Abstract
Hyperoxia can cause substantial reductions in peripheral and coronary blood flow at rest and during exercise, which may be caused by reactive oxygen species (ROS) generated during hyperoxia. The aim of this study was to investigate the role of ROS in hyperoxia-induced reductions in skeletal muscle blood flow during forearm exercise. We hypothesized that infusion of vitamin C would abolish the effects of hyperoxia on the forearm blood flow (FBF) responses to exercise. Twelve young healthy adults performed rhythmic forearm handgrip exercise (10% of maximum voluntary contraction for 5 min) during normoxia and hyperoxia. For each condition, two trials were conducted with intra-arterial administration of saline or vitamin C. FBF was measured using Doppler ultrasound. During hyperoxia with saline, FBF and forearm vascular conductance (FVC) were 86.3 ± 5.1 and 86.8 ± 5.2%, respectively, of the normoxic values (100%) (P < 0.05). During vitamin C, hyperoxic FBF and FVC responses were 90.9 ± 4.2 and 90.9 ± 4.1%, respectively, of the normoxic values (P = 0.57 and 0.59). Subjects were then divided into three subgroups based on their percent decrease in FBF (>20, 10–20, and <10%) during hyperoxia. In the subgroup that demonstrated the greatest hyperoxia-induced changes (>20%), FBF and FVC during hyperoxia were 67.1 ± 4.0 and 66.8 ± 3.6%, respectively, of the normoxic values. Vitamin C abolished these effects on FBF and FVC with values that were 102.0 ± 5.2 and 100.8 ± 6.1%, respectively. However, vitamin C had no effect in the other two subgroups. This analysis is consistent with the idea that ROS generation blunts the FBF responses to exercise in the subjects most affected by hyperoxia.
Keywords: forearm blood flow, vascular conductance, ascorbic acid
hyperoxia causes a decrease in blood flow and vascular conductance in contracting muscles. This is consistent with the general observation that a change in O2 content can affect blood flow to the exercising muscle. This observation has been reported with both normobaric and hyperbaric environments (1, 2, 6, 20). Similarly, coronary blood flow is reduced by ∼20%, and coronary vascular resistance is increased by 23% during hyperoxia (14). The exact mechanism for this increase in vascular resistance and reduced skeletal and cardiac muscle blood flow during hyperoxia is unclear. However, one of the potential mechanisms is an increase in reactive oxygen species (ROS) during hyperoxia. To our knowledge, this idea has not been tested in human skeletal muscles during exercise under normobaric but hyperoxic conditions.
The administration of supplemental O2 in young healthy subjects increases ROS formation (11) and forearm vascular resistance under resting conditions (4). Along these lines, McNulty et. al. (14) have reported the restoration of coronary blood flow and reduction in resistance in response to hyperoxia following infusion of ascorbic acid (vitamin C) in humans. This is consistent with the idea that the constricting effects of hyperoxia on vascular function are related to acute oxidative stress. In addition to hyperoxia, it has been shown that acute administration of vitamin C in healthy older adults, a population known to exhibit increased ROS, increases forearm blood flow (FBF) and vascular conductance (FVC) during dynamic exercise (12).
With this information as a background, the primary aim of this study was to investigate the role of ROS in hyperoxia-induced reductions in skeletal muscle blood flow during forearm exercise in humans. We hypothesized that infusion of vitamin C would abolish the effects of hyperoxia on the FBF responses to exercise.
METHODS
Subjects.
A total of 13 young healthy subjects were consented for the study; however, arterial catheterization was successful in 12 subjects (7 men and 5 women) who completed the study. All participants were free of acute cardiovascular or respiratory disease, not taking any medications, nonobese (body mass index < 28 kg/m2), and nonsmoking. Studies were performed between 7 AM to 10 AM following an overnight fast. All participants refrained from exercise and caffeine for at least 24 h before the start time of the study. Female subjects were studied during the early follicular phase of the menstrual cycle (n = 1) or the placebo phase of oral contraceptives (n = 4). All subjects provided written, informed consent before participation, and all study procedures were approved by the Institutional Review Board of the Mayo Clinic.
Arterial and venous catheterization.
A 20-gauge, 5-cm (model RA-04020; Arrow International, Reading, PA) catheter was placed in the brachial arterial of the exercising arm under aseptic conditions after local anesthesia (2% lidocaine) for measurement of arterial pressure, arterial blood gases, and administration of vitamin C, and was continuously flushed (3 ml/h) with saline. An 18-gauge, 3-cm catheter was inserted retrograde in an antecubital vein of the exercising arm under aseptic conditions for measurement of venous blood gases.
Arterial and venous blood O2 content.
Arterial and venous blood samples were collected under each condition during rest and the last minute of exercise. Blood samples were then analyzed by the Immunochemistry Core Laboratory of the Clinical Research Unit of the Mayo Clinic Clinical and Translational Science Awards using a blood-gas analyzer (Radiometer ABL 825, Westlake, OH).
Heart rate and systemic blood pressure.
Heart rate (HR) was recorded via continuous three-lead electrocardiogram. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5; Datex-Ohmeda, Louisville, CO).
FBF.
Brachial artery mean blood velocity (MBV) and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole between contractions during steady-state conditions. This method has been previously used for several other studies in our laboratory (3, 17). FBF was calculated as the product of MBV (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min).
Hyperoxia.
Participants were exposed to a hyperoxic condition at rest and during the handgrip exercise. Hyperoxia was generated by having participants breathe 100% O2 through a closed face mask connected to a non-rebreathing valve during the experimental protocol. A one-way valve at the exhalation port prevented room air from being inspired during inhalation.
Forearm exercise.
Participants performed rhythmic forearm exercise with a handgrip dynamometer at a moderate (∼10% of one repetition maximum) level of exercise. This workload elicits only minor increases in arterial pressure or fatigue and allows the study of steady-state vascular responses in the exercising forearm over multiple trials. In addition, this technique was adapted to facilitate beat-to-beat measurements of brachial artery blood flow via Doppler ultrasound. The weight was lifted 4 to 5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation using a metronome.
Vitamin C infusion.
A loading dose of 8 mg·100 ml forearm volume−1·min−1 was administered for 10 min. This was followed by a maintenance dose of 4 mg·100 ml forearm volume−1·min−1.
Experimental protocol.
A schematic diagram of the general experimental timeline is illustrated in Fig. 1. All participants completed four separate exercise trials in the supine position in a temperature-controlled room. Exposure to normoxia or hyperoxia was alternated and randomized. Measurements were performed with the subjects breathing either normoxic air (21% O2) or inspired oxygen (100% O2) using a tight-fitting mask. All of the participants rested for 15 min postinstrumentation and before the beginning of the first trial. Each trial consisted of a 2-min resting baseline period, followed by 5 min of handgripping exercise. A rest period of at least 20 min was used between trials to allow FBF to return to baseline levels between exercise bouts (Fig. 1). Rest and exercise under the saline infusion served as the control for the normoxic and hyperoxic conditions, while vitamin C infusion was used to test the potential role of ROS in the blood flow response to hyperoxic exercise.
Fig. 1.
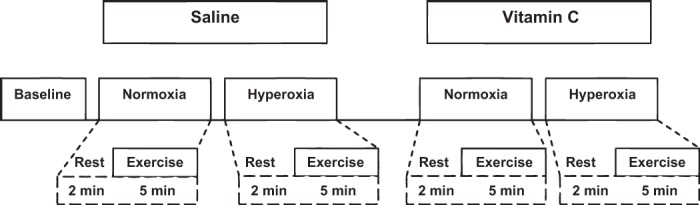
Detailed experimental timeline in minutes. All exercise trials (normoxic and hyperoxic) consisted of a 2-min rest (baseline) period, followed by 5 min of rhythmic handgrip exercise (∼10% of maximum voluntary contraction). Additionally, each hyperoxia trial consisted of a 5-min Po2 normalization period before the rest measurements. Saline and vitamin C loading was performed 10 min before the baseline measures while the participant was under resting condition. There was no time gap between loading and maintenance dose for saline and/or vitamin C.
Data analysis and statistics.
Data were collected at 250 Hz, stored on a computer, and analyzed off-line with signal processing software (WinDaq; DATAq Instruments, Akron, OH). HR and mean arterial pressure (MAP) were analyzed from the electrocardiogram and the brachial artery pressure waveform, respectively. MBV during exercise was determined by averaging the last 45 s of the exercise trial. Arterial diameters were measured at baseline and in the last 15 s of the exercise trials.
FVC was calculated as (FBF/MAP) × 100 and expressed as ml·min−1·100 mmHg−1 so that FVC will be quantitatively similar to the standard units of FBF (19). Blood flow values were normalized to 100% with normoxia, as it is a control treatment.
We were assessing the effect of hyperoxia and vitamin C on FBF and FVC during exercise, and hence normoxia was treated as the control condition, and all of the values were normalized to 100% under normoxia. This allowed us to eliminate the variability introduced by differences in FBF during normoxic exercise and provided better insight into the changes on vascular function due to hyperoxia. This approach has been used previously for similar data representation (16).
All data are reported as means ± SE. A priori significance was set at P < 0.05. Normality of distribution was assessed. Analysis of variance with repeated measures was used to assess changes in continuous outcome variables of blood pressure and HR between gas (hyperoxia vs. normoxia) and drug (saline vs. vitamin C) conditions during exercise. Paired sample t-tests were used to assess differences between FBF and FVC as a percent change from normoxia. Data analysis was carried out using SigmaPlot 12.0.
RESULTS
All 12 subjects completed the protocol. The subjects were 28 ± 2 yr of age, 177 ± 3 cm in height, and weighed 78 ± 4 kg (body mass index: 25 ± 1 kg/m2).
Systemic hemodynamic variables.
The group data for systemic hemodynamic variables are presented in Table 1. There were no significant differences in HR and MAP between different oxygen conditions and between saline and vitamin C trials.
Table 1.
Systemic variables
| Normoxia |
Hyperoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline |
Vitamin C |
Saline |
Vitamin C |
|||||
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| MAP, mmHg | 95 ± 1 | 97 ± 2 | 96 ± 1 | 97 ± 1 | 96 ± 1 | 96 ± 1 | 96 ± 1 | 97 ± 1 |
| HR, beats/min | 64 ± 1 | 65 ± 1 | 64 ± 1 | 63 ± 1 | 64 ± 1 | 64 ± 1 | 64 ± 1 | 63 ± 1 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate.
Arterial and venous O2 content.
The group data for arterial and venous partial pressure of oxygen are presented in Table 2. There was a significant difference in the normoxia vs. hyperoxia conditions.
Table 2.
Partial pressure of oxygen during normoxia and hyperoxia
| Normoxia |
Hyperoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline |
Vitamin C |
Saline |
Vitamin C |
|||||
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| PaO2, Torr | 127 ± 6 | 131 ± 5 | 128 ± 2 | 126 ± 3 | 437 ± 7† | 430 ± 6† | 388 ± 10† | 393 ± 9† |
| PvO2, Torr | 49 ± 11 | 27 ± 2‡ | 45 ± 6 | 27 ± 2‡ | 63 ± 17 | 32 ± 2†‡ | 54 ± 6† | 31 ± 3†‡ |
| PaCO2, Torr | 38 ± 1 | 34 ± 1‡ | 36 ± 1 | 37 ± 1 | 38 ± 1 | 33 ± 1†‡ | 35 ± 1 | 36 ± 1 |
| PvCO2, Torr | 48 ± 1 | 53 ± 2 | 42 ± 3* | 49 ± 4‡ | 42 ± 3 | 50 ± 5‡ | 44 ± 3 | 49 ± 5‡ |
| CO2 tot, ml/dl | 17 ± 1 | 17 ± 1 | 17 ± 1 | 17 ± 1 | 19 ± 1† | 18 ± 1†‡ | 17 ± 1* | 18 ± 1* |
Values are means ± SE. PaO2, arterial partial pressure of oxygen; PvO2, venous partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; PvCO2, venous partial pressure of carbon dioxide; CO2 tot, concentration of total oxygen in blood.
P < 0.05 from saline.
P < 0.05 vs. normoxia.
P < 0.05 from rest.
FBF and FVC.
Presented in Table 3 are group data for forearm hemodynamics at rest and with exercise intensity during saline and vitamin C infusion. To calculate the percent change for FBF and FVC, normoxic-saline condition served as a control and was normalized to 100%. There was a difference in the FBF (P < 0.001) and FVC (P < 0.05) between the normoxia-saline condition (mean = 100%) and the hyperoxia-saline condition (86.3 ± 5.1 and 86.8 ± 5.2%, respectively).
Table 3.
Absolute FBF and FVC values under each condition
| Normoxia |
Hyperoxia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline |
Vitamin C |
Saline |
Vitamin C |
|||||
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| FBF, ml/min | 93 ± 21 | 259 ± 46* | 114 ± 20 | 299 ± 46* | 91 ± 14 | 242 ± 43* | 103 ± 13 | 258 ± 33*† |
| FVC, ml·min−1·mmHg−1 | 94 ± 22 | 295 ± 48* | 114 ± 20 | 297 ± 44* | 94 ± 14 | 243 ± 36*† | 105 ± 14 | 259 ± 32*† |
Values are means ± SE. FBF, forearm blood flow; FVC, forearm vascular conductance.
P < 0.05 vs. rest.
P < 0.05 vs. normoxia.
However, infusion of vitamin C did not restore FBF and FVC in the entire group during hyperoxic exercise (90.9 ± 4.2 and 90.9 ± 4.1%, respectively) compared with saline (P = 0.57 and 0.59), and there was no difference in FBF (P = 0.168) and FVC (P = 0.597) between the hyperoxia-saline condition (mean = 80.75 and 86.75%) and the hyperoxia-vitamin condition (mean = 91.07 and 90.91% respectively).
There was considerable variability in the absolute FBF and FVC values (Fig. 2) in response to hyperoxia between subjects, which prompted further study. Hence, to study the effect of vitamin C on hyperoxia, the cohort was divided into three subgroups based on the percent decrease in FBF (>20, 10–20, and <10% decrease) during hyperoxia compared with normoxia. Previous studies have shown that hyperoxia results in ∼20% decrease in blood flow (14). Hence, the primary group included individuals with a decrease in flow >20%. There was a significant difference in FBF (Fig. 3A) and FVC (Fig. 3B) in this group between hyperoxia-saline and hyperoxia-vitamin conditions. However, this was not observed in the other two subsets that demonstrated a <20% decrease in FBF or FVC.
Fig. 2.
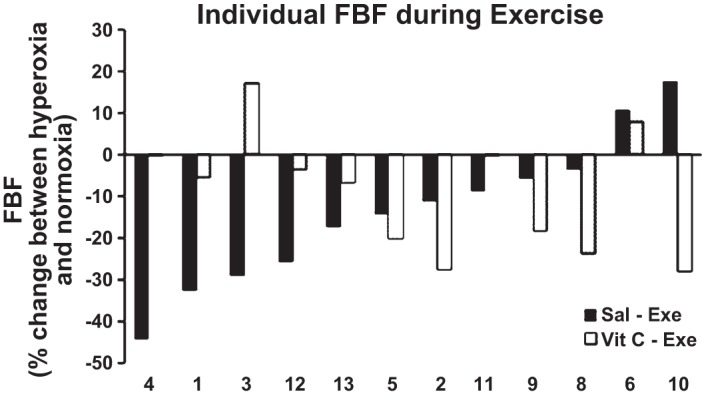
Percent (%) change between hyperoxic and normoxic exercise forearm blood flow (FBF) under each drug condition for each individual subject. The figure is representative of the intersubject variability to hyperoxia. Sal-Exe refers to %change in FBF (hyperoxic-normoxic) during saline infusion, whereas Vit C-Exe refers to the %change in FBF during vitamin C infusion.
Fig. 3.
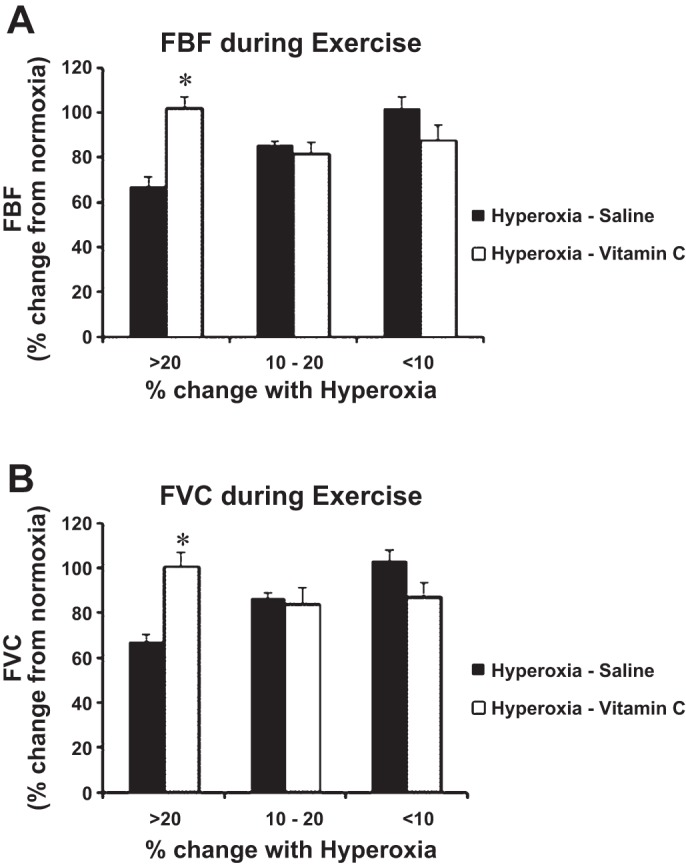
%Change in FBF (A) and forearm vascular conductance (FVC; B) during hyperoxic exercise with and without vitamin C. The figure represents the “responders” vs. “nonresponders”. Vitamin C significantly improved the FBF (A) and FVC (B) only in the group that had a >20% change with hyperoxic exercise. Values are means ± SE; n = 4 (>20% group), n = 3 (10–20% group), and n = 5 subjects (<10% group). *P < 0.05 vs. hyperoxia saline.
DISCUSSION
When considering the entire group of subjects, the primary finding of the present study was that intra-arterial infusions of vitamin C did not have a restorative effect on the hyperoxic-induced reductions in FBF and FVC during exercise. That is, FBF and FVC during hyperoxic exercise remained significantly lower than the values observed under normoxic conditions, despite administration of vitamin C (Table 3). Furthermore, the FBF and FVC responses to hyperoxic exercise were not enhanced with vitamin C compared with the saline trials (Table 3). However, due to variable FBF responses to hyperoxic exercise observed in the present study, we examined whether certain individuals demonstrated greater increases in FBF and FVC in response to vitamin C administration. Interestingly, we found that vitamin C appears to have a restorative effect on FBF only when there is substantial vasoconstriction during hyperoxic exercise (i.e., decrease in FBF ≥20%). These findings are novel because, while vitamin C has been shown to modulate hyperoxia-ROS-mediated vasoconstriction at rest, there is very little information regarding how this vasoconstrictor mechanism operates during exercise, and little is known about the subject-to-subject variability of any such responses.
Responders vs. nonresponders.
A number of previous studies have shown that muscle blood flow and vasodilation are reduced with hyperoxia, under both normobaric and hyperbaric environments, at rest and during exercise, compared with normoxia (1, 2, 8, 15). Indeed, FVC was significantly lower during hyperoxic compared with normoxic exercise in the present study; however, there was no significant difference in FBF between hyperoxic and normoxic exercise when compared as a whole group. However, there was considerable between-subject variability in the magnitude of the hyperoxia-induced vasoconstriction during exercise. Therefore, we divided our data into three distinct groups based on the percent FBF decrease during the hyperoxia-saline condition compared with the normoxia-saline condition. The distinction for each group was based on previous studies where McNulty et al. (14) reported that, compared with room air, breathing 100% oxygen reduced coronary blood velocity by 20%. Hence, participants in the group with a reduction in FBF of 20% or greater were categorized as responders, whereas the participants in the group with a reduction in FBF of 10% or lower were categorized as nonresponders. In the present study, only the responders demonstrated a restoration of FBF with vitamin C administration during hyperoxic exercise.
Interestingly, in the case of nonresponders, there was a trend (P = 0.18) for reduction in FBF following vitamin C administration during exercise under hyperoxia. It has been reported that acute antioxidant supplementation can have negative effects on exercise-induced vasodilation in young adults (5). Similar findings were present in the group of participants who had less than a 10% reduction in FBF during the exercise and hyperoxic conditions in the present study. This may suggest that a certain amount of ROS may be essential for “normal” function of vasodilation during exercise in young, healthy individuals.
It has been speculated that hyperoxia-induced vasoconstriction is associated with increased production of ROS. Mak et al. (13) studied the effect of vitamin C infusion on hyperoxic vasoconstriction and blunted acetylcholine-mediated vasodilation in patients with congestive heart failure. Although the FBF responses to acetylcholine following hyperoxia were not significantly reduced, the authors reported a significant increase in vascular resistance. Additionally, administration of vitamin C during hyperoxia increased the FBF responses to acetylcholine. Hence, there seems to be a connective link between hyperoxia-induced ROS and hyperoxia-mediated vasoconstriction. The results from the present study suggest that hyperoxia significantly reduces FBF (%) during forearm handgrip exercise compared with normoxia (Fig. 2). However, contrary to our hypothesis, vitamin C infusion during hyperoxic exercise did not completely ameliorate the hyperoxic-induced vasoconstriction. Our results reinforce the idea that hyperoxia-induced vasoconstriction could be partially ROS mediated in certain individuals.
One of the potential pathways that ROS could lead to decreased vascular response may lie in the stimulation of vasoconstrictor endothelin-1 (ET-1). In vitro and in vivo animal studies have shown that hyperoxia can elicit the release of ET-1 (7, 9). In addition, it has been shown that there is a significant amount of ET-1-mediated vasoconstriction even during rhythmic exercise in humans (21). Although the role of ET-1 in the exercise hyperemic response has only been demonstrated under normoxic conditions (21), it may be safe to speculate that there could be similar or higher stimulation of ET-1 during hyperoxic exercise. Finally, ROS could potentially shift the balance of NO bioavailability by scavenging NO. Zhilyaev et al. (22) have shown that hyperoxia-induced vasoconstriction in the brain is due to inactivation of NO, primarily driven by an increase in superoxide anions.
Hyperoxia and other mechanisms.
It has also been shown that hyperoxia can augment muscle sympathetic nerve activity (MSNA) in response to isometric handgrip exercise compared with normoxic conditions (10). Yet others have demonstrated a hyperoxic-induced attenuation of MSNA during rhythmic handgrip exercise (18). If hyperoxia does indeed increase MSNA during exercise (relative to normoxic exercise), as suggested by Houssiere and colleagues (10), one of the possible explanations for the hyperoxia-induced vasoconstriction in contracting skeletal muscle could be a greater α-adrenergic-mediated vasoconstriction and/or attenuation of functional sympatholysis. However, our laboratory has previously demonstrated that changes in the vasoconstrictor responsiveness (i.e., functional sympatholysis) during hyperbaric hyperoxic exercise did not explain the substantial reductions in FBF and FVC compared with normoxic exercise (2). Moreover, the FBF and FVC response to hyperoxic exercise remained significantly lower during α-adrenergic blockade, compared with normoxic exercise under α-adrenergic blockade (2). Taken together, these findings suggest that hyperoxia-induced vasoconstriction during exercise likely does not result from enhanced vasoconstrictor responsiveness.
Experimental considerations.
In the present study, we were unable to discern any specific characteristics between responders and nonresponders that might explain our observations. However, some potential explanations include, but are not limited to: 1) differences in antioxidant pathways that might be influenced by hyperoxia; 2) sex-related differences; 3) differences in sympathetic nervous system activity (both at baseline and/or in response to exercise and hyperoxia); and 4) physical activity levels. Studies to examine these potential mechanisms will likely need to include younger and older adults, including individuals with known high and low circulating ROS or differences in antioxidant capacity. In this context, we did not directly measure ROS or antioxidant capacity in the present study. Therefore, the interpretation that vitamin C restores the FBF and FVC responses to hyperoxic exercise by reducing ROS in certain individuals (i.e., responders) is speculative. Given that the number of individuals in each of the groups is low and considering the subject-to-subject variability observed in the present findings, it would be interesting to determine whether a greater hyperoxic stimulus (i.e., hyperbaric hyperoxia) evokes more consistent responses. If this is indeed the case, then the blood flow responses to handgripping would be more uniformly reduced in all subjects, and the effect of vitamin C might limit the hyperoxia-mediated reductions in blood flow to a greater extent. Similarly, it would be interesting to conduct similar studies in patient groups known to have increased baseline levels of ROS.
Lastly, the findings from this study are limited to young adults. There is strong evidence that vitamin C administration can have differential effects on FBF in young vs. older adults during dynamic exercise (12). Along these lines, intra-arterial infusion of vitamin C increased FBF and FVC ∼30–35% during rhythmic forearm exercise in older adults, whereas it had no effect in young healthy adults (12).
Conclusions.
The present study demonstrates that hyperoxia-induced vasoconstriction may be due to ROS generation during forearm exercise only in certain individuals. Hence, when antioxidant (vitamin C) is administered during hyperoxic exercise, the FBF seems to be restored only in the individuals who have a 20% or more decrease in FBF. The present study fails to demonstrate that hyperoxia-induced vasoconstriction maybe due to ROS during rhythmic forearm in all individuals.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-46493 (M. J. Joyner) and HL-105467 (D. P. Casey) and by CTSA UL1 TR000135.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M.R., M.J.J., and D.P.C. conception and design of research; S.M.R., B.G.W., J.L.T., and D.P.C. performed experiments; S.M.R., B.G.W., and J.L.T. analyzed data; S.M.R., M.J.J., J.L.T., and D.P.C. interpreted results of experiments; S.M.R. prepared figures; S.M.R. drafted manuscript; S.M.R., M.J.J., B.G.W., J.L.T., and D.P.C. edited and revised manuscript; S.M.R., M.J.J., B.G.W., J.L.T., and D.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christopher Johnson, Pamela Engrav, and Luke Matzek for technical assistance.
REFERENCES
- 1.Casey DP, Joyner MJ, Claus PL, Curry TB. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol 300: H1892–H1897, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey DP, Joyner MJ, Claus PL, Curry TB. Vasoconstrictor responsiveness during hyperbaric hyperoxia in contracting human muscle. J Appl Physiol (1985) 114: 217–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol (1985) 107: 1128–1137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford P, Good PA, Gutierrez E, Feinberg JH, Boehmer JP, Silber DH, Sinoway LI. Effects of supplemental oxygen on forearm vasodilation in humans. J Appl Physiol (1985) 82: 1601–1606, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Habre W, Petak F, Ruchonnet-Metrailler I, Donati Y, Tolsa JF, Lele E, Albu G, Beghetti M, Barazzone-Argiroffo C. The role of endothelin-1 in hyperoxia-induced lung injury in mice. Respir Res 7: 45, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen M, Madsen J. Estimation of relative changes in resting muscle blood flow by 133Xe washout: the effect of oxygen. Scand J Clin Lab Invest 31: 133–139, 1973. [DOI] [PubMed] [Google Scholar]
- 9.Higgins RD, Hendricks-Munoz KD, Caines VV, Gerrets RP, Rifkin DB. Hyperoxia stimulates endothelin-1 secretion from endothelial cells; modulation by captopril and nifedipine. Curr Eye Res 17: 487–493, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Houssiere A, Najem B, Cuylits N, Cuypers S, Naeije R, van de Borne P. Hyperoxia enhances metaboreflex sensitivity during static exercise in humans. Am J Physiol Heart Circ Physiol 291: H210–H215, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol 48: 703–719, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol 282: H2414–H2421, 2002. [DOI] [PubMed] [Google Scholar]
- 14.McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol (1985) 102: 2040–2045, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Reich T, Tuckman J, Naftchi NE, Jacobson JH. Effect of normo- and hyperbaric oxygenation on resting and postexercise calf blood flow. J Appl Physiol (1985) 28: 275–278, 1970. [DOI] [PubMed] [Google Scholar]
- 16.Schrage WG, Dietz NM, Joyner MJ. Effects of combined inhibition of ATP-sensitive potassium channels, nitric oxide, and prostaglandins on hyperemia during moderate exercise. J Appl Physiol (1985) 100: 1506–1512, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol (1985) 99: 1866–1871, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977. [DOI] [PubMed] [Google Scholar]
- 21.Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Zhilyaev SY, Moskvin AN, Platonova TF, Gutsaeva DR, Churilina IV, Demchenko IT. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci Behav Physiol 33: 783–787, 2003. [DOI] [PubMed] [Google Scholar]


