Abstract
Chronic fatigue syndrome (CFS) with orthostatic intolerance is characterized by neurocognitive deficits and impaired working memory, concentration, and information processing. In CFS, upright tilting [head-up tilt (HUT)] caused decreased cerebral blood flow velocity (CBFv) related to hyperventilation/hypocapnia and impaired cerebral autoregulation; increasing orthostatic stress resulted in decreased neurocognition. We loaded the baroreflex with phenylephrine to prevent hyperventilation and performed n-back neurocognition testing in 11 control subjects and 15 CFS patients. HUT caused a significant increase in heart rate (109.4 ± 3.9 vs. 77.2 ± 1.6 beats/min, P < 0.05) and respiratory rate (20.9 ± 1.7 vs. 14.2 ± 1.2 breaths/min, P < 0.05) and decrease in end-tidal CO2 (ETCO2; 42.8 ± 1.2 vs. 33.9 ± 1.1 Torr, P < 0.05) in CFS vs. control. HUT caused CBFv to decrease 8.7% in control subjects but fell 22.5% in CFS. In CFS, phenylephrine prevented the HUT-induced hyperventilation/hypocapnia and the significant drop in CBFv with HUT (−8.1% vs. −22.5% untreated). There was no difference in control subject n-back normalized response time (nRT) comparing supine to HUT (106.1 ± 6.9 vs. 97.6 ± 7.1 ms at n = 4), and no difference comparing control to CFS while supine (97.1 ± 7.1 vs 96.5 ± 3.9 ms at n = 4). However, HUT of CFS subjects caused a significant increase in nRT (148.0 ± 9.3 vs. 96.4 ± 6.0 ms at n = 4) compared with supine. Phenylephrine significantly reduced the HUT-induced increase in nRT in CFS to levels similar to supine (114.6 ± 7.1 vs. 114.6 ± 9.3 ms at n = 4). Compared with control subjects, CFS subjects are more sensitive both to orthostatic challenge and to baroreflex/chemoreflex-mediated interventions. Increasing blood pressure with phenylephrine can alter CBFv. In CFS subjects, mitigation of the HUT-induced CBFv decrease with phenylephrine has a beneficial effect on n-back outcome.
Keywords: chronic fatigue syndrome, orthostatic challenge, n-back testing, cerebral blood flow, cognition
chronic fatigue syndrome (CFS) is characterized by incapacitating fatigue, inability to concentrate, and short-term memory loss. It is accompanied by joint pain and generalized myalgias, unrefreshing sleep, tender lymph nodes, sore throat, and headache. Patients often experience postexertional malaise and exacerbation of symptoms after physical or mental exertion. Additional symptoms include difficulties with word-finding and reading comprehension, inability to calculate numbers and speech impairments, visual disturbances, psychological problems, depression, irritability, anxiety, and panic attacks; symptoms are highly variable and fluctuate in severity (1, 16).
Neurocognitive impairment in CFS, subjectively described as mental fog or cloudiness, causes impaired working memory and concentration and difficulty in processing complex information (1, 25, 27, 36, 39). Many CFS patients have an inability to plan and order responses and exhibit working memory deficits during demanding tasks that require attention and switching between mental processing routines (10–12, 28, 29, 39) and impaired speed in information processing (10). Evidence for the role of cerebral perfusion is unclear, as some studies support this (4, 8, 24) while other studies fail to support (15, 31) the hypotheses of global or regional deficits of perfusion in patients with CFS. However, our prior studies using transcranial Doppler ultrasound (TCD) showed reduced cerebral blood flow and cognitive loss in postural tachycardia syndrome (POTS) patients compared with healthy control subjects during 70° upright tilt but not while supine (38).
We have shown that chronic orthostatic intolerance [OI, the inability to remain upright because of signs and symptoms relieved by recumbency (43)] is present in a large subset of CFS patients (48, 49). OI takes the form of postural tachycardia, postural hyperventilation, or both (50), each of which can result in significant reductions of cerebral blood flow velocity (CBFv) (24, 42, 61). Prior work provides evidence of excessive pooling of blood in the splanchnic (gut) circulation while upright as one cause of central hypovolemia and OI (33). There is also evidence for intermittent reductions in cerebral blood flow causing the accumulation of cerebral lactate (46) and reactive oxygen species (ROS) (32) and neurocognitive loss in CFS (14, 18).
CFS is frequently associated with OI as POTS (23, 49). POTS is an increase in heart rate (HR) of >30 beats/min (bpm) upon standing upright or a maximum HR of at least 120 bpm in those aged 14 yr and older and at least 130 bpm for those 13 yr and younger (47), with fatigue, nausea, headache, visual disturbances, hypocapnia, and/or cognitive impairments as well. A cause for this neurocognitive impairment has not been established, but it may be due to decreased cerebral blood flow (30, 38), due in part to a direct effect of CO2 on cerebrovascular flow, altered cerebrovascular regulation, or a combination of these (38, 39). We and others have shown that in CFS/POTS orthostasis changes CO2, resulting in hypocapnia and hyperventilation (35, 50).
We have also shown an increased respiratory chemoreflex response to hypoxia and a decreased respiratory chemoreflex response to hypercapnia in POTS compared with control, even while supine (56). These differences were enhanced by orthostasis. From this we inferred that peripheral chemoreflex sensitivity measured by the hypoxic ventilatory response was increased while central chemoreflex sensitivity measured by the hypercapnic ventilatory response was reduced in POTS. Therefore in CFS/POTS, baroreflex unloading during head-up tilt (HUT) stimulates peripheral oxygen-dependent chemoreflexes, causing hyperventilation that is unopposed by the restraining effects of hypocapnia. Upright hyperventilation and hypocapnia reduces cerebral blood flow (37, 38) and likely contributes to light-headedness and to cognitive impairment while upright.
The purpose of this study was therefore to test the hypothesis that impaired upright cognition occurs in CFS patients and that it can be improved by loading the baroreflex with phenylephrine to increase cerebral blood flow.
STUDY DESIGN AND METHODOLOGY
We recruited CFS subjects with reported neurocognitive impairment and hyperventilation/hypocapnea (n = 15) likely due to their POTS and healthy control subjects (n = 11). CFS subjects were between 15 and 29 yr old and fulfilled CDC criteria for CFS (16). CFS subjects had normal physical examination, were free of systemic illness, and included those with persistent or relapsing chronic fatigue of new or definite onset and four or more of the following concurrent symptoms that persisted or recurred during 6 consecutive months and had not predated the fatigue: 1) impairment in memory or concentration severe enough to cause substantial reduction of previous levels of educational, social, or personal activities; 2) muscle pain or multiple joint pain; 3) headaches of a new type, pattern, or severity; 4) unrefreshing sleep; and 5) postexertional malaise lasting >24 h. Healthy volunteer control subjects had normal physical examination and were free from systemic illnesses.
CFS subjects were evaluated for OI by use of upright tilt to 60° for 10 min. Symptoms of OI included dizziness, headache, fatigue, neurocognitive or sleep disorders, exercise intolerance, hyperventilation, nausea/abdominal pain, and/or sweating. All subjects were instrumented with a finger plethysmograph using a Finometer (Finapres Medical Systems) for beat-to-beat blood pressure and ECG for heart rate and rhythm. End-tidal carbon dioxide (ETCO2) was measured with a nasal cannula using capnography and arterial oxygen saturation measured by pulse oximetry (BCI Capnocheck Plus, Smiths Medical, Dublin, OH). We monitored respirations with a respiratory plethysmograph (Respitrace, NIMS, Miami, FL) that was calibrated against a pneumotachogram (Hans Rudolph, Shawnee, KS). We also used TCD (Neurovision, Yonkers, NY) of the right middle cerebral artery (MCA) to measure CBFv (38, 39). All parameters were recorded during initial evaluation and continuously during all subsequent testing. All data were acquired continuously to computer through an A/D converter (DATAQ; Milwaukee, WI) using custom computer software.
Once identified, CFS subjects with OI and hyperventilation/hypocapnea were asked to continue with n-back testing of their neurocognitive function. Use of medication was exclusionary for all subjects. If medication had been used, it was discontinued at least 2 wk prior to their study date. Prior to acceptance into the study, all subjects filled out a screening questionnaire concerning orthostatic intolerance, review of systems, and cognitive, physical, and social functioning. This study was approved by the Institutional Review Board of New York Medical College. All subjects 18 yr and older signed informed consent forms prior to participation in the study. Subjects less than 18 yr of age gave informed assent, and their legal guardians signed informed consent.
n-Back testing of neurocognitive function.
Prior to the start of the study, subjects filled out the Edinburgh Handedness Inventory to determine their dominant hand, which was used for responding during the n-back task. In addition, subjects completed the WTAR (Wechsler Test of Adult Reading) test to assess baseline reading ability, intelligence, and memory and to estimate any cognitive deficits prior to initiation of the study. Cognitive deficits were exclusionary for participation (39).
After the initial tilt during which CFS subjects were evaluated for OI, qualifying subjects were returned to a supine position for 30 min and then testing was performed with the n-back test, which challenges working memory, attention, concentration, and information processing. This was performed both while subjects were supine and during an upright tilt to 60° as previously described (6, 39, 41). The “n” refers to a number that indicates increasing difficulty. We used 0-, 1-, 2-, 3-, and 4-back levels as progressively increasing mental challenges.
At each n-back level of testing, a sequence of alphabetical characters is displayed on a video screen and the subject is asked to respond based on the following. During the 0-back level, the subject responds by activating a recording device when a particular character is displayed. We used the letter “O” as the response character. During the 1-back level, the subject responds if the current character displayed on the screen is the same as that previously displayed “1” character back. During the 2-back level, the subject responds if the current character displayed on the screen is the same as that previously displayed “2” characters back. This scheme is repeated incrementally for the “3”- and “4”-back steps. The stimulus duration (the amount of time the character was displayed on the screen) was 1 s, and the interstimulus duration (the amount of time between the display of any characters) was 1 s. Between each n-back level, there was a 10-s pause. To prevent confusion, the video screen displayed which n-back level was next during this pause. A computer generated a random sequence of 29 capital letters, excluding vowels, for each n-back level. Only the 0-back sequences contained the letter “O.” Each level of the n-back had its own individual, nonrepetitive sequence, and for continuity all subjects saw identical sequences in identical order. n-Back levels were presented in sequential order (0-back, then 1-back, then 2-back, then 3-back, and then 4-back). Subjects responded during each n-back task by pressing a switch placed in their dominant hand.
Protocol.
Throughout the entire protocol, a script was read to all subjects to ensure standardization of testing. After instrumentation, all subjects lay supine on a tilt table and awake with their eyes open for 5 min to accommodate. Subjects then practiced responding with the handheld switch to the beat of a metronome and then rested for 5 min. Next, subjects underwent three n-back task practice sessions with a 2-min rest between sessions. Subjects were allowed to ask questions during these practice sessions and were monitored to assess whether they correctly understood the n-back task. After successful completion of the n-back practice sessions, no more questions were allowed. After completion, subjects lay supine and awake with their eyes open for 15 min. Baseline measurements were taken throughout this period. The subjects then completed an n-back task while supine. After the task, the subjects were tilted to 60° for 10 min. The first 1 min of data was excluded to allow for hemodynamic stabilization to occur. Minutes 2–4 were taken as the baseline values, and the n-back task was administered during minutes 4–10. Subjects were instructed to inform us if they felt ill, nauseated, or presyncopal as a result of HUT. Presyncope was defined as a fall in systolic blood pressure of 20 mmHg or a systolic blood pressure below 80 mmHg with signs and symptoms of OI such as nausea, diaphoresis, pallor, and/or headache. Each subject completed the full tilt unless he/she requested to be returned to the supine position, at which point the test was ended. If significant hypotension below 80 mmHg and/or bradycardia below 50 bpm occurred, subjects were immediately lowered to the supine position and the test was ended. At the end of the tilt-table testing, subjects returned to the supine position and remained still for 10 min.
Data analysis and statistics.
For each n-back, the numbers of correct responses (C), missed responses, and incorrect false responses (F) were recorded. Correct responses were defined as the subject appropriately responding to the n-back challenge. Missed responses were defined as a subject not responding to an n-back repeat when he/she should have. False responses were defined as a subject responding inappropriately when an n-back repeat was not presented. Absolute response time (RT) was calculated in milliseconds as the difference between the time the letter first appeared (t0) on the video screen and the time the subject responded (tr). Thus RT = tr − t0. For each level of the n-back, we recorded the mean RT. The normalized response time (nRT) was calculated as the mean absolute RT per total number of responses, or the mean time per response, and nRT = RT/(C + F). This takes into account the number of times a subject responded. Data were recorded continuously at 200 Hz. NCSS 2007 (LCC) statistical software was used. Study group means for physiological measures were compared with unpaired t-tests. n-Back measures were analyzed with a repeated-measures ANOVA, including a group × period (i.e., n-back) interaction. When the interactions were significant, post hoc Student's t-tests using Bonferroni's adjustment were used to determine differences only between groups (21). Results are reported as means ± SE. Significance was set at P < 0.05.
Reversal of decreased CBFv and neurocognition.
To establish whether phenylephrine infusion could mitigate the decrease in neurocognition related to decreased CBFv, the following was used. Subjects returned for an additional evaluation following the initial screening tilt in which cardiovascular, pulmonary, and CBFv data were measured supine and during a tilt/n-back evaluation. We then loaded the baroreflex with the α1-adrenergic receptor agonist phenylephrine to prevent hyperventilation in these CFS subjects. Intravenous phenylephrine was initially infused at 0.2 μg·kg−1·min−1 and increased until systolic blood pressure was elevated by ∼10% (5, 44, 53, 56); once this elevation was achieved, a maintenance dose was infused during all subsequent testing.
RESULTS
Study population demographics.
There were no statistical differences in age, height, or weight between the CFS group (n = 15) and the control group (n = 11). The characteristics of the two groups are shown in Table 1.
Table 1.
Study population demographics
| Characteristics | Control Subjects | CFS Subjects |
|---|---|---|
| Age, yr | 24.1 ± 0.6 | 21.8 ± 1.4 |
| Weight, kg | 67.4 ± 3.9 | 61.8 ± 3.7 |
| Height, cm | 168.3 ± 1.2 | 165.7 ± 2.6 |
| Sex (F/M) | 7/4 | 14/1 |
Values are means ± SE. CFS, chronic fatigue syndrome.
Supine cardiorespiratory characteristics.
Although control subjects had a significantly lower HR while supine compared with CFS patients (54.6 ± 1.9 vs. 74.6 ± 2.1 bpm, P < 0.05), there were no differences while supine in mean blood pressure (71.8 ± 3.3 vs. 71.4 ± 3.1 mmHg), ETCO2 (44.9 ± 0.9 vs. 41.1 ± 0.5 Torr), or respiratory rate (16.7 ± 1.3 vs. 18.2 ± 1.0 breaths/min) comparing control subjects to CFS patients, respectively.
Cardiorespiratory effects of 60° HUT.
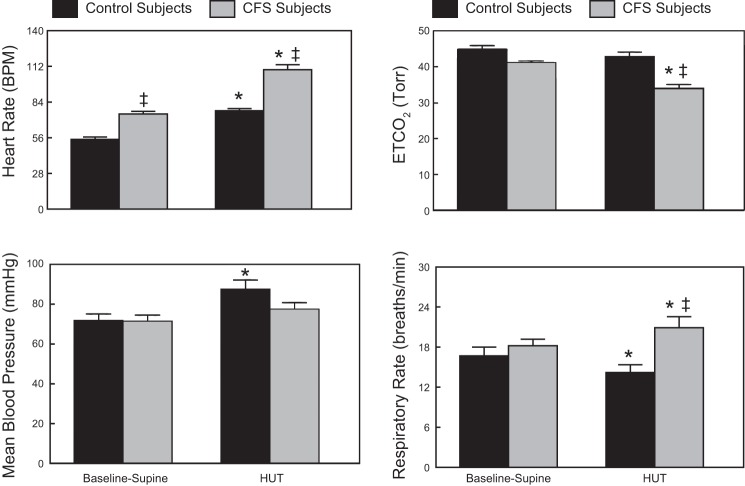
The response of both CFS and control subjects to a 60° HUT is shown in Fig. 1. HUT resulted in a significant increase in HR (109.4 ± 3.9 vs. 77.2 ± 1.6 bpm, P < 0.05) and respiratory rate (20.9 ± 1.7 vs. 14.2 ± 1.2 breaths/min, P < 0.05) and a significant decrease in ETCO2 (33.9 ± 1.1 vs. 42.8 ± 1.2 Torr, P < 0.05) in CFS patients compared with control subjects. During HUT, control subjects had a significant increase in HR (77.2 ± 1.6 vs. 54.6 ± 1.9 bpm, P < 0.05) and blood pressure (87.6 ± 4.5 vs. 71.8 ± 3.3 mmHg, P < 0.05) compared with the supine condition.
Fig. 1.
Comparison of Baseline-Supine and 60° head-up tilt (HUT) cardiorespiratory dynamics in control and chronic fatigue syndrome (CFS) subjects showing mean blood pressure, heart rate in beats/minute (bpm), respiratory rate, and end-tidal carbon dioxide (ETCO2). Data are shown as means ± SE. *Significantly different comparing Baseline-Supine to HUT (P < 0.05); ‡significantly different comparing control to CFS (P < 0.05).
In addition, a 60° HUT caused a significant decrease in CBFv in CFS compared with control subjects (58.0 ± 1.4 vs. 68.8 ± 2.5 cm/s, P < 0.05), as shown in Fig. 2. While control CBFv was reduced <10% by orthostasis, CFS subjects experienced a CBFv reduction that was >20%. This is similar to results that we have shown previously in response to a 70° HUT in POTS subjects (38).
Fig. 2.
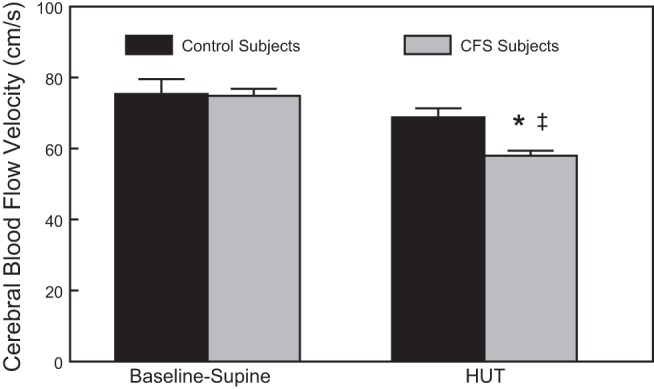
Comparison of Baseline-Supine and 60° HUT cerebral blood flow velocity in control and CFS subjects. Data are shown as means ± SE. *Significantly different comparing Baseline-Supine to HUT (P < 0.05); ‡significantly different comparing control to CFS (P < 0.05).
Cardiorespiratory response to phenylephrine.
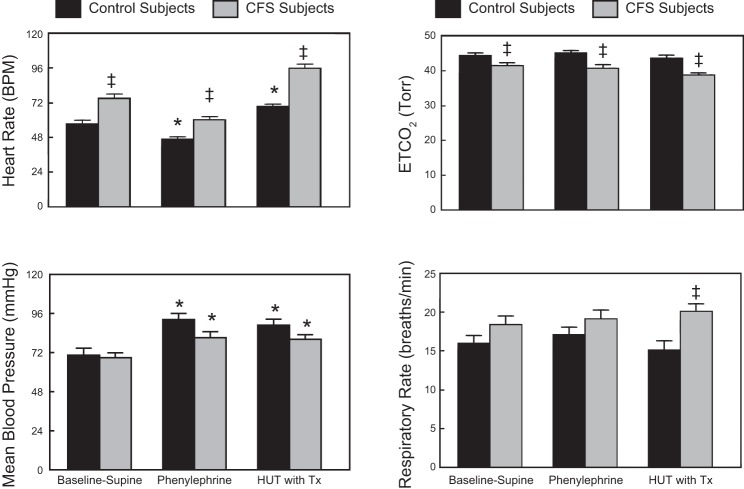
When phenylephrine was infused intravenously to achieve an ∼10% increase in supine blood pressure, as shown in Fig. 3, there was an anticipated compensatory significant decrease in HR in both control and CFS subjects. HUT during phenylephrine resulted in a significant increase in HR in both groups but no increase in blood pressure beyond that resulting from the drug alone. While ETCO2 was significantly lower (P < 0.05) and respiratory rate significantly higher (P < 0.05), comparing CFS to control, there were no changes in ETCO2 or respiratory rate comparing supine, supine plus phenylephrine, and HUT plus phenylephrine within groups.
Fig. 3.
Comparison of Baseline-Supine to Baseline-Supine with phenylephrine and 60° HUT with phenylephrine (HUT with Tx) cardiorespiratory dynamics in control and CFS subjects showing mean blood pressure, heart rate, respiratory rate, and ETCO2. Data are shown as means ± SE. *Significantly different comparing Baseline-Supine to HUT (P < 0.05); ‡significantly different comparing control to CFS (P < 0.05).
Effect of phenylephrine and 60° HUT on brain blood flow.
As shown in Fig. 4, phenylephrine caused a significant (P < 0.05) increase in supine CBFv only in control subjects (10.6% control; 3.0% in CFS patients). During orthostatic challenge (HUT), however, the phenylephrine-induced increase in CBFv mitigated the significant decrease on CBFv in both control subjects (0.2% increase) and CFS subjects (−8.1% vs. −22.5% untreated). Interestingly, HUT with phenylephrine resulted in a diminished CBFv in CFS subjects that was the same as healthy control subjects in the absence of treatment.
Fig. 4.
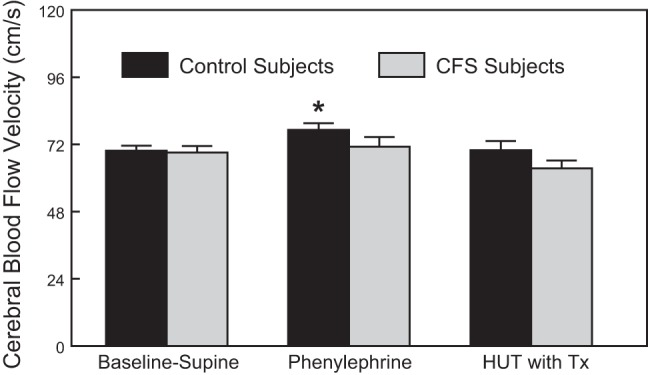
Comparison of Baseline-Supine to Baseline-Supine with phenylephrine and 60° HUT with phenylephrine (HUT with Tx) cerebral blood flow velocity in control and CFS subjects. Data are shown as means ± SE. *Significantly different comparing Baseline-Supine to phenylephrine (P < 0.05).
n-Back testing of neurocognitive function.
To evaluate the effect of CBFv restoration during HUT with phenylephrine on neurocognitive function nRT, n-back testing was performed, as it is a measure of performance that also takes into account the number of times a subject responded. This parameter increases with increasing task difficulty with the escalation from n = 0 to n = 4. Accordingly, as shown in Fig. 5, top, nRT increased as n increased, and when determined in control subjects there was no difference in n-back performance comparing supine to HUT (106.1 ± 6.9 vs. 97.6 ± 7.1 ms for n = 4, not significant). However, as shown in Fig. 5, bottom, CFS subjects performed more poorly, as their nRT increased significantly with increasing n-back difficulty (at n > 2) during HUT compared with supine (148.0 ± 9.3 vs. 96.4 ± 6.0 ms for n = 4, P < 0.05). Although not shown, n-back testing results in CFS subjects while supine were not different from results in control subjects either supine or upright. Thus the CFS subjects' neurocognitive performance was diminished by orthostasis.
Fig. 5.
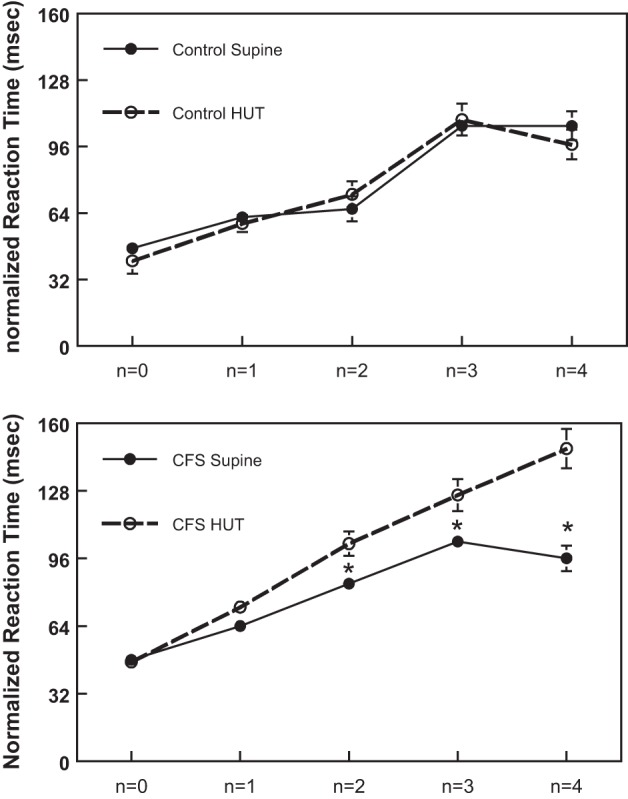
Neurocognitive function evaluated by n-back testing comparing normalized reaction time measured in control subjects (top), supine and during HUT, to that measured in CFS subjects (bottom), supine and during HUT. *Significantly different, comparing responses measured supine with those during HUT (P < 0.05).
Effect of phenylephrine on n-back testing of neurocognitive function.
To establish the influence of maintaining CBFv during orthostasis with phenylephrine (8.1% decrease with phenylephrine vs. −22.5% without) on neurocognitive performance, we performed n-back testing during HUT in the absence and presence of phenylephrine. Figure 6 shows that during HUT CFS patients' performance of n-back testing was significantly improved by phenylephrine for all values > n = 0, (114.6 ± 7.1 vs. 148.0 ± 9.3 ms for n = 4, P < 0.05). This is indicated by the reduction in nRT values for n-back levels 1–4 and a significant reduction in the slope of this relationship compared with control. In data not shown, there was no difference in the results of nRT in control subjects with or without phenylephrine and n-back in CFS following phenylephrine while upright was similar to that measured in control subjects.
Fig. 6.
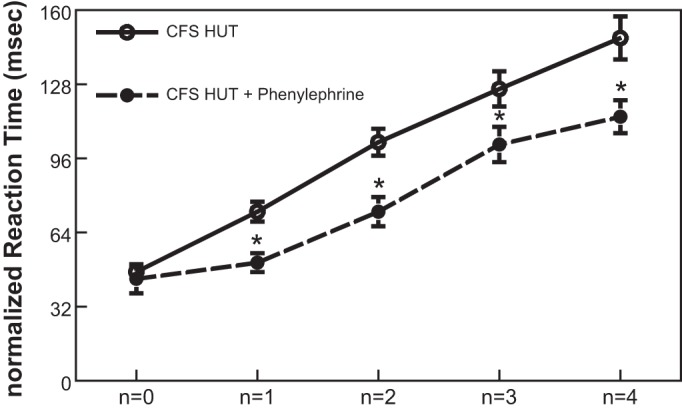
Neurocognitive function evaluated by n-back testing measured in CFS subjects during HUT, with and without phenylephrine. *Significantly different, comparing responses with and without phenylephrine (P < 0.05).
DISCUSSION
The present study shows that in CFS subjects mitigation of the HUT-induced decrease in CBFv with phenylephrine has a beneficial effect on neurocognitive function as measured by n-back performance. Neurocognitive impairment in CFS, subjectively described as mental fog or cloudiness, may be among the most debilitating aspects of this syndrome (1, 25, 27, 36, 39). CFS patients have impaired working memory and concentration and difficulty in processing complex information. When tested, CFS patients also have an inability to plan and order responses. Similarly, working memory deficits in CFS patients have been described during demanding tasks that required attention and switching between mental processing routines (10–12, 28, 29). We and others have shown that in CFS/POTS, orthostasis changes CO2, resulting in hypocapnia and hyperventilation (35, 50). While a cause for this neurocognitive impairment has not been established, it may be due to decreased cerebral blood flow (30, 50), as a direct effect of CO2 on cerebrovascular flow, altered cerebrovascular regulation, or a combination of these (38, 39).
The role of cerebral perfusion in neurocognitive dysfunction is unclear, as some studies support (4, 8, 24) while other studies fail to support (15, 31) findings of global or regional deficits of perfusion in patients with CFS. Our prior studies suggest this impaired perfusion hypothesis, as TCD demonstrated reduced cerebral blood flow (38) and cognitive loss in CFS/POTS patients (41) compared with healthy control subjects during 70° upright tilt but not while supine. In other experiments, we showed an increased respiratory chemoreflex response to hypoxia and a decreased respiratory chemoreflex response to hypercapnia in POTS compared with control, even while supine (56). These differences were enhanced by orthostasis.
Prior investigations have examined cognitive performance in CFS/POTS. While some studies were performed supine, and sometimes while seated, posture was never altered. However, the use of different positions (seated or supine) in prior studies could account for different results. An n-back study performed supine with BOLD fMRI found no n-back performance differences in CFS compared with control subjects; however, brain activation was reduced by 2-back and 3-back (7). On the other hand, cognitive deficits were not demonstrated by other investigators using a variety of cognitive testing tools (10, 12). Most cognitive testing modalities are unsuitable for time-delimited orthostatic testing. Differences of CFS from control appear to segregate with posture, such that testing shows no difference in cognition compared with control subjects when supine but does show a difference in cognition compared with control subjects while seated (10, 11, 59). This is consistent with orthostatic-dependent results, which likely are related to other orthostatic-dependent changes in CFS subjects that we have observed.
We have shown that peripheral chemoreflex sensitivity, measured by the hypoxic ventilatory response, was increased in POTS while central chemoreflex sensitivity, measured by the hypercapnic ventilatory response, was reduced (56). In CFS/POTS, baroreflex unloading during HUT stimulates peripheral oxygen-dependent chemoreflexes, causing hyperventilation that is unopposed by the restraining effects of hypocapnia. Hyperventilation causing decreased arterial partial pressure of CO2 and decreased ETCO2 is commonly observed when CFS/POTS subjects undergo tilt-table testing (35, 37, 50). Therefore in the present study we chose to load the baroreflex with phenylephrine to oppose this mechanism and to evaluate its ability to mitigate the effects of orthostasis on neurocognition.
Effects of phenylephrine on cardiorespiratory measurements.
In these studies, HUT in CFS subjects resulted in significant decreases in ETCO2 and increases in respiratory rate. Infusion of phenylephrine, however, prevented these changes, and ETCO2 and respiratory rate were the same as supine both during phenylephrine infusion supine and during HUT. Phenylephrine also prevented the significant drop in CBFv that is measured during HUT in CFS subjects. Since neurocognitive impairment due to reduced cerebral blood flow may be due in part to a direct effect of CO2 on cerebrovascular flow, altered cerebrovascular regulation, or a combination of these (38, 39), prevention of the orthostasis-induced decreases of CBFv and CO2 should result in improved upright neurocognitive testing in CFS subjects.
Changes in cerebral blood flow are tightly linked to alterations in CO2, and this cerebrovascular reactivity maintains central pH and thus affects the respiratory central chemoreceptor response. There are also studies that show that reduction of CBF-CO2 reactivity may lead to an enhanced ventilatory reactivity to CO2 through greater sensitivity of the central chemoreceptors (2). The exact nature of these relationships, however, remains somewhat controversial, as recent publications suggest that elevations in the sensitivity of peripheral and central chemoreflexes can either cause an increase in ventilation (a hyperadditive effect) or a decrease in ventilation (a hypoadditive effect) (13, 57, 60).
In the present study n-back testing while supine showed that in both control subjects and CFS subjects nRT increased with increasing challenge difficulty as n increased from 0 to 4. Control subjects performed equally, whether supine or during HUT. n-Back testing of supine CFS subjects was the same as that of control subjects. However, during HUT nRT was significantly longer compared with the supine condition, indicating increased difficulty in information processing while upright.
Effects of phenylephrine on n-back testing.
When n-back testing was done during infusion of phenylephrine, there was no difference in the performance of control subjects, supine or during HUT. However, in CFS subjects n-back results with phenylephrine during HUT were the same as those while supine. That is, in CFS subjects phenylephrine reversed the orthostasis-induced decrease in neurocognitive performance as measured by n-back testing.
We have previously shown that n-back outcome is impaired in CFS/POTS during orthostasis and that there is a progressive decrease in the number of correct identifications and increase in the reaction time of the n-back tasks measured during incremental tilt (39). We now show that phenylephrine, perhaps related to its ability to minimize the orthostatic reduction in brain blood flow velocity, significantly improved n-back performance in CFS subjects while upright and resulted in neurocognitive function similar to that measured in control subjects while upright. The mechanism for this effect has yet to be determined but may involve the effect of neural tasks on local functional hyperemia, so-called neurovascular coupling, as we have previously shown (52), or of blood pressure on the synchronization of CBFv affecting cerebral autoregulation.
Effects of phenylephrine on cerebral blood flow.
We have recently reported, based on cerebral near-infrared spectroscopy measurements during a modified Oxford maneuver [infusion of the NO donor sodium nitroprusside (SNP) followed by phenylephrine], that increased MCA blood flow after SNP was likely due to vasodilation (9). Cerebral blood flow measured in the MCA increased after phenylephrine and then returned to baseline. This is similar to previous findings in which CBFv decreased during SNP and increased during phenylephrine (51). In the present study, in contrast to the effect of SNP, it is possible that phenylephrine causes vasoconstriction of the cerebral vasculature mediated via elevation in perfusion pressure, an important determinant of cerebral reactivity (20). And, since phenylephrine does not cross the blood-brain barrier (19, 40), this vasoconstriction is likely a myogenic response to the increase in cerebral perfusion pressure.
Neuronal activity and cerebral blood flow are tightly coupled in both the resting and the activated brain, and neuronal activity causes increased cerebral blood flow (3), denoted as “functional hyperemia.” The coupling is sufficiently tight that local neuronal activity can be assessed by measuring regional blood flow (17). In studies employing incremental tilt, while CBFv decreased with tilt angle to a similar extent in CFS/POTS and control subjects, the expected increase of CBFv during cognitive and neuronal activation, denoted “neuronal activated CBFv,” was absent in CFS/POTS subjects. This is in contrast to control subjects, in whom progressive tasking difficulty enhances task-dependent blood flow, which is also orthostasis independent (52).
Increased cerebral blood flow during mental tasks is expected, and reflects enhanced neuronal activity. Phenylephrine, perhaps by maintaining cerebral blood flow during orthostatic challenge, may have avoided disruption in neurovascular coupling in CFS and maintained neuronal activity, restoring task-dependent blood flow and mental abilities. Elevating blood pressure during orthostasis with another α1-adrenergic agonist, midodrine, improves aspects of neurocognitive function in some subjects with spinal cord injury (40). Disruption in neurovascular coupling has been shown in diabetes, depression, hypertension, stroke, and Alzheimer disease (17). The hypothesis of disrupted neurovascular coupling receives support from the reduction in n-back performance with angle of tilt in CFS/POTS as we have previously reported (39).
The large drop in CBFv in CFS subjects during HUT also suggests that both dynamic and static cerebral autoregulation in these subjects are diminished and is consistent with the increase in coherence between mean arterial pressure (MAP) and CBFv that indicates reduced autoregulation (38). The increased coherence and synchronization between MAP and CBFv suggest that baroreflex-mediated fluctuations in pressure may be related to fluctuations in CBFv. Morita et al. (34) demonstrated that the microvasculature in the region of the MCA has sympathetic and parasympathetic innervation, and the removal of each, especially sympathetic nerves, greatly affects autoregulation (34). This supports the idea that baroreflex and sympathetic activity may be translated to changes in CBFv. Talman et al. also demonstrated that the baroreflex affects autoregulation by showing that disruption of the baroreflex attenuates the response of CBFv to increases in MAP (54). Caution must be exercised when comparing different studies of cerebral autoregulatory function, because descriptive parameters of autoregulation may not be comparable and because cerebral autoregulation is asymmetric when measured during different physiological states such as exercise and chronic hypotension (58).
Thus phenylephrine may alter neurovascular coupling in CFS subjects, either directly or by altering the cerebral autoregulation. This may be achieved by influencing the baroreflex “set point” of this response either directly or through changing peripheral resistance and systemic blood pressure. Since phenylephrine was able to significantly reduce the orthostatic fall in CBFv in CFS subjects and prevent the decreased n-back performance during HUT, understanding its mechanism of action is important in suggesting therapies directed at preventing so-called brain or mental fog in this population. The exact nature of these relationships is the subject of ongoing determinations.
Limitations.
Transcranial Doppler data should be interpreted cautiously because it measures global CBFv and is likely insensitive to small localized changes. However, its use is complementary to tilt methodology because of its rapid temporal resolution.
Changes in CBFv with phenylephrine may not represent changes in flow, as TCD measures CBFv rather than cerebral blood flow. CBF is dependent on the diameter of the insonated artery, and the diameter of the MCA may be resistant to change during orthostatic stress (45).
In the present study, the majority of CFS subjects were female (14:1 F:M), this because most CFS patients are female, with estimates ranging from 75% to 80% (26). The majority of our subjects were all menstruating women, but we did not determine the menstrual phase in any of our CFS subjects or control subjects. However, while previous studies have demonstrated that hormonal fluctuations that occur during the normal menstrual cycle may alter autonomic regulation of arterial pressure during various environmental stimuli (55), there is no apparent hormonal effect on orthostatic tolerance or related symptoms (22). Because of the skewed sexual distribution of CFS, it is unlikely that separate studies comparing like sexes can be accomplished.
GRANTS
This study was supported by a grant from the Chronic Fatigue and Immune Deficiency Syndrome Association of America (M. S. Medow) and National Heart, Lung, and Blood Institute grants RO1 HL-112736 and RO1 HL-074873 (J. M. Stewart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.M., C.T., and J.M.S. conception and design of research; M.S.M., S.S., Z.R.M., and S.D. performed experiments; M.S.M., S.S., Z.R.M., S.D., and J.M.S. analyzed data; M.S.M., S.S., Z.R.M., S.D., C.T., and J.M.S. interpreted results of experiments; M.S.M. prepared figures; M.S.M. drafted manuscript; M.S.M., S.S., Z.R.M., S.D., C.T., and J.M.S. edited and revised manuscript; M.S.M. approved final version of manuscript.
REFERENCES
- 1.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 160: 221–236, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci 301: 9–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110: 3193–3198, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Caseras X, Mataix-Cols D, Giampietro V, Rimes KA, Brammer M, Zelaya F, Chalder T, Godfrey EL. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med 68: 947–955, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman B, Biaggioni I. Intravascular source of adenosine during forearm ischemia in humans: implications for reactive hyperemia. Hypertension 33: 1453–1457, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Del Pozzi AT, Pandey A, Medow MS, Messer ZR, Stewart JM. Blunted cerebral blood flow velocity in response to a nitric oxide donor in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 307: H397–H404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc 10: 101–109, 2004. [DOI] [PubMed] [Google Scholar]
- 11.DeLuca J, Johnson SK, Ellis SP, Natelson BH. Cognitive functioning is impaired in patients with chronic fatigue syndrome devoid of psychiatric disease. J Neurol Neurosurg Psychiatry 62: 151–155, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobbs BM, Dobbs AR, Kiss I. Working memory deficits associated with chronic fatigue syndrome. J Int Neuropsychol Soc 7: 285–293, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Duffin J, Mateika JH. CrossTalk opposing view: Peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol 591: 4351–4353, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evengard B, Schacterle RS, Komaroff AL. Chronic fatigue syndrome: new insights and old ignorance. J Intern Med 246: 455–469, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Fischler B, D'Haenen H, Cluydts R, Michiels V, Demets K, Bossuyt A, Kaufman L, De Meirleir K. Comparison of 99mTc HMPAO SPECT scan between chronic fatigue syndrome, major depression and healthy controls: an exploratory study of clinical correlates of regional cerebral blood flow. Neuropsychobiology 34: 175–183, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 121: 953–959, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 100: 328–335, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Glass JM. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: new trends and future directions. Curr Rheumatol Rep 8: 425–429, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hansson E, Schmiterlow CG. A comparison of the distribution, excretion and metabolism of a tertiary (promethazine) and a quaternary (Aprobit) phenothiazine compound labelled with S35. Arch Int Pharmacodyn Ther 131: 309–324, 1961. [PubMed] [Google Scholar]
- 20.Harper AM, Glass HI. Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry 28: 449–452, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hintze JL. General Linear Models (GLM) In: NCSS Help Systems (www.ncss.com). Kaysville, UT: NCSS, 2007. [Google Scholar]
- 22.Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab 87: 1569–1575, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hoad A, Spickett G, Elliott J, Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. QJM 101: 961–965, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Ichise M, Salit IE, Abbey SE, Chung DG, Gray B, Kirsh JC, Freedman M. Assessment of regional cerebral perfusion by 99Tcm-HMPAO SPECT in chronic fatigue syndrome. Nucl Med Commun 13: 767–772, 1992. [PubMed] [Google Scholar]
- 25.Jain SS, DeLisa JA. Chronic fatigue syndrome: a literature review from a physiatric perspective. Am J Phys Med Rehabil 77: 160–167, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, McCready W, Huang CF, Plioplys S. A community-based study of chronic fatigue syndrome. Arch Intern Med 159: 2129–2137, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen R. Chronic fatigue: an evolutionary concept analysis. J Adv Nurs 63: 199–207, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Joyce E, Blumenthal S, Wessely S. Memory, attention, and executive function in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 60: 495–503, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komaroff AL. Clinical presentation of chronic fatigue syndrome. Ciba Found Symp 173: 43–54, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Low PA, Novak V, Spies JM, Novak P, Petty GW. Cerebrovascular regulation in the postural orthostatic tachycardia syndrome (POTS). Am J Med Sci 317: 124–133, 1999. [DOI] [PubMed] [Google Scholar]
- 31.MacHale SM, Lawrie SM, Cavanagh JT, Glabus MF, Murray CL, Goodwin GM, Ebmeier KP. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry 176: 550–556, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Medow MS, Aggarwal A, Baugham I, Messer Z, Stewart JM. Modulation of the axon-reflex response to local heat by reactive oxygen species in subjects with chronic fatigue syndrome. J Appl Physiol (1985) 114: 45–51, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 15: 67–75, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular sympathetic, parasympathetic, and sensory nerves on autoregulation and spontaneous vasomotion. Acta Physiol Scand 154: 121–130, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Natelson BH, Intriligator R, Cherniack NS, Chandler HK, Stewart JM. Hypocapnia is a biological marker for orthostatic intolerance in some patients with chronic fatigue syndrome. Dyn Med 6: 2, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natelson BH, Lange G. A status report on chronic fatigue syndrome. Environ Health Perspect 110, Suppl 4: 673–677, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 297: H664–H673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 122: 227–238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olesen J. The effect of intracarotid epinephrine, norepinephrine, and angiotensin on the regional cerebral blood flow in man. Neurology 22: 978–987, 1972. [DOI] [PubMed] [Google Scholar]
- 41.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razumovsky AY, DeBusk K, Calkins H, Snader S, Lucas KE, Vyas P, Hanley DF, Rowe PC. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J Neuroimaging 13: 57–67, 2003. [PubMed] [Google Scholar]
- 43.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, Medow MS, Natelson BH, Stewart JM, Mathew SJ. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 25: 1073–1087, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 160: 222–226, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart J, Weldon A, Arlievsky N, Li K, Munoz J. Neurally mediated hypotension and autonomic dysfunction measured by heart rate variability during head-up tilt testing in children with chronic fatigue syndrome. Clin Auton Res 8: 221–230, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr 135: 218–225, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904–H913, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart JM, Medow MS, DelPozzi A, Messer ZR, Terilli C, Schwartz CE. Middle cerebral O2 delivery during the modified Oxford maneuver increases with sodium nitroprusside and decreases during phenylephrine. Am J Physiol Heart Circ Physiol 304: H1576–H1583, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 302: H1185–H1194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300: H1492–H1500, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talman WT, Dragon DN, Ohta H. Baroreflexes influence autoregulation of cerebral blood flow during hypertension. Am J Physiol Heart Circ Physiol 267: H1183–H1189, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol 285: R1091–R1097, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Taneja I, Medow MS, Clarke DA, Ocon AJ, Stewart JM. Baroreceptor unloading in postural tachycardia syndrome augments peripheral chemoreceptor sensitivity and decreases central chemoreceptor sensitivity. Am J Physiol Heart Circ Physiol 301: H173–H179, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teppema LJ, Smith CA. CrossTalk opposing view: Peripheral and central chemoreceptors have hyperadditive effects on respiratory motor control. J Physiol 591: 4359–4361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 56: 268–273, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Vollmer-Conna U, Wakefield D, Lloyd A, Hickie I, Lemon J, Bird KD, Westbrook RF. Cognitive deficits in patients suffering from chronic fatigue syndrome, acute infective illness or depression. Br J Psychiatry 171: 377–381, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Wilson RJ, Day TA. CrossTalk opposing view: Peripheral and central chemoreceptors have hypoadditive effects on respiratory motor output. J Physiol 591: 4355–4357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging 26: 83–86, 2006. [DOI] [PubMed] [Google Scholar]