction reserve might be accessed during SVR cycling. Although Adami et al. (1) measured and reported [HHb] under both conditions, it is unclear whether peak values differed in their study (see Table 1 therein).
Table 1.
Parameter estimates for double-linear and sigmoid regression models of the [HHb] profile during RI as a function of %WR
| VLD | VLP | RF | ||
|---|---|---|---|---|
| Double-linear | m1 | 0.38 ± 0.19 | 0.37 ± 0.26 | 0.29 ± 0.27 |
| b1 | 28.5 ± 7.1 | 35.7 ± 8.3* | 44.3 ± 12.7* | |
| m2 | −0.10 ± 0.33 | 0.12 ± 0.14 | 0.71 ± 0.44*# | |
| b2 | 69.7 ± 38.1 | 56.6 ± 22.3 | 12.6 ± 27.6*# | |
| BP | 80.7 ± 10.3 | 78.1 ± 10.2 | 73.6 ± 19.0 | |
| R2 | 0.91 ± 0.04 | 0.91 ± 0.10 | 0.90 ± 0.04 | |
| Sigmoid | f0 | 30.5 ± 5.2 | 32.5 ± 10.2 | 43.1 ± 15.6 |
| A | 57.6 ± 71.0 | 64.2 ± 72.3 | 322.1 ± 431.3 | |
| c | 2.96 ± 0.51 | 2.11 ± 0.41* | 4.05 ± 1.84# | |
| d | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02* | |
| c/d | 73.4 ± 63.7 | 74.5 ± 68.7 | 136.1 ± 80.6*# | |
| R2 | 0.93 ± 0.04§ | 0.93 ± 0.07† | 0.91 ± 0.05 |
Values are means ± SD. m1 and m2 = slope of linear regression before and after the breakpoint (BP), respectively; b1 and b2 = y-intercept of linear regression before and after BP, respectively; R2 = coefficient of determination; f0 = baseline (i.e., 20 W) [HHb]; A = [HHb] amplitude; d = slope of sigmoid regression; c = constant dependent upon d where: c/d = x-value corresponding to 50% of A. Significantly different (P < 0.05) from:
VLD,
VLP,
Double-linear. Trend (P = 0.05) for difference from:
double-linear.
[HHb], muscle deoxygenation; RI, ramp incremental exercise; WR, work rate; VLD, distal vastus lateralis; VLP, proximal vastus lateralis.
Dynamic heterogeneities in the [HHb] responses throughout the quadriceps complex have been described during both constant load (25, 26) and ramp incremental (RI) exercise (14). Such heterogeneities persist even after correction for subcutaneous adipose tissue thickness (ATT), which has been shown to affect the NIRS signal quality (14, 26). Given that [HHb] responses do not appear to be uniform across the quadriceps muscles, it is reasonable to question whether SVR responses may also differ, both in terms of intersite comparisons during exercise (i.e., dynamic heterogeneities) as well as intrasite comparisons with maximal exercise, submaximal exercise, and OCC. Therefore, the purpose of the present paper was to compare ATT-corrected [HHb] responses at three quadriceps muscle sites during RI and constant-load SVR and moderate-intensity (MOD) exercise, as well as during OCC.
METHODS
Participants.
Seven young, healthy men (25 ± 4 yr, 1.78 ± 0.02 m, 64 ± 9 kg; mean ± SD) provided written informed consent to participate in the study after the experimental protocol, including possible risks and benefits associated with the testing procedures, was explained. All volunteers were nonsmokers; were free of known respiratory, cardiovascular, and metabolic diseases; and were involved in regular exercise (ranging from recreational activity to amateur competitive sport). The study was approved by the Human Subjects Committee of Kobe Design University, in accordance with the Declaration of Helsinki.
Protocol.
On day one, participants reported to the laboratory to perform an RI test (20 W/min) to the limit of tolerance on a cycle ergometer (Combi124 232C, Tokyo, Japan) for determination of peak O2 uptake (V̇o2peak) and the gas exchange threshold (GET). The ramp portion of the protocol was initiated following 4 min of cycling at 20 W. Subjects were instructed to maintain a pedal cadence of 60 rpm; tests were terminated when pedal cadence could not be maintained above ∼50 rpm, despite strong verbal encouragement. Following the ramp portion of the RI test, subjects completed 6 min of recovery cycling at 20 W. GET was determined by visual inspection as the VO2 at which CO2 output (V̇co2) began to increase out of proportion in relation to VO2, with a systematic rise in minute ventilation-to-V̇o2 ratio and end-tidal Po2, whereas minute ventilation-to-V̇co2 ratio and end-tidal Pco2 were stable (6, 36).
From the RI tests, both an MOD and an SVR exercise WR were identified for each subject. The MOD WR was selected to elicit a V̇o2 corresponding to ∼80% of the VO2 at GET; the SVR WR corresponded to 120% of the WR at which V̇o2peak was attained [i.e., after accounting for the pulmonary V̇o2 (V̇o2p) mean response time as previously described (15)]. Subsequent to the RI test, and on separate days, subjects completed: 1) three successive step-transitions (34) from a 6 min baseline WR of 20 W to 6 min at the calculated MOD WR (i.e., 36 min of continuous cycling) and 2) a single step-transition from a 6 min baseline WR of 20 W to the calculated SVR WR. As with the RI test, SVR tests were terminated when pedal cadence could not be maintained above ∼50 rpm, despite strong verbal encouragement, and the SVR portion of the test was followed by 6 min of recovery cycling at 20 W. Subjects' time to fatigue (TTF) was recorded for the SVR exercise bout. The MOD and SVR tests were completed in a randomized order. As with RI tests, subjects were encouraged to maintain a pedal cadence of ∼60 rpm during step exercise.
In addition to the exercise testing, subjects also underwent an ischemic OCC of their right quadriceps as described previously (14). Briefly, subjects were seated in an upright position, with a pressure cuff placed around the upper thigh of the right leg. Following 2 min of seated rest, the cuff was immediately inflated (E-20 Rapid Cuff Inflator; Hokanson, Bellevue, WA) to and held at 250 mmHg, for a duration sufficient to elicit a near-plateau in the muscle deoxygenation signals (see below for details); subjects remained relaxed throughout OCC.
Each test was conducted at the same time of day for each subject, with a minimum of 24 h between laboratory visits. Subjects were advised to be postprandial (2–3 h) and to refrain from strenuous activity (24 h), caffeine (3 h), and alcohol consumption (48 h) prior to testing.
Equipment and measurements.
All tests were performed in a climate-controlled laboratory, where the ambient temperature, relative humidity, and atmospheric pressure remained constant at ∼22°C, ∼50%, and ∼750 mmHg, respectively. Exercise was performed on an electromagnetically braked cycle ergometer, with power output and cadence recorded continuously by computer. Heart rate (HR) was measured every 5 s by short-range radio telemetry (Polar RS800CX N; Polar Electro Japan, Tokyo, Japan).
Pulmonary gas exchange was measured breath by breath with a flow meter and gas analyzer system (Aeromonitor AE-300S; Minato Medical Science, Osaka, Japan). Inspiratory and expiratory flows were determined by a low-resistance, hot-wire flow meter. Respired gases were sampled continuously from a mouthpiece and analyzed by paramagnetic O2 and infrared CO2 analyzers. Gas concentrations and volume signals were digitized every 13 ms and time aligned for breath-by-breath calculation of alveolar gas exchange and ventilatory variables with established algorithms (5). The system was calibrated before each experiment with precision-analyzed gas mixtures and a 2-liter syringe over a range of flow rates (Minato Medical Science).
Absolute skeletal muscle deoxygenation of the right quadriceps was quantified by multichannel, time-resolved near-infrared spectroscopy (TRS-NIRS; TRS-20, Hamamatsu Photonics KK, Hamamatsu, Japan) at three muscle sites: the distal (VLD, 13 ± 1 cm proximal to patella) and proximal (VLP, 23 ± 2 cm proximal to patella) regions of the vastus lateralis and at the midregion of the rectus femoris (RF, 21 ± 3 cm proximal to patella). The measurement sites on the quadriceps were shaved and cleaned with alcohol before each experiment. Measurement sites were also marked on the skin with permanent ink to ensure consistent probe positioning throughout the study. Each TRS-20 probe, consisting of a detector fixed at 3 cm from the emitter, was secured to the skin by double-sided adhesive tape and enclosed within a black rubber casing. Picosecond light pulses (repetition frequency of 5 MHz at a full width at half-maximum of 100 picoseconds) were provided at three different wavelengths (760, 795, and 830 nm) to measure absolute muscle deoxygenation {[deoxy(Hb+Mb)]; [HHb]}, oxygenation {[oxy(Hb+Mb)]; [HbO2]}, and total hemoglobin ([Hb+Mb], [Hbtot]), each sampled at 0.5 Hz. Temporal light intensity profiles at each measurement site were fitted with a photon diffusion equation (32) for estimation of mean optical path-length, and scattering and absorption coefficients allowing quantification of NIRS chromophores in micromoles (see also Refs. 14, 24, 26). Before each test, the equipment was calibrated in line with the manufacturer's recommendations.
To account for the influence of subcutaneous ATT on the NIRS signals (14, 26), we utilized a correction factor based on the relationship between [Hbtot] and ATT as described by Bowen et al. (9). For this, subjects reported to the laboratory in a rested state and were seated in an upright position (i.e., in a similar position to that during cycling) to determine the ATT at each of the three muscle sites where NIRS probes were placed; the ATT (in mm) was determined with B-mode Doppler ultrasound (Logiq 400; GE-Yokogawa Medical Systems, Tokyo, Japan). Resting [Hbtot] at each muscle site was calculated from the 2 min resting measure prior to OCC. The [Hbtot]-ATT correction method was based on the NIRS signal being derived predominantly from the muscle and skin (assuming minimal signal from Hb and Mb in adipose tissue), such that the attenuation in the [Hbtot] signal should be proportional to the volume of adipose within the sample, represented by the ATT. This assumption was supported by the demonstration of a strong and inverse correlation between resting [Hbtot] and ATT (R2 = 0.64, P < 0.05, Fig. 1).
Fig. 1.
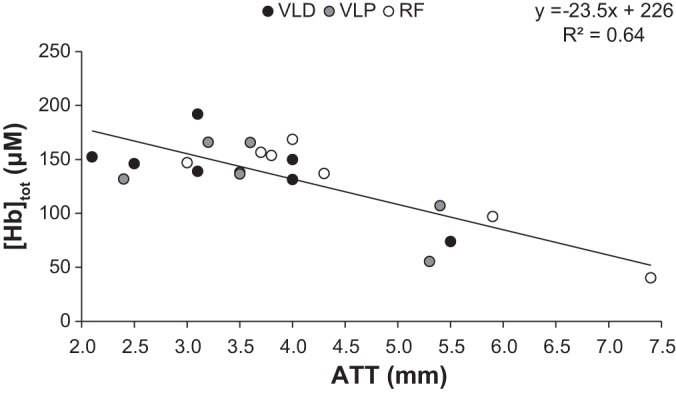
Relationship between subcutaneous adipose tissue thickness (ATT) and time-resolved near-infrared spectroscopy (TRS-NIRS)-derived resting total hemoglobin concentration ([Hbtot]) in the distal (VLD) and proximal (VLP) vastus lateralis and rectus femoris (RF) in 7 subjects. The linear regression (R2 = 0.64, P < 0.05) was used to normalize the absolute muscle deoxygenation responses during exercise and occlusion between muscle sites to an ATT of 0.
ATT was similar (P > 0.05) between VLD (3.5 ± 1.1 mm) and VLP (3.8 ± 1.1 mm) but lower than RF (4.6 ± 1.5 mm). Thus, to normalize the main variable of interest, [HHb], to an ATT of 0 mm, we applied a linear regression to the relationship between [Hbtot] and ATT and solved this in each subject using the measured ATT at each individual site: [Hbtot] = −23.5·(ATT) + 226 (Fig. 1). Absolute [HHb] responses between subjects and muscle sites were then normalized to an ATT of 0 mm by: 1) taking the ratio of the measured [Hbtot] at the given ATT and the [Hbtot] at 0 mm ATT (i.e., 226 μM) (both derived from the linear regression), which provided a correction factor, and then 2) multiplying this correction factor by the raw [HHb] signal (9). This approach to ATT correction differs from some earlier studies from our laboratory (14, 26); however, in those studies, the subject-specific corrections were generated by regressing ATT data and the slope of the [HHb] profile during OCC across multiple (i.e., 4) sites. In the present study, only three sites were measured; this is consistent with Bowen et al. (9). All references to [HHb] hereafter represent ATT-corrected values unless otherwise stated.
Data analysis.
Peak values for [HHb], V̇o2p, and HR were given as the highest continuous 20 s average during RI, SVR, and MOD; peak [HHb] values were calculated in a similar manner during OCC. Note that the peak [HHb] value did not necessarily occur at end-exercise in all conditions. We edited breath-by-breath V̇o2p data from the MOD and SVR transitions by removing aberrant data points that lay outside 4 SD of the local mean; the justification for this editing process was provided by Lamarra et al. (28), who demonstrated that “noise” observed within the V̇o2p signal conformed to a predictable Gaussian distribution, independent of WR. The data for each transition were linearly interpolated to 1 s intervals. The ensemble-averaged V̇o2p and [HHb] data were then further time-averaged into 5 s bins.
The [HHb] profile has been described to consist of a time delay at the onset of exercise, followed by an increase in the signal with an “exponential-like” time course (16); as such, the [HHb] responses were modeled with the following equation:
| (1) |
where Y(t) represents the [HHb] for any given time; YB is the baseline value; A is the amplitude of the response; t is a given amount of time; τ represents the time required to attain 63% of the steady-state amplitude; and TD represents the mathematical time delay. The physiologically calculated time delay for the [HHb] response (TD [HHb]) during both MOD and SVR was determined using second-by-second data and corresponded to the time, after the onset of exercise, at which the [HHb] signal began a systematic increase from its nadir value. Determination of the TD [HHb] was made on individual trials and averaged to yield a single value for each individual. For MOD, the [HHb] data were modeled from the end of the TD [HHb] to 90 s of the transition using an exponential model as described in Eq. 1; for SVR, [HHb] data were modeled from the end of the TD [HHb] to 90 s or to the end of exercise for those subjects unable to complete 90 s; in one subject, the dramatic drop in [HHb] prior to end-exercise led us to model the response for only the first 70 s. As previously described by duManoir et al. (18), different fitting strategies (i.e., 90–180 s) resulted in minimal differences (< 2 s) in estimates of the [HHb] time constant (τ[HHb]), and the early exponential increase in [HHb] was well characterized in the 90 s following exercise onset. The τ[HHb] described the time course for the increase in [HHb], whereas the overall change of the effective [HHb] (τ′[HHb] = TD [HHb] + τ[HHb]) described the overall time course of the [HHb] from the onset of exercise.
Since the duration of the RI, SVR, and OCC tests varied from subject to subject, physiological response profiles were averaged in the time domain such that each subject's responses comprised an equal number of data points (i.e., n = 101 for RI and OCC; n = 37 for SVR because of shorter duration). This approach, which has been used previously (35), was employed to directly compare individual subject responses and to generate an overall grand mean response.
It was previously reported that the profile of normalized (i.e., 0–100%) [HHb] (plotted as a function of relative WR; %WR) during RI is better described by a piecewise equation that includes two linear segments [hereafter referred to as “double-linear” as described by Spencer et al. (35)] compared with a symmetrical sigmoid function previously proposed (7, 8, 14, 17, 19, 21, 29); however, this has not been confirmed by TRS-NIRS. Thus, both the sigmoid and double-linear functions were tested and compared in the present study. First, the entire [HHb] response was modeled from the onset of ramp exercise until exercise cessation with the following sigmoid function:
| (2) |
where f0 represents the baseline [HHb]; A is the amplitude of the response, d is the slope of the sigmoid, c is a constant that is dependent on d where c/d is the x value corresponding to 50% of the total amplitude. Second, the double-linear model characterizes the predominant increase in [HHb] observed throughout the middle portion of the exercise protocol (beginning at the point where the [HHb] signal began a systematic increase above baseline as determined by visual inspection) and the “near-plateau” that follows. The equation used to fit two intersecting lines is:
| (3) |
where y1 is defined by the slope (m1) and intercept (b1) of the first line segment; the intercept {b2, substituted in Eq. 3 with “(m1*BP) + b1]”} of the second component is derived from the model parameter estimates and is given as the y value of the first line segment at the breakpoint (BP). Thus, y2 is given by b2 and the slope (m2) from the BP to the end of exercise. The overall function defines y for the full range of x values, where, if x < BP, then y = y1; otherwise, y = y2. The model parameters were estimated by least-squares linear and nonlinear regression (Origin; OriginLab, Northampton, MA) in which the best fit was defined by minimization of the residual sum of squares and minimal variation of residuals around the y-axis (y = 0); the number of iterations performed was ≤100.
Statistical analyses.
Descriptive data are presented as means ± SD. Comparisons of peak V̇o2p and HR across exercise interventions (i.e., RI, SVR, MOD), as well as V̇o2p, HR, and [HHb] kinetics parameters during MOD and SVR, were made by one-way repeated-measures ANOVA; comparisons of peak [HHb] were analyzed by two-way (muscle region * intervention) repeated-measures ANOVA. Tukey's post hoc analyses were used where appropriate. The possible effect of spatial heterogeneity on [HHb] was determined by calculating the coefficient of variation [CV (%) = 100*SD/mean of 3 muscle regions] for each subject; this was assessed for both the periods before and during the interventions as well as during the 6 min recovery cycling following RI and SVR. The sigmoid and double-linear models were compared by computing the change in corrected Akaike information criterion (2, 10) scores (ΔAICC); see Spencer et al. (35). Pearson's product-moment correlation coefficients were used to quantify the strength of relationships between variables. All statistical analyses were performed with SPSS version 20.0 (SPSS, Chicago, IL). Statistical significance was accepted at P < 0.05.
RESULTS
During RI exercise, subjects attained a peak WR of 306 ± 44 W; this yielded a V̇o2peak of 3.4 ± 0.5 l/min (53.7 ± 10.2 ml·kg−1·min−1) and a GET of 2.0 ± 0.4 l/min. Both an MOD (117 ± 30 W, 38 ± 7% of peak from RI) and an SVR (337 ± 49 W, 110 ± 2% of peak from RI) WR were prescribed based upon the results of the RI test. Group mean responses for V̇o2p, HR, and [HHb] (as a function of %WR) during RI are illustrated in Fig. 2. Table 1 includes parameter estimates from the double-linear and sigmoid regressions of [HHb] at the VLD, VLP, and RF. Although the sigmoid regression models yielded similar (if not higher, see Table 1) coefficients of determination (i.e., R2), based upon lower AICC scores in 20/21 (i.e., >95%) of individual cases, the double-linear function was preferred over the sigmoid model. Accordingly, the double-linear regression models (i.e., derived from Table 1) are superimposed on the data in Fig. 2. Both the initial slope of increase in [HHb] (i.e., m1) and the BP were similar (P > 0.05) across quadriceps measurement sites. Whereas the slope after BP (i.e., m2) was near zero for VLD and VLP (P > 0.05 between sites and from m2 = 0), it was greater (P < 0.05 from VLD, VLP, m2 = 0, and m1) in the RF (Table 1, Fig. 2); thus, [HHb] does not appear to plateau during RI but, rather, accelerates as a function of %WR in the RF.
Fig. 2.
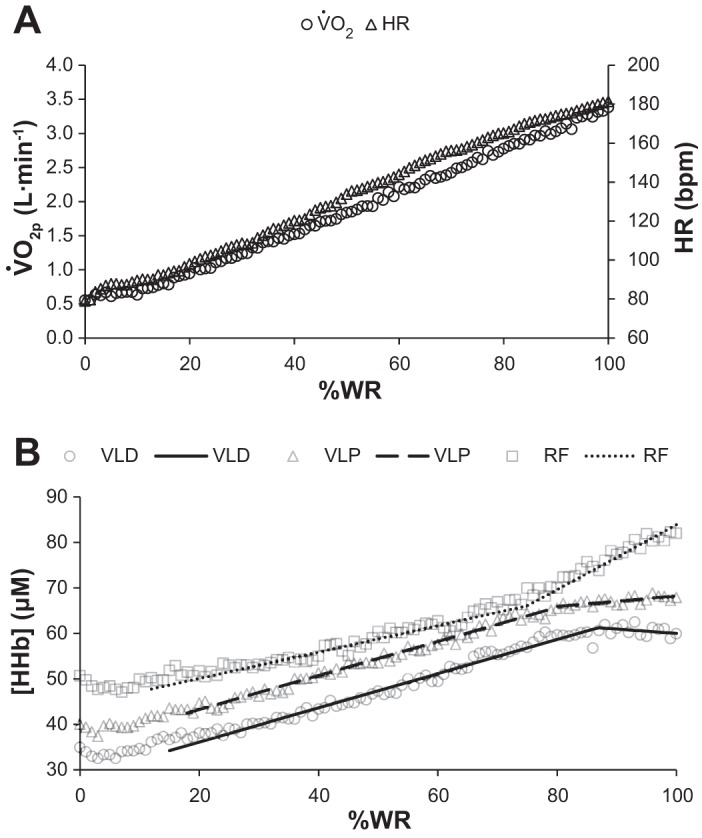
Group mean pulmonary O2 consumption (V̇o2p), heart rate (HR) (A), and muscle dexoygenation ([HHb]) (B) responses as a function of relative work rate (%WR) during ramp incremental exercise. Double-linear regression models (derived from Table 1) of [HHb] responses are superimposed.
During SVR exercise, the TTF was 111 ± 28 s. The V̇o2peak observed during SVR was 3.1 ± 0.3 l/min, which was less than the V̇o2peak observed during RI (ΔV̇o2 = 0.3 ± 0.3 l/min, P < 0.05). There was a strong association (r = 0.84, P < 0.05) between subjects' TTF and the percentage of RI V̇o2peak attained during SVR [i.e., (SVR V̇o2peak/RI V̇o2peak) * 100]. Whereas the V̇o2p response progressively increased until the end of SVR (Fig. 3), [HHb] at all three muscle sites appeared to reach an apex (VLD: 38 ± 13 s = VLP: 52 ± 16 s < RF: 96 ± 32 s) and then began to fall before the end of exercise {i.e., peak [HHb] was greater (P < 0.05) than end-exercise at all 3 sites}. [HHb] kinetics parameter estimates for SVR are presented in Table 2. HR rose continuously until the end of exercise, peaking at 166 ± 17 beats/min (bpm) (P = 0.08 from RI).
Fig. 3.
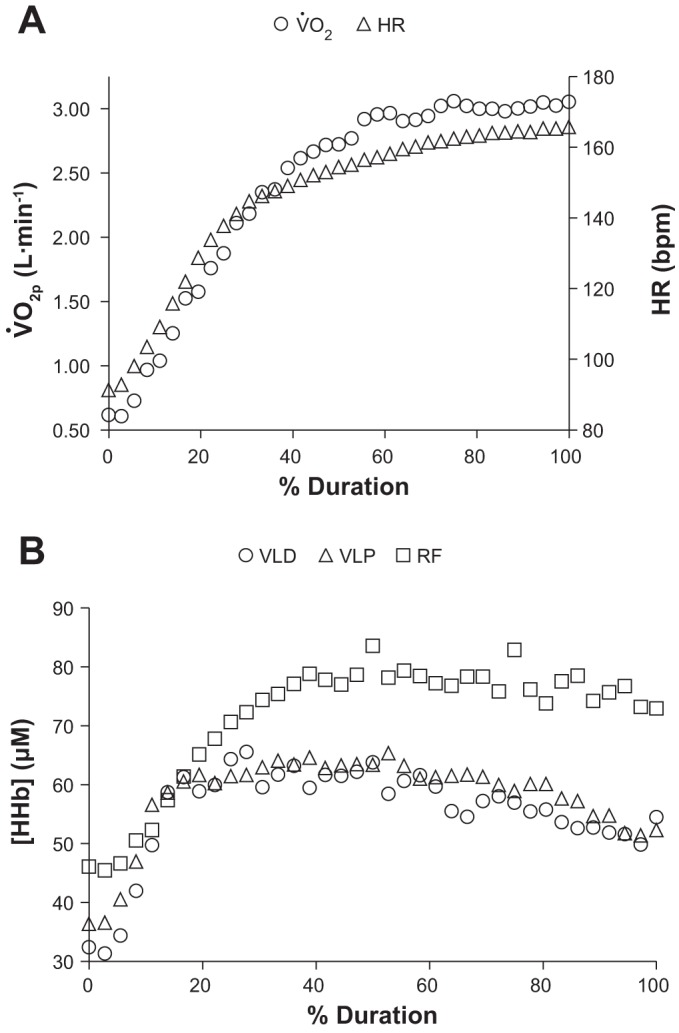
Group mean V̇o2p, HR (A), and [HHb] (B) responses as a function of relative duration (% Duration) during severe-intensity exercise. At all 3 sites, [HHb] reaches an apex before beginning a progressive drop as exercise cessation approaches.
Table 2.
On-transient [HHb] kinetic parameter estimates during moderate- and severe-intensity exercise
| [HHb] (μM) |
|||
|---|---|---|---|
| VLD | VLP | RF | |
| MOD | |||
| Baseline | 35.9 ± 7.2 | 34.4 ± 7.1 | 50.7 ± 10.3*# |
| Amplitude | 14.6 ± 6.8 | 9.7 ± 6.0* | 10.3 ± 6.2* |
| Steady state | 50.5 ± 10.8 | 44.1 ± 11.5† | 60.9 ± 15.5*# |
| τ, s | 9 ± 3§ | 9 ± 4§ | 21 ± 15†‡ |
| 95% CI for τ, s | 3 ± 1 | 3 ± 1 | 5 ± 1*# |
| Time delay, s | 9 ± 1 | 7 ± 2 | 9 ± 2 |
| τ′ [HHb], s | 17 ± 3§ | 17 ± 4§ | 30 ± 16#† |
| SVR | |||
| Baseline | 34.3 ± 5.2 | 39.7 ± 9.0$ | 48.8 ± 7.0* |
| Amplitude | 26.8 ± 12.1$ | 23.3 ± 20.1$ | 30.6 ± 14.1$ |
| τ[HHb], s | 5 ± 2$ | 5 ± 3$ | 9 ± 3*#$ |
| 95% CI for τ, s | 1.8 ± 0.5$ | 1.2 ± 0.5$ | 2.0 ± 0.5$ |
| Time delay, s | 6 ± 1$ | 5 ± 2$ | 9 ± 3*# |
| τ′ [HHb], s | 11 ± 2$ | 10 ± 3$ | 19 ± 6*#$ |
Values are means ± SD. Significantly different (P < 0.05) from:
VLD,
VLP,
τV̇o2p,
MOD. Trend (0.05 ≤ P ≤ 0.10) for difference from:
VLD,
VLP.
RF, rectus femoris; MOD, moderate-intensity exercise; SVR, severe-intensity exercise; CI, confidence interval.
In the present group, the OCC lasted 8 ± 1 min to elicit an apparent plateau in [HHb] at all three muscle sites; the [HHb] response profiles for all three sites are illustrated in Fig. 4. The peak [HHb] from all three sites during OCC, RI, SVR, and MOD is illustrated in Fig. 5; comparisons are made both among sites (Fig. 5, top) and among conditions (Fig. 5, bottom). The peak [HHb] observed during MOD was lower (P < 0.05) than that observed under all other conditions for all three sites. Peak [HHb] was higher (P < 0.05) during OCC than RI and SVR at the VLD and RF but was similar (P > 0.05) across these conditions at the VLP. During both OCC and MOD, peak [HHb] was lower (P < 0.05) at the VLP than VLD and RF. Spatial heterogeneities in the [HHb] responses are evidenced by intersite CVs for baseline, peak, end-exercise/end-occlusion, and recovery [HHb] (Table 3).
Fig. 4.
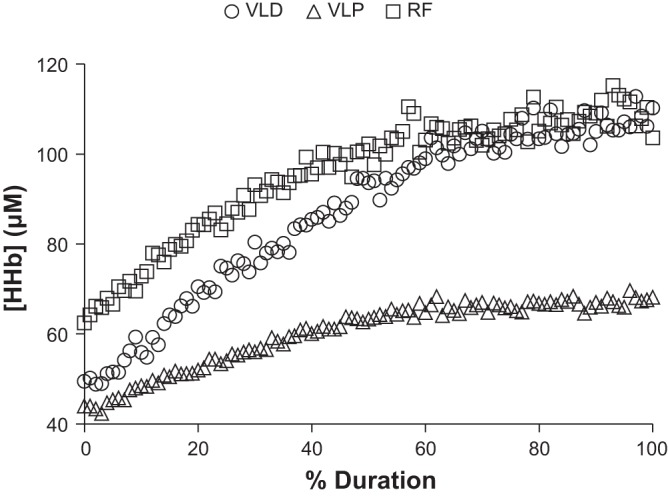
Group mean [HHb] responses as a function of relative duration (% Duration) during ischemic occlusion. Note: the pressure cuff was positioned proximal to the TRS-NIRS probes on the right thigh.
Fig. 5.
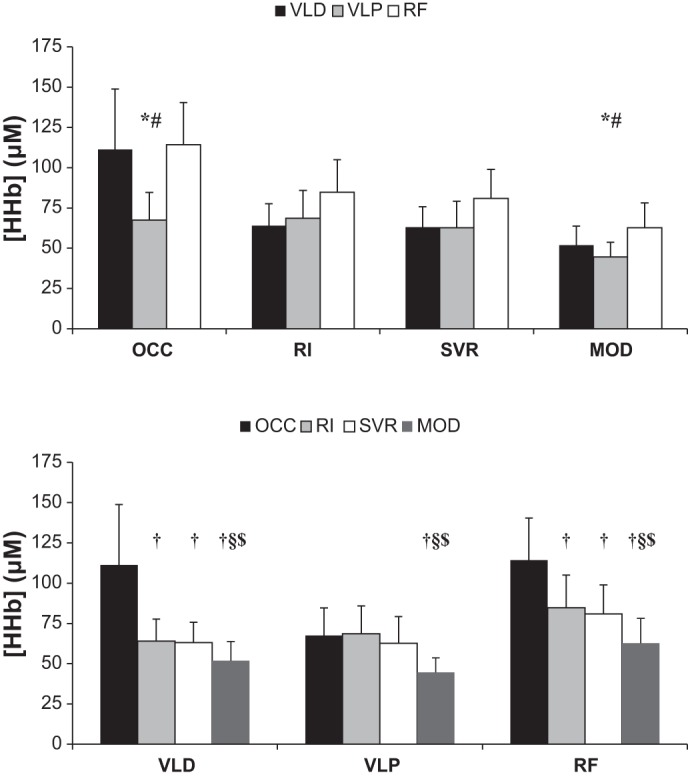
Peak [HHb] at VLD and VLP and RF. Top: group mean data grouped by occlusion (OCC), ramp incremental exercise (RI), severe-intensity exercise (SVR), moderate-intensity exercise (MOD). Bottom: group mean data grouped by muscle site. Significantly different (P < 0.05) from *VLD, #RF, †OCC, §RI, and $SVR.
Table 3.
Intersite coefficients of variation (%) for [HHb]
| RI | SVR | MOD | OCC | |
|---|---|---|---|---|
| Baseline | 19.8 ± 8.2 | 19.8 ± 7.5 | 24.2 ± 6.0 | 20.4 ± 10.4 |
| Peak | 18.6 ± 5.0 | 17.6 ± 6.3 | 17.6 ± 5.8 | 27.9 ± 10.9 |
| End of test | 20.2 ± 5.6 | 22.4 ± 11.0 | 17.5 ± 5.3 | 26.3 ± 11.9 |
| Recovery | 35.2 ± 13.6 | 32.9 ± 15.7 |
Values are means ± SD. Note: Baseline occlusion (OCC) was calculated from resting measures; baseline RI, SVR, and MOD were calculated from 20 W cycling measures; peak values were calculated from the highest continuous 20 s average; end-of-test values were calculated from the final 20 s of exercise or OCC; and recovery values were calculated from the final 20 s of the 6 min recovery cycling at 20 W.
During MOD, V̇o2p and HR increased from 0.60 ± 0.06 l/min and 85 ± 14 bpm to 1.55 ± 0.33 l/min and 119 ± 17 bpm, respectively. The [HHb] kinetics parameter estimates from both MOD as well as SVR are described in Table 2. Both the baseline and steady-state [HHb] were elevated (P < 0.05) at the RF compared with VLD and VLP during MOD. SVR transitions were characterized by a greater [HHb] amplitude (and thus greater [HHb] “steady state”) and reduced τ[HHb] and τ′[HHb] values (i.e., faster adjustments) compared with MOD at all three sites; the TD [HHb] was also smaller in the VLD and VLP (but not RF) during SVR compared with MOD (Table 2).
DISCUSSION
The purpose of the present paper was to compare peak ATT-corrected [HHb] responses at three quadriceps muscle sites during RI, SVR, and MOD exercise, as well as during ischemic OCC. The main findings were as follows: 1) attainment of a maximal [HHb] (i.e., as measured in OCC) during intense cycling is site specific. Maximal [HHb] was not attained during RI and SVR at either the VLD or at the RF; however, it was attained at the VLP. Thus, despite exercise continuing to the limit of tolerance and the attainment of near-maximal V̇o2p, an apparent “reserve” in O2 extraction (as reflected by an inability to match maximal deoxygenation levels established during OCC) was still evident in VLD and RF, but not VLP; 2) the adjustment of [HHb] during SVR was faster than during MOD and was characterized by the attainment of an apex followed by a gradual drop in the signal before exercise cessation; 3) heterogeneities exist in both the amplitude and dynamic profile of [HHb] responses throughout the quadriceps complex during cycling exercise; 4) the adjustment of [HHb] during RI is better characterized by two intersecting lines (i.e., double-linear) compared with a symmetrical sigmoid function at all three muscle sites; this had not previously been shown with TRS-NIRS. However, the near-plateau previously described to occur near the end of exercise (35) was not observed at the RF, where the trajectory of [HHb] was shown to be increasing.
In the present study, the highest [HHb] values were observed under OCC conditions in two of three muscles sites, and these values could not be attained during intense cycling. Therefore, these results suggest that, in the VLD and RF, there is an O2 extraction reserve that cannot be tapped during dynamic, rhythmic exercise; the presence of such a reserve suggests that the lowest venous O2 content levels observed during intense cycling would not be as low as those attained during OCC. It is unclear, however, from the present data whether such a reserve is the result of sufficient (during RI) or even surplus (during SVR where [HHb] falls after an apex) blood flow through the capillaries or the result of reduced transit times leading to insufficient time for equilibration (i.e., O2 diffusion) between capillaries and muscle cells (or some other mechanism). In the former instance, the rise in blood flow exceeds that of muscle O2 utilization, resulting in an attenuated fractional O2 extraction; in that latter instance, red blood cell transit through the capillary is too rapid (reduced red cell transit time) for full equilibration of O2 off-loading from Hb. Another possibility that has been explored recently (13) relates to the notion of an inhibition of motor outflow or muscle activation during large muscle mass exercise as the limit of exercise tolerance is approached. Cannon et al. (13) illustrate that only a fraction of the quadriceps muscle volume was “metabolically challenged” during peak bilateral knee-extension exercise; thus, under circumstances where exercise intolerance occurs with an apparent energy reserve, perhaps it is not surprising that a fractional O2 extraction reserve should also exist. Unfortunately at this time, it seems that resolving this issue will have to await advances in capillary blood flow measures, as these are still not possible in exercising humans.
In contrast to the VLD and RF sites, peak [HHb] values at the VLP did not differ among OCC, RI, and SVR conditions, perhaps suggesting that the true maximal values are attained, and thus, no fractional O2 extraction reserve is present in this muscle region during intense cycling. To our knowledge, this is the first study to report such a finding. However, it is important to note that the peak [HHb] during both RI and SVR did not differ among sites (Fig. 5A); therefore, if the ATT-corrected [HHb] truly allows for valid comparisons among different muscle regions, it would appear that the peak reliance on fractional O2 extraction was similar throughout the quadriceps complex during intense cycling. As a result, the peak [HHb] observed during OCC becomes important within the context of the present study, as this is the standard against which exercise values are to be compared within a given site. (It is important to note that the present study does not address the issue of “normalizing” [HHb] responses to peak values observed during OCC; this was not the focus of the present study and is not a recommendation herein.) Notably, however, the peak [HHb] during OCC was lowest at the VLP in all seven subjects (i.e., compared with the other 2 sites). Although we cannot preclude the possibility that the proximity of the cuff somehow affected the NIRS signal at the VLP, without a compelling reason to doubt the accuracy and validity of these measures, we are left to question what physiological conditions might have led to a reduced [HHb] during OCC only at one site (i.e., VLP), particularly given the distinct plateau in the VLP illustrated in Fig. 4. Notwithstanding uncertainty regarding the mechanism(s) underpinning a reduced peak [HHb] during OCC relative to other sites, the present data do not support the suggestion of an O2 extraction reserve at the VLP during intense cycling. Considering that measures of venous Po2 and O2 content during maximal exercise are very low (i.e., ∼12–15 mmHg and ∼13–25 ml/l, respectively), even in mixed venous blood sampled at the femoral vein (11), perhaps it should not come as a surprise that near-maximal O2 extraction is occurring at certain muscle sites, even during intense cycling when microvascular O2 delivery and blood flow velocity are presumably high. The observation that the attainment of maximal fractional O2 extraction during intense cycling is site specific reinforces the importance of considering the matching of blood flow to O2 utilization at the level of the microvasculature (i.e., rather than simply across the exercising limb) and also reinforces the importance of considering dynamic heterogeneities throughout various regions of the active muscle tissues (27).
In light of the fact that V̇o2peak during SVR was only 92 ± 7% of that observed during RI (P < 0.05), it may seem that higher peak [HHb] values should not be expected during SVR compared with RI. Two points seem pertinent here, however: first, at all three quadriceps sites, [HHb] reached an apex before the end of SVR and then gradually fell until exercise cessation. Thus, the attainment of peak [HHb] during SVR is independent of TTF as well as whole body V̇o2. Second, Bangsbo et al. (3, 4) concluded that the time required for attainment of peak O2 extraction at the onset of intense exercise (i.e., measured across the muscle during one-legged knee extension) was ∼45–50 s; thus, our findings (particularly in the VL; average of VLD and VLP = ∼45 s, average of 3 sites = ∼62 s) are in line with these previous measures and are considerably faster than the expected time to reach V̇o2peak. Recall that in the present study, those subjects who were able to tolerate SVR for longer periods reached higher percentages of V̇o2peak from RI (r = 0.82).
One advantage of the TRS-NIRS system used in the present study was that the multiple channels allowed for simultaneous measures in distinct regions of the quadriceps muscle complex. As has been reported previously during submaximal exercise (25, 26), the present study illustrated heterogeneities in the [HHb] responses; these heterogeneities existed both in the amplitude of the responses (e.g., peak [HHb] during OCC) as well as the time course of adjustment of [HHb]. Regrettably, we did not measure integrated electromyography (iEMG) in the present study [see Chin et al. (14) for further discussion]; doing so might have allowed us to further elucidate the mechanism(s) contributing to different times required to reach the peak [HHb] during SVR (i.e., the apex) in the VL vs. RF muscles. Camata et al. (12), for example, demonstrated that the EMG profile (normalized root mean square) within the RF muscle significantly differed from those of the other quadriceps muscles [vastus lateralis (VL) and vastus medialis] during SVR cycling (i.e., at a power output equivalent to 110% of V̇o2 max); however, at present, it is unclear whether the “delayed apex attainment” of [HHb] in the RF during SVR exercise is related to this distinct activation pattern of the muscle or perhaps is reflective of a more rapid adjustment of microvascular O2 delivery (or some combination of the two).
Although not a central focus of the present article, this study also compared the [HHb] kinetic responses between MOD and SVR exercise. Naturally the amplitude was larger during SVR compared with MOD exercise (which is in line with the peak responses discussed above). However, to our knowledge, this is the first study to have shown that the adjustment (τ[HHb] and τ′[HHb]) of [HHb] is faster during SVR than MOD at each of the three muscle sites measured. Interestingly, the TD [HHb] was shorter during SVR compared with MOD at the two VL sites, but not in the RF. Taken together with the significantly slower adjustment of [HHb] in the RF, this may suggest an altered O2 demand in this muscle region compared with the VL; this interpretation is also consistent with the unique [HHb] profile observed during incremental exercise (14, 27).
Several studies have sought to describe the profile of [HHb] during incremental (both step and ramp) cycling (7, 8, 14, 17, 19, 21, 30, 31, 35) under a variety of conditions. In short, the response profile has been characterized using a symmetrical sigmoid regression as well as a double-linear model; in either case, three phases are generally described. The present study has shown, for the first time using TRS-NIRS, that the previously proposed double-linear model (35) provides a more suitable description of these profiles than the symmetrical sigmoid model more often used in the past. Whereas the profiles at the VLD and VLP were characterized by a linear increase in [HHb] with increasing intensity followed by a near plateau, the RF signal comprised a shallow increase early in exercise, but a steeper increase later. This upward trajectory of [HHb] at higher intensities has not been noted previously but was observed in all seven subjects in the present study. Interestingly, the only other study to have characterized the profile of [HHb] in the RF muscle during incremental exercise is that of Chin et al. (14); although they do not discuss the absence of a clear plateau in the RF, Fig. 2B therein appears to display a similar profile to that illustrated in the present study. Furthermore, they present evidence of both a delayed and blunted increase in the iEMG signal, suggesting that the RF muscle may not be highly involved in the cycling effort until higher intensities are reached; this could help to explain the unique [HHb] profile observed at this measurement site. Given the absence of a clear plateau (based upon the upward trajectory described in Table 1 and Fig. 2) in the RF during incremental exercise, it is perhaps somewhat surprising that the peak [HHb] observed during SVR was not greater by comparison.
Limitations
In the present study, we used resting ischemic OCC to elicit a plateau in the [HHb] signal at all three muscle sites. This decision was based upon previous observations, primarily in the VLD. Based on these previous observations, we expected this [HHb] plateau to occur at a level greater than that observed during incremental exercise, and thus the central question we sought to address was whether this expected reserve could be accessed at higher (i.e., severe) intensities. Under conditions in which OCC was accompanied by voluntary muscle contractions in the region of NIRS interrogation, it is highly possible that the addition of voluntary contractions would elicit greater peak [HHb] values than those observed during OCC alone, given that the muscular demand for O2 would likely have been increased. Recognizing that the [HHb] signal attained a plateau during OCC (i.e., a period in which O2 supply is constant) at all three muscle sites, it is also possible, however, that this plateau might have occurred at the same site-specific absolute [HHb] but would simply have been attained more rapidly.
The [HHb] signal is understood to represent the balance between O2 delivery and utilization. However, it must be acknowledged that the exercise and OCC conditions elicit physiological responses that contribute differently to the eventual rise in [HHb]; the former causes increases in both O2 delivery and O2 utilization, whereas the latter reduces the O2 delivery but does so under conditions of much lower O2 utilization (i.e., resting muscle V̇o2 is lower during OCC). Nevertheless, OCC has been used previously as a “physiological calibration” for NIRS-derived data collected at rest and during exercise (9, 14).
Finally, while we acknowledge that the sample size of seven might be considered “small” by some standards, it is in the range of participants that normally are used with these types of exercise studies. Importantly, one advantage of the present study design is the fact that all of our conditions (i.e., quadriceps site * exercise/occlusion) were compared in a within-subjects (i.e., repeated measures) model. The important risk to consider in this case is the possibility of a type II error: in this study, such an error would be present only if the OCC value were actually greater than the RI or SVR values in the VLP, and we were unable to detect such a difference. Given the very small “effect size” (∼0.065), we believe that this is highly unlikely.
Conclusions
The present study supports the notion of an inaccessible O2 extraction reserve at the VLD and RF, but not at the VLP during intense cycling exercise. It is unclear whether this reserve is the product of abundant microvascular blood flow, insufficient time to allow for equilibration between capillary and muscle fibers, or perhaps is related to submaximal neuromuscular activation. The adjustment of [HHb] during severe exercise was characterized by the attainment of an apex followed by a gradual drop in the signal before exercise cessation; thus, a failure to attain 100% of the ramp incremental V̇o2peak should not necessarily have precluded access of the O2 extraction reserve. In light of the differing response profiles during ischemic OCC, RI, SVR, and MOD exercise, the presence of meaningful heterogeneities in both the amplitude and dynamic profile of [HHb] is confirmed in the present study.
GRANTS
S. Koga was supported by Japan Society for the Promotion of Science Grant KAKENHI-24650401.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.S., N.K., J.M.K., and S.K. conception and design of research; M.D.S., T.A., and S.K. performed experiments; M.D.S. analyzed data; M.D.S., T.A., N.K., J.M.K., and S.K. interpreted results of experiments; M.D.S. prepared figures; M.D.S. drafted manuscript; M.D.S., T.A., N.K., J.M.K., and S.K. edited and revised manuscript; M.D.S., T.A., N.K., J.M.K., and S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our gratitude to the subjects in this study and acknowledge the technical assistance provided by Mikio Miwa. We also express our gratitude to Drs. D. H. Paterson, J. M. Murias, H. B. Rossiter, and T. S. Bowen for very helpful insights during the preparation of the manuscript.
REFERENCES
- 1.Adami A, Pogliaghi S, De Roia G, Capelli C. Oxygen uptake, cardiac output and muscle deoxygenation at the onset of moderate and supramaximal exercise in humans. Eur J Appl Physiol 111: 1517–1527, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Akaike H. A new look at statistical model identification. IEEE Transact Automatic Control Ac19: 716–723, 1974. [Google Scholar]
- 3.Bangsbo J. Muscle oxygen uptake in humans at onset of and during intense exercise. Acta Physiol Scand 168: 457–464, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol 279: R899–R906, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Beaver WL, Lamarra N, Wasserman K. Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol 51: 1662–1675, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Boone J, Koppo K, Barstow TJ, Bouckaert J. Effect of exercise protocol on deoxy[Hb + Mb]: incremental step versus ramp exercise. Med Sci Sports Exerc 42: 935–942, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Boone J, Koppo K, Barstow TJ, Bouckaert J. Pattern of deoxy[Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol 105: 851–859, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Bowen TS, Rossiter HB, Benson AP, Amano T, Kondo N, Kowalchuk JM, Koga S. Slowed oxygen uptake kinetics in hypoxia correlate with the transient peak and reduced spatial distribution of absolute skeletal muscle deoxygenation. Exp Physiol 98: 1585–1596, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Burnham KP, Anderson DR. Multimodel inference - understanding AIC and BIC in model selection. Soc Meth Res 33: 261–304, 2004. [Google Scholar]
- 11.Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 289: R1448–R1458, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Camata TV, Altimari LR, Bortolotti H, Dantas JL, Fontes EB, Smirmaul BP, Okano AH, Chacon-Mikahil MP, Moraes AC. Electromyographic activity and rate of muscle fatigue of the quadriceps femoris during cycling exercise in the severe domain. J Strength Cond Res 25: 2537–2543, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Cannon DT, Howe FA, Whipp BJ, Ward SA, McIntyre DJ, Ladroue C, Griffiths JR, Kemp GJ, Rossiter HB. Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol (1985) 115: 839–849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin LM, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T, Koga S. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol 111: 1259–1265, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis JA, Whipp BJ, Lamarra N, Huntsman DJ, Frank MH, Wasserman K. Effect of ramp slope on determination of aerobic parameters from the ramp exercise test. Med Sci Sports Exerc 14: 339–343, 1982. [PubMed] [Google Scholar]
- 16.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95: 113–120, 2003. [DOI] [PubMed] [Google Scholar]
- 17.DiMenna FJ, Bailey SJ, Jones AM. Influence of body position on muscle deoxy[Hb+Mb] during ramp cycle exercise. Respir Physiol Neurobiol 173: 138–145, 2010. [DOI] [PubMed] [Google Scholar]
- 18.duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Kinetics of VO2 limb blood flow and regional muscle deoxygenation in young adults during moderate intensity, knee-extension exercise. Eur J Appl Physiol 108: 607–617, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 103: 1999–2004, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, Vago P, Marconi C, Morandi L. Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve 35: 510–520, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Gravelle BM, Murias JM, Spencer MD, Paterson DH, Kowalchuk JM. Adjustments of pulmonary O2 uptake and muscle deoxygenation during ramp incremental exercise and constant-load moderate-intensity exercise in young and older adults. J Appl Physiol 113: 1466–1475, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill AV. Muscular Activity. Oxford, UK: Bailliere, 1925, p. 125. [Google Scholar]
- 23.Hughson RL, O'Leary DD, Betik AC, Hebestreit H. Kinetics of oxygen uptake at the onset of exercise near or above peak oxygen uptake. J Appl Physiol 88: 1812–1819, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M, Poole DC. Kinetics of muscle deoxygenation and microvascular PO2 during contractions in rat: Comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol 112: 26–32, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Koga S, Poole DC, Fukuoka Y, Ferreira LF, Kondo N, Ohmae E, Barstow TJ. Methodological validation of the dynamic heterogeneity of muscle deoxygenation within the quadriceps during cycle exercise. Am J Physiol Regul Integr Comp Physiol 301: R534–R541, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 62: 2003–2012, 1987. [DOI] [PubMed] [Google Scholar]
- 29.McNarry MA, Welsman JR, Jones AM. Influence of training and maturity status on the cardiopulmonary responses to ramp incremental cycle and upper body exercise in girls. J Appl Physiol 110: 375–381, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Murias JM, Keir DA, Spencer MD, Paterson DH. Sex-related differences in muscle deoxygenation during ramp incremental exercise. Respir Physiol Neurobiol 189: 530–536, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Murias JM, Spencer MD, Keir DA, Paterson DH. Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. Am J Physiol Regul Integr Comp Physiol 304: R720–R725, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oda M, Yamashita Y, Nakano T, Suzuki A, Shimizu K, Hirano I, Shimomura F, Ohmae E, Suzuki T, Tsuchiya Y. Nearinfrared time-resolved spectroscopy system for tissue oxygenation monitor. Proc SPIE 3597: 611–617, 1999. [Google Scholar]
- 33.Scheuermann BW, Barstow TJ. O2 uptake kinetics during exercise at peak O2 uptake. J Appl Physiol 95: 2014–2022, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Spencer MD, Murias JM, Lamb HP, Kowalchuk JM, Paterson DH. Are the parameters of VO2, heart rate and muscle deoxygenation kinetics affected by serial moderate-intensity exercise transitions in a single day? Eur J Appl Physiol 111: 591–600, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Spencer MD, Murias JM, Paterson DH. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol 112: 3349–3360, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Whipp BJ, Ward SA, Wasserman K. Respiratory markers of the anaerobic threshold. Adv Cardiol 35: 47–64, 1986. [DOI] [PubMed] [Google Scholar]


