Abstract
Background
Antiretroviral therapy (ART) reduces the risk of tuberculosis, but the incidence still exceeds that in HIV-uninfected people. Isoniazid preventive therapy (IPT), which decreases the risk of tuberculosis in people not on ART, may offer additional protection.
Methods
Pragmatic randomized double-blind placebo-controlled trial to evaluate the effect of 12 months IPT among participants established on or newly starting ART, in Khayelitsha, South Africa (NCT00463086, Lancet D-09-02885). Tuberculosis was excluded at screening by sputum culture. Incident tuberculosis was the primary endpoint.
Findings
1,329 participants contributed 3,227 person-years (PY) of follow up in the modified intention-to-treat analysis; 662 on IPT and 667 on placebo. There were 95 incident tuberculosis cases: 2.3 (95%CI 1.6-3.1) versus 3.6 (95%CI 2.8-4.7) per 100 PY in the IPT and placebo arms respectively (hazard ratio 0.63, 95%CI 0.41-0.94). Study drug was discontinued due to grade 3 or 4 raised ALT in 19/662 in the IPT and 10/667 in the placebo arm, risk ratio=1.9 (95%CI 0.90-4.09). In secondary analyses, there was no evidence that the effect of IPT was restricted to those who were positive on tuberculin skin test (TST) or interferon gamma release assay (IGRA): adjusted hazard ratio for those with negative tests 0.43 (95%CI 0.21-0.86) and 0.43 (95%CI 0.20-0.96); for positive tests 0.86 (95%CI 0.37-2.00) and 0.55 (95%CI 0.26-1.24) respectively. No all cause mortality benefit of IPT was demonstrated
Interpretation
IPT reduced the incidence of tuberculosis in HIV-infected individuals on ART. In this high incidence setting, individuals on ART who have TST or IGRA negative results may also benefit from IPT. IPT can easily be implemented in ART clinics.
Background
Tuberculosis is a major cause of morbidity and mortality in HIV-infected people. The burden is greatest in sub-Saharan Africa, especially in Southern Africa where more than 50% of new tuberculosis cases are co-infected with HIV-1 (1). Meta-analysis of randomized controlled trials show that isoniazid preventive therapy (IPT) decreases the risk of tuberculosis by 32% in HIV-1 infected people not on combination antiretroviral therapy (ART), but strong statistical evidence for benefit was only seen in those who are tuberculin skin test (TST) positive.(2) However, there was heterogeneity of IPT duration and follow up between the included studies. ART independently reduces the risk of tuberculosis by 65%,(3) but the incidence still exceeds that in HIV-uninfected people(4).
Three retrospective observational cohort studies suggested a greater impact of the combined effect of ART and IPT on the risk of tuberculosis than of ART alone. (5-7) The proportion of individuals that received both treatments concurrently was unclear in the Brazilian study, and IPT preceded ART in the South African study.(6, 7) In the BOTUSA study, a randomized controlled trial of 36 versus 6 months of IPT conducted in Botswana, IPT provided for 36 months was shown to be beneficial in those who started ART.(8) However, ART was prescribed in some of the participants at variable times during follow-up when participants fulfilled criteria for ART initiation. Thus the independent effect of IPT in patients on ART could not be determined. Some participants in an Indian study comparing the effect of six months of ethambutol plus isoniazid with 36 months of isoniazid started ART during the study, but the effect of concomitant ART and IPT on efficacy and toxicity was not reported.(9)
Concurrent IPT and ART could result in shared toxicity, notably hepatitis and neuropathy. Furthermore, isoniazid inhibits several cytochrome P450 isoenzymes(10) that metabolise many antiretrovirals. This may cause increased antiretroviral concentrations, which may exceed the toxic threshold. Therefore it is important to establish if IPT further reduces the risk of tuberculosis in people on ART, and quantify any additional toxicity.
We therefore conducted a pragmatic randomized double-blind placebo-controlled trial to evaluate the effect of IPT on the risk of active tuberculosis in HIV-1 infected people concurrently receiving ART. Our secondary objectives included determining drug toxicity and all-cause mortality.
Methods
Study setting, design and intervention
A pragmatic 1:1 individually randomized double-blind placebo-controlled trial of IPT in HIV-1 infected persons on ART was conducted at the Ubuntu Clinic in Khayelitsha, Cape Town, South Africa. The intervention was daily self-administered isoniazid or matching placebo dosed according to body weight (200 mg/day for <50 kg or 300 mg/day for ≥50 kg) together with 25mg of pyridoxine for 12 months (could be completed over a 15 month period).
Randomisation and masking
Participants were randomly allocated to isoniazid or placebo using random number generator software. Randomisation was stratified by ART status at baseline: newly on ART (start-ART) versus established on ART (on-ART). Participants, clinicians and local pharmacy staff were blinded to treatment allocations. Further details on randomisation and masking are described in the study protocol (webappendix B).
Inclusion and exclusion criteria
Adults (≥18 years) were recruited amongst ART clinic attendees consecutively listed in study screening logs. All participants had baseline tuberculosis symptom screening and sputum mycobacterial culture performed.(11) Exclusion criteria were: 1) active tuberculosis or suspicion of active tuberculosis as determined by symptom screening; 2) Current or previous treatment of latent tuberculosis infection; 3) Current treatment with fluoroquinolones or other antibiotics with significant antituberculous activity; 4) History of intolerance to isoniazid; 5) Grade 3/4 baseline alanine transaminase (ALT); 6) Grade 3/4 peripheral neuropathy; 7) Pregnancy or <6 weeks post-partum. The AIDS Clinical Trials Group (ACTG) tables for grading drug toxicity for persons on ART were used.
Follow-up procedures
At each visit participants were asked about symptoms of drug adverse events: nausea, vomiting, or right upper quadrant pain, rashes, and new or worsening peripheral neuropathy. ALT was performed at baseline, monthly for the first three months and 3 monthly thereafter. Protocol-specified reasons for permanently stopping study drug due to toxicity were new or worsening peripheral neuropathy of grade 2 or more, grade 3/4 raised ALT or clinical hepatitis, new rash grade 2 or more. CD4+ lymphocyte counts and viral loads were performed according to clinic protocols: initially 6-monthly then yearly beyond the first year on ART from 2010. Clinic nurses and doctors followed up participants routinely; schedules were aligned with ART appointments to aid participant retention. Pharmacy staff dispensed the study drug along with other routine prescriptions. Pharmacy refill records were used to monitor adherence to ART and the study drug. Screening started on 1 November 2007, the first participant was randomized on 31 January 2008, the last completed the study drug on 31 October 2010 and study closure was on 30 September 2011.
Ascertainment of incident active tuberculosis
A standard tuberculosis symptom screen was done at each clinic visit. Two sputum specimens were obtained from tuberculosis suspects for microscopy by auramine staining and for mycobacterial culture, with species identification and drug sensitivity testing for isoniazid and rifampicin of all positive isolates (using BACTEC Mycobacterial Growth Indicator Tube, Becton Dickinson Microbiology Systems, Cockeysville, MD, USA). Specimens were processed at the National Health Laboratory Service. Sputum induction was performed on individuals unable to expectorate spontaneously. Needle aspiration biopsy was performed in cases presenting with suspected tuberculosis lymphadenitis. Cases suspected with extra-pulmonary tuberculosis (EPTB) and requiring further investigation were referred to an HIV specialist clinic. Urine for acid fast bacilli smear and culture was requested in all participants with suspected EPTB. Infectious disease specialists (GM & RJW) were consulted for difficult cases. The provincial electronic tuberculosis register and the database of the National Health Laboratory Service were searched for tuberculosis cases at study closure. Database linking was via a unique patient number and/or national identity numbers. At each clinic visit, participants were also asked about tuberculosis investigations or treatment initiated outside the Ubuntu clinic. The provincial electronic tuberculosis register and the database of the National Health Laboratory Service were searched at study closure for tuberculosis cases to verify completeness of ascertainment and identify cases diagnosed at other sites in patients who might have left care. All incident cases were verified prior to study unblinding. Tuberculosis treatment was commenced for all those who met the case definition for tuberculosis: definite if compatible clinical features plus culture positive for M tuberculosis; probable if based on microscopy, or possible if based only on radiology and/or clinical features.
Analysis
Results of this trial are reported in accordance with the CONSORT and Pragmatic Trials in Healthcare guidelines for reporting pragmatic trials.(12) A modified intention-to-treat analysis was conducted: any randomized participants who withdrew from the study before receiving the study drug or those whose baseline sputum culture results indicated prevalent tuberculosis after randomisation were excluded. The primary endpoint was time to development of incident tuberculosis (definite, probable or possible) during the study period. Secondary endpoints were time to death or the risk adverse drug reaction. For the primary outcome person-time at risk was calculated from date of randomisation to earliest of (i) tuberculosis (the clinic visit date tuberculosis was diagnosed and registered in the clinic database or notified in the tuberculosis register); (ii) death (death ascertainment was by report to the clinic staff and augmented from the national death register); (iii) loss to follow up (participants were defined as lost to follow up if their last clinic contact was more than 6 months before the date of closure of the study database-these were censored at their last contact date); (iv) transferred out (excluding participants transferred out for care at another clinic in the district since outcome data is available on the province-wide patient electronic register and from the national TB register); (v) study closure (30 September 2011). The log-rank test was used to compare survival curves by treatment arm. The hazard ratio for the treatment effect and the associated 95% confidenceintervals (CI) were calculated by Cox proportional hazards regression. To assess the durability of the treatment effect, follow-up time was split into three time periods, 0-11 months, 12-23 months, and ≥24 months, and the likelihood ratio test used to test for effect modification by time interval. Tuberculin skin tests (TST; 2TU RT23 PPD, Statens Serum Institut, Denmark) and interferon gamma release assays (IGRA; QuantiFERON Gold In-Tube, Cellestis, Australia) were performed as part of a nested study.(13) TST and IGRA were performed at the baseline screening visit, prior to application of RCT-specific inclusion/exclusion criteria, in persons who indicated willingness to return for TST results. Manufacturer’s criteria for IGRA positivity were used (≥ 0.35 IU/ml); TST induration of ≥ 5 mm was deemed positive. Those who did not accept TST/IGRA testing did not differ from those who did with respect to age, gender, CD4+ count, previous history or ART status (webappendix A). Effects of isoniazid by period since randomisation and by tuberculosis infection status at enrolment were specified at the time of the analysis. Time from randomisation to death was compared by study arm and hazard ratios were calculated from the Cox proportional hazards model.
A final sample size of 1,368 had 80% power to detect a 35% reduction in the incidence of tuberculosis in the intervention versus control group assuming a rate of 8.5 per 100 person years in the control group, a type I error of 0.05 and a 30% loss to follow up rate in each arm (see protocol in webappendix B for further details).(14, 15) All analyses were performed using STATA version 12.0 (Stata Corp, College Station, TX, USA).
Ethics approval was obtained from the ethics review boards of the University of Cape Town, Médecins Sans Frontières, and the London School of Hygiene and Tropical Medicine. Written consent or a thumb-print was required from all participants prior to screening. A four-member data safety and monitoring board provided oversight during the study period. The trial was registered at www.clinicaltrials.gov (HAART-IPT Study NCT00463086), and the protocol peer-reviewed and approved by the Lancet (Lancet D-09-02885).
Results
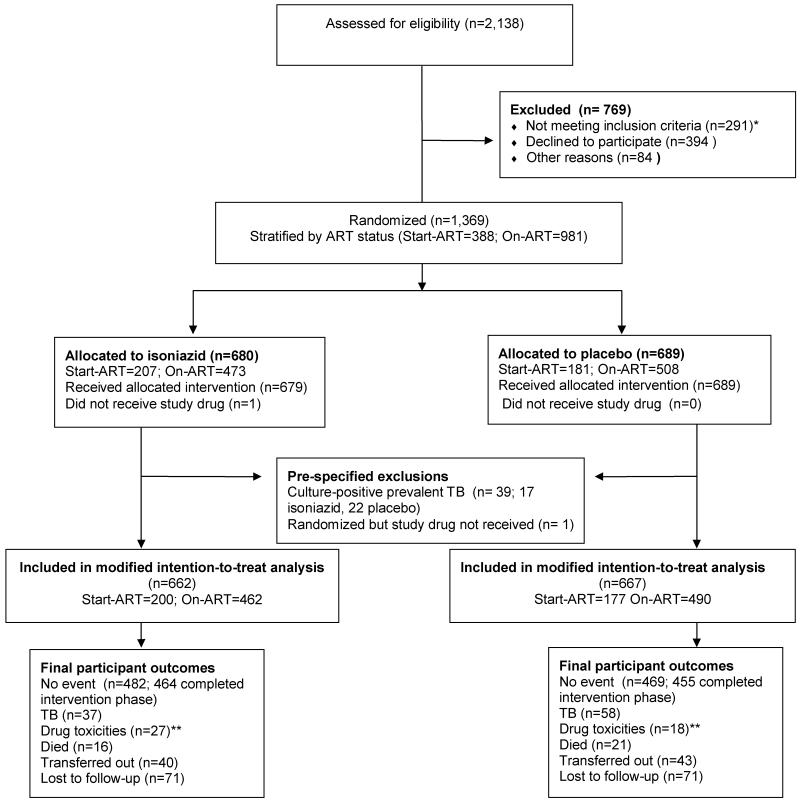
2,138 HIV infected individuals were assessed for eligibility. Following 769 exclusions, 291 due to failure to meet the inclusion criteria, 1,369 participants were randomized to either placebo or isoniazid (Figure 1-CONSORT flow diagram). Characteristics at screening between the 478 participants who were screened but were not randomized and the 64% who were are given in Table S1: the proportion already established on ART was lower amongst those who were not randomized and the proportion with previous TB was higher amongst those randomized (42.2% vs. 34.5%). 39 culture-positive prevalent tuberculosis cases were diagnosed after randomisation, and one person did not receive the study drug, leaving 1,329 in the modified intention-to-treat analysis (ITT); 667 placebo and 662 isoniazid. Baseline characteristics at enrolment for those in the modified ITT were similarly distributed between the arms (Table 1). The median time on ART was 357 days (interquartile range (IQR): 139-798) in the on-ART group and 14 days (IQR: 4-25) in the start-ART group. Reasons for stopping placebo or isoniazid during the active intervention phase are given by study arm in Table S2, webappendix A. The maximum follow up time was 3.7 years, with a median of 2.5 years (IQR: 2.1-3.1). Numbers at study closure that transferred out or were lost to follow-up were comparable between study arms. The proportion lost to follow up in each arm was 11%; far less than the 30% initially assumed.
Figure 1. CONSORT Flow Diagram.
*Reasons for excluding 291 individuals that did not meet the inclusion criteria: 211 prevalent TB diagnosed, 13 prior IPT, 19 pregnancy, 23 pre-existing Grade 3 toxicity (ALT, peripheral neuropathy or rash), 1 age under 18 years, 24 already on TB treatment. **Drug toxicities include: ALT ≥ grade 3, clinical hepatitis, ≥ grade 2 rash or peripheral neuropathy.
Table 1. Baseline characteristics at enrolment of those included in intention to treat analysis, by study arm.
| Characteristic | 667 1Placebo | 662 2Isoniazid | 1,329 Total |
|---|---|---|---|
| Median age (IQR), years | 34 (29-40) | 34 (30-40) | 34 (30-40) |
| Female | 74.7% (498) | 75.5% (500) | 75.1% (998) |
| Established on ART | 73.5% (490) | 69.8% (462) | 71.6% (952) |
| Median days on ART (IQR), On-ART | 330 (137-727) | 394 (139-889) | 357 (139-798) |
| Median days on ART (IQR), Start ART | 14 (4-20) | 14 (4-25) | 14 (4-25) |
| Median CD4+ count (IQR), cells/mm 3 | 214 (154-355) | 218 (150-373) | 216 (152-360) |
| N=602 | N=585 | N=1187 | |
| Previous TB | 41% (271/657) | 44% (289/652) | 42.8% (560/1,309) |
| Tuberculin Skin Test (TST) | |||
| Positive (≥5mm) | 30.3% (202) | 28.7% (190) | 29.5% (392) |
| Negative (<5mm) | 42.4% (283) | 40.6% (269) | 41.5% (552) |
| Unknown | 27.3% (182) | 30.7% (203) | 29.0% (385) |
| Interferon Gamma Release Assay (IGRA) | |||
| Positive | 30.7% (205) | 27.8% (184) | 29.3% (389) |
| Negative | 39.1% (261) | 41.4% (274) | 40.3% (535) |
| Indeterminate | 5.7% (38) | 5.4% (36) | 5.6% (74) |
| Unknown | 24.4% (163) | 25.4% (168) | 24.9% (331) |
| Discordant/Concordant TST/IGRA pairs | |||
| TST+/IGRA+ | 17.8% (119) | 17.4% (115) | 17.6% (234) |
| TST−/IGRA− | 28.5% (190) | 29.9% (198) | 29.2% (388) |
| TST+/IGRA− | 10.9% (73) | 10.0% (66) | 10.5% (139) |
| TST−/IGRA+ | 9.6% (64) | 8.2% (54) | 8.9% (118) |
| Unknown | 33.1% (221) | 34.6% (229) | 33.9% (450) |
| Combination ART regimen | |||
| D4T/3TC/EFV | 40.8% (272) | 38.2% (253) | 39.5% (525) |
| AZT/3TC/EFV | 5.4% (36) | 8.0% (53) | 6.7% (89) |
| D4T/3TC/NVP | 33.4% (223) | 32.0% (212) | 32.7% (435) |
| AZT/3TC/NVP | 17.7% (118) | 19.6% (130) | 18.7% (248) |
| *Other | 2.7% (18) | 2.1% (14) | 2.4% (32) |
| Median baseline ALT (IQR), IU/ml | 28 (21-43) | 30 (22-41) | 29 (22-42) |
IQR interquartile range; ALT Alanine transaminase; D4T stavudine; AZT azidothymidine or zidovudine; 3TC lamivudine; EFV efavirenz; NVP nevirapine
Other ART regimen: AZT/didanosine/lopinavir/ritonavir (2-placebo), 3TC/EFV/tenofovir (9-placebo; 12-isoniazid), D4T/3TC/ lopinavir/ritonavir (1-placebo; 1-isoniazid), AZT/3TC/ lopinavir/ritonavir (2-placebo), 3TC/NVP/ tenofovir (3-placebo; 1-isoniazid), 3TC/ tenofovir / lopinavir/ritonavir (1-placebo)
Adherence to the study drug
For those participants in each arm who completed 12 months of the study drug phase (N=1,100), a median of 360 doses (IQR: 330-390) were dispensed over a median of 12 months on the study drug (IQR: 11-13 months) (Figure S1a & Sb, webappendix A). Median months on study drug and total doses dispensed did not differ by study arm.
Primary endpoint: Incidence of tuberculosis (all diagnoses)
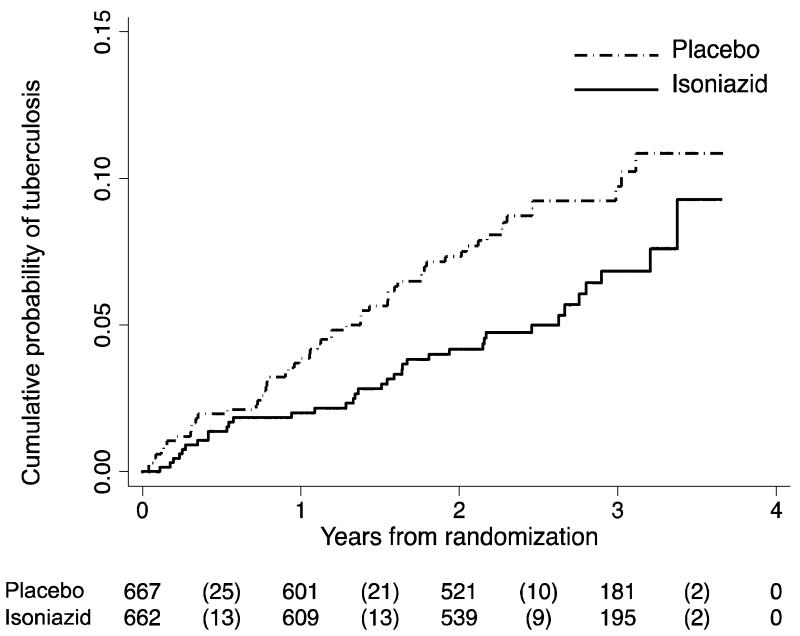
There were 95 cases of tuberculosis that developed over 3,226.5 person-years of follow up; 58 on placebo and 37 on isoniazid (Table 2); 36% (34/95) culture-confirmed. 59% were diagnosed at the study site and 39% at satellite clinics (of whom 7% were identified through linkage to national health laboratory service and provincial electronic tuberculosis notifications data). 75% of cases developed during the first two years of follow-up after randomisation (Figure 2a). The overall rate of tuberculosis was 2.9 per 100 person-years; 2.3 (95%CI 1.6-3.) in the isoniazid arm vs. 3.6 (95%CI 2.8-4.7) per 100 person-years in the placebo arm (unadjusted hazard ratio (HR) 0.63, 95% CI 0.41-0.94; Logrank P=0.02; 1-beta=0.80) (Figure 2a). Adjusting for time-updated CD4+ count did not significantly change the overall hazard ratio for tuberculosis, HR=0.64, (95% CI 0.42 - 0.96). Eight of the 95 tuberculosis cases subsequently died during that treatment episode (two isoniazid and six placebo). Drug sensitivity testing was performed on 25 of the 34 culture-confirmed cases of tuberculosis: four had multi-drug resistant tuberculosis (three placebo and one isoniazid), and two had isoniazid mono-resistance (both on isoniazid).
Table 2. Effect of isoniazid on rate of tuberculosis or death, or risk of stopping the study drug due to adverse events.
| Overall | Placebo | Isoniazid | Effect | ||||
|---|---|---|---|---|---|---|---|
| Endpoint | Events /PY |
Rate/ 100 PY |
Events /PY |
Rate/ 100 PY |
Events /PY |
Rate/ 100 PY |
HRu (95% CI) |
| Tuberculosis | |||||||
| All tuberculosis | 95/ 3226.5 | 2.9 | 58/ 1597.2 | 3.6 | 37/ 1629.3 | 2.3 | 0.63* (0.41 - 0.94) |
| Definite | 34/ 3226.5 | 1.1 | 22/1597.2 | 1.4 | 12/ 1629.3 | 0.7 | 0.54 (0.27 - 1.08) |
| Probable/possible | 61/ 3226.5 | 1.9 | 36/ 1597.2 | 2.3 | 25/ 1629.3 | 1.5 | 0.68 (0.41 - 1.10) |
| Deaths | |||||||
| All causes | 37/3579.12 | 1.0 | 21/1792.8 | 1.2 | 16/1786.3 | 0.9 | 0.72 (0.34 - 1.34) |
| Stopping drug for adverse events | Events/n | Risk | Events/n | Risk | Events/n | Risk | RRu 95% CI |
| 1All reasons | 196/ 1,329 | 14.7% | 94/667 | 14.1% | 102/ 662 | 15.4% | 1.1 (0.84 - 1.42) |
| ALT ≥ grade 3 | 29/ 1,329 | 2.2% | 10/ 667 | 1.5% | 19/ 662 | 2.9% | 1.9 (0.90 - 4.09) |
| ALT ≥ grade 3, clinical hepatitis, ≥ grade 2 rash or peripheral neuropathy | 45/ 1,329 | 3.4% | 18/ 667 | 2.7% | 27/ 662 | 4.1% | 1.5 (0.84 - 2.72) |
PY person-years; HRu unadjusted hazard ratio; CI confidence interval; RRu unadjusted relative risk; ALT alanine transaminase. Maximum time at risk=3.7 years. PY for death is > PY for TB: Four individuals died after developing TB. Total numbers enrolled, risks in those enrolled (%), RRu unadjusted risk ratio for INH versus placebo.
All reasons excluding tuberculosis.
P < 0.05
Figure 2a. Time to tuberculosis from randomisation.
Placebo group was prescribed ART plus placebo and isoniazid group was prescribed ART plus isoniazid. Numbers show the numbers being followed at each time point, and the numbers in parentheses indicate new tuberculosis cases in each period. Logrank test p-value for equality of survival curves =0.02
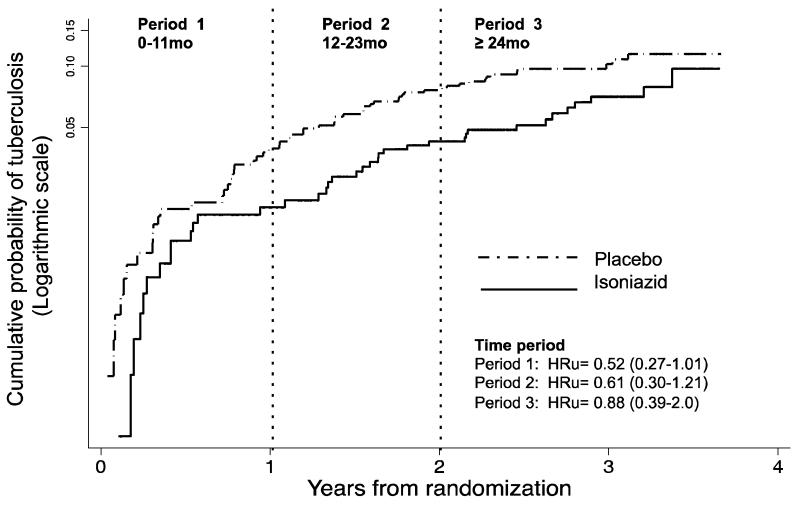
The effect of isoniazid on the risk of tuberculosis appeared to be greatest in the first year of follow-up when individuals were still on treatment; unadjusted HR=0.52 (95% CI 0.27-1.01) (Figure 2b). The effect appeared to wane over time: HR=0.61 (95% CI 0.30-1.21) 12-23 months, and HR=0.78 (95% CI 0.39-2.0) ≥ 24 months. However, there was insufficient statistical power to demonstrate formal interaction by time period since randomisation (P=0.34, assuming linear effect for period). Tuberculosis rates per study period since randomisation are shown in Table S3, webappendix A.
Figure 2b. Cumulative hazard plot for ART vs. ART plus IPT effect by time period since randomisation.
Nelson-Aalen cumulative hazard plot on a logarithmic y-scale to indicate proportionality of hazards over time periods. HRu= unadjusted hazard ratio (95% confidence interval indicated in parentheses). Treatment ended 1 year after randomisation. Likelihood ratio test for interaction of treatment arm with time period P=0.61; & assuming linear trend for time period P=0.34.
Effect by tuberculosis infection status at enrolment
Analyses to examine the effect of ART with isoniazid on time to tuberculosis stratified by TST or IGRA status are shown in Table 3. The effect of isoniazid on tuberculosis incidence appeared greater in TST or IGRA negative groups than in those who were TST or IGRA positive, but there was weak statistical evidence that the effects were different (Interaction P=0.58 and P=0.24, respectively) after adjustment for baseline CD4+ count and ART status on enrolment.
Table 3. Effect of isoniazid on the rate of all tuberculosis stratified by markers of M.tuberculosis infection status at enrolment.
| Placebo | Isoniazid | Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Cases / PY |
Rate/ 100 PY |
n | Cases / PY |
Rate/ 100 PY |
3HRu (95% CI) |
5Pi |
6HRa (95% CI) |
8Pi | |
| 1 IGRA | ||||||||||
| Negative | 261 | 21/631.9 | 3.3 | 274 | 9/ 687.9 | 1.3 | 0.40 (0.18-0.87) | 0.43* (0.20 - 0.96) | ||
| Positive | 205 | 19/ 481.7 | 3.9 | 184 | 13/ 427.3 | 3.0 | 0.76 (0.38-1.5) | 0.22 | 0.55 (0.26 - 1.24) | 0.58 |
| 2 TST | ||||||||||
| Negative | 283 | 27/ 660.0 | 4.1 | 269 | 11/ 656.3 | 1.7 | 0.41** (0.20-0.83) | 0.43* (0.21 - 0.86) | ||
| Positive | 202 | 14/ 496.4 | 2.8 | 190 | 12/ 461.1 | 2.6 | 0.92 (0.43-1.97) | 0.13 | 0.86 (0.37 - 2.0) | 0.24 |
indeterminate interferon-gamma release assay (IGRA) results included with negatives.
TST=tuberculin skin test negative if induration <5mm and positive if ≥ 5mm.
Unadjusted hazard ratio comparing INH vs placebo.
Pi=Likelihood ratio test p-value for interaction in the unadjusted model.
Hazard ratio comparing INH vs placebo, adjusted for CD4+ count and ART status at enrolment.
Pi=Likelihood ratio test p-value for interaction in adjusted model.
P < 0.05,
for P < 0.01.
Secondary endpoints: All-cause mortality and drug toxicity
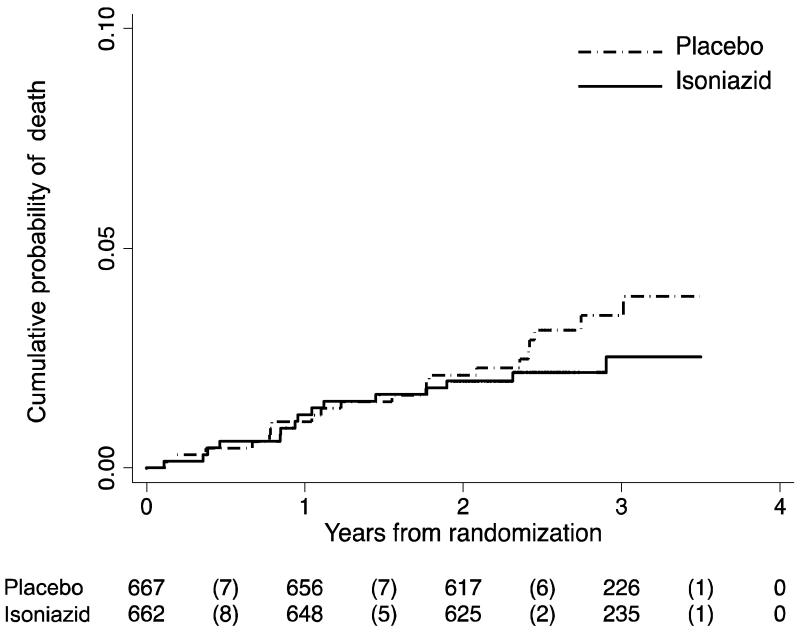
There were 37 deaths from all causes during 3,579 person-years of follow-up; 21 on placebo and 16 on isoniazid (Table 2). The overall rate of all cause mortality was 1 per 100 person-years; rates were slightly lower in the isoniazid (0.9 per 100 person-years) compared with the placebo arm (1.2 per 100 person-years); HR=0.72, 95% CI 0.34-1.34, logrank P=0.32 (Figure 2c). Further details on the 37 deaths were obtained from hospital records or family reports; eight were tuberculosis deaths (two isoniazid and six placebo), 13 were due to non-TB reasons deemed unrelated to the study drug (six isoniazid and seven placebo; eight occurred during the intervention phase), and the rest reasons were unknown (eight isoniazid and eight placebo; two occurred during the intervention phase). Table S4 summarises specific causes of death by study arm.
Figure 2c. Time to death from randomisation.
Placebo group was prescribed ART plus placebo and isoniazid group was prescribed ART plus isoniazid. Logrank test p-value for equality of survival curves= 0.32
196 participants, 102 on isoniazid and 94 on placebo, had the study drug discontinued due to any adverse events; 15.4% isoniazid vs. 14.1% placebo, relative risk (RR)=1.1 (95% CI 0.84-1.42) (Table 2 and Table S2, webappendix A). Twenty-seven on isoniazid and 18 on placebo stopped the study drug due to presumed drug toxicity (any of grade 3/4 ALT, clinical hepatitis, new or worsening grade 2 or more rash or peripheral neuropathy), RR= 1.5, 95% CI 0.84-2.7 (Table 2). Thirty-four participants had grade 3 or more raised ALT, which resulted in stopping the study drug in 29 participants (in two participants grade 3 ALT occurred only in month 12 and results were obtained after study drug was completed, subsequent ALT in three participants were all <grade 3). The risk of developing any grade 3 or more raised ALT was 3.2% if on isoniazid and 1.9% on placebo; RR=1.6 (95% CI 0.82-3.22). The risk of stopping the study drug due to ≥ grade 3 raised ALT alone was 2.9% on isoniazid compared with 1.5% on placebo, RR=1.9 (95% CI 0.90-4.09). The risk of stopping the drug was 2.8% if on isoniazid and 1.6% on placebo (RR=1.7, 95% CI 0.72-4.12) for the on-ART group, and 3.0 % and 1.1% for the start-ART group (RR=2.7, 95% CI 0.54-12.98).
Number needed to treat to benefit versus harm
Table S5, webappendix A, estimates the numbers needed to treat or harm. Twenty-five individuals on ART need to be treated with isoniazid to prevent a case of tuberculosis. One hundred individuals on ART need to be treated with isoniazid to result in harm as defined by stopping study drug due to presumed drug toxicity.
Discussion
Twelve months of IPT independently reduced the incidence of tuberculosis among participants concurrently on ART by 37%. There was a favourable risk to benefit ratio with the number needed to harm four times higher than the number needed to treat to benefit with IPT. The greatest benefit from IPT appeared to be in the first year, however evidence for the effect waning with time was weak. IPT benefit appeared to be stronger in those with negative TST or IGRA tests, although with only weak statistical evidence for interaction. Implementing IPT in an ART clinic is likely to be easier than in pre-ART care as individuals on ART are receiving regular medication and more frequent follow up visits. A strength of our study was the use of sputum cultures to exclude tuberculosis at baseline. It is possible that some of the effect of IPT seen in studies that did not rigorously exclude tuberculosis may have occurred as a result of the effect of isoniazid monotherapy on subclinical disease.(16)
The benefit of IPT in individuals on ART appeared to wane gradually over time rather than rebound rapidly soon after stopping. However, our study was underpowered to evaluate duration of benefit beyond the study period. The risk of active tuberculosis decreases over time due to ART-mediated improvements in CD4+ counts,(3) which may have masked loss of effect of IPT after completion. Studies of IPT in pre-ART cohorts have shown that 6 months IPT has a short-term benefit.(17, 18) A similar, but more dramatic, loss of effect was seen in the 6 month IPT arm of the BOTUSA study, in which 45% of the participants started ART according to need during the study period(8). Longer courses of IPT for all HIV-infected individuals are now recommended by WHO.(19) Our findings, together with those of the BOTUSA study(8), support the use of longer term IPT in individuals on ART living in moderate or high incidence areas. However, in routine clinical practice the risk of non-adherence and treatment discontinuations may be higher in individuals prescribed extended IPT compared with shorter courses.(20) In our study TST negative participants on ART appeared to benefit more from IPT than TST positive participants. Similar trends in effect were observed by IGRA status. This was an unexpected finding, as a meta-analysis of randomized controlled trials of IPT without ART showed significant benefit only in those with positive TST(2). It is not clear why TST positive individuals not on ART may benefit more from IPT than individuals with TST negative results. TST negatives may not benefit as much because they may not be latently infected with M tuberculosis. However, in areas with a high prevalence of tuberculosis many of the negative TST results in HIV-infected individuals are likely to be false negatives, especially in those with lower CD4 counts (21). IPT may be effective in ART-naïve patients with a positive TST because effective IPT requires some acquired immunity to M tuberculosis, for which TST is a crude measure. Isoniazid has been shown to modify the immune response to tuberculosis, potentially by releasing mycobacterial antigens (22). In TST positive individuals ART is likely to augment pre-existing immunity to M tuberculosis, and the incremental benefit of IPT may be small or non-existent. By contrast, in individuals with TST negative results ART improves acquired immunity to M tuberculosis in those individuals who have been exposed to M tuberculosis, as shown by positive results on repeat TST. A recent Ugandan study reported a high negative to positive TST conversion rate of 30.9/100 person years during the first 6 months after starting ART (23). IPT may be effective in this setting of early restoration of acquired immunity. Among patients on ART, the efficacy of IPT may not be strongly linked to TST/IGRA status. Thus, in the absence of a more predictive test or a multivariate algorithm that predicts benefit, IPT should be recommended to all patients receiving ART in moderate or high incidence areas regardless of TST/IGRA status.
Stopping study drug for presumed toxicity occurred more commonly in the isoniazid arm, with a relative risk of 1.5 (95% CI 0.84-2.7), which is similar to the risk ratio of 1.66 (95% CI 1.09, 2.51) reported in the Cochrane meta-analysis of IPT trials in pre-ART patients.(2) We also did not find strong evidence for increased risk of stopping study drug for toxicity in those newly starting ART. These findings suggest that there may be no additive toxicity of IPT in patients concurrently receiving ART.
Our study had some limitations. First, the study lacked sufficient statistical power to show clear differences in effect estimates by TST or IGRA status or to establish the duration of benefit of IPT in individuals on ART. This would have required a larger sample size and/or longer duration of follow up. Second, although we aimed to confirm as many cases of incident tuberculosis as possible using culture at the study clinic, 39% of cases were diagnosed at satellite clinics where diagnostic confirmation may not have been as rigorous. Thus the incidence of culture confirmed tuberculosis may have been underestimated. Third, we did not repeat IGRA and TST in the early study period. The prospective utility of a single test result obtained at screening may be reduced with increasing study follow-up and time on ART. Repeat testing after several months of ART may have allowed more accurate determination of the presence of latent tuberculosis infection. (23),(24, 25) (26, 27), (28) Fourth, the observed rate of tuberculosis in the placebo arm was lower than the rate assumed, which we had estimated from two local cohort studies(29, 30) available to us when the study started. Possible explanations for the lower than expected tuberculosis incidence include high case ascertainment by screening with sputum cultures, which has been shown to lower tuberculosis incidence after starting ART,(31) and increasing CD4+ counts at ART initiation(32), which is the most important risk factor for incident tuberculosis after ART initiation(33). However, person years of observation were higher than we had assumed as loss to follow up was lower than expected and duration of follow up was longer than planned, due to slow recruitment. Our study had 80% statistical power for the observed effect size of 37% of IPT for the primary endpoint, suggesting that the longer period of observation compensated for the lower than expected tuberculosis incidence. Fifth, the study revealed a more modest effect than previously shown in mostly observational studies. (5-7) Methodological issues in previous studies prevented an independent evaluation of the effect of IPT with ART. (5-8) Modest effects described, and the resultant high rate of tuberculosis in the intervention arm, suggest ART plus IPT alone may not be adequate to control tuberculosis at the population level. Sixth, our study results may not be generalizable to settings of lower tuberculosis incidence where background rates of M tuberculosis exposure are low. Finally, we conducted a pragmatic trial in a busy ART/TB integrated clinic. Study procedures would likely have been more rigorous in a dedicated fully staffed study clinic, but conducting the study in a busy ART clinic with minimal additional staffing allowed us to evaluate implementation of IPT.
Conclusion
Twelve months of IPT reduced the incidence of tuberculosis in HIV-infected individuals established on ART or newly starting ART and seemed well tolerated. In this high incidence setting, individuals on ART who have TST or IGRA negative results may also benefit from IPT. It is feasible to implement IPT in busy ART clinics in settings with high rates of HIV and tuberculosis co-morbidity.
Research In Context Panel
Systematic Review
We searched PubMed for studies published from 1996 up to January 1, 2014, that conducted systematic reviews, meta-analyses or randomized controlled trials of the efficacy of tuberculosis preventive therapy on incident tuberculosis in HIV-1 infected adults on combination antiretroviral therapy (ART). We included the following search terms: “Antiretroviral Therapy” OR “HIV” AND “Tuberculosis” AND “Isoniazid” OR “Preventive therapy”. We identified one recent meta-analysis(2), and four subsequent trials that were not included in the meta-analysis(8, 9, 20, 34), one was a cluster randomized trial of a complex intervention(34). No randomized controlled trials of tuberculosis preventive therapy in adults on ART were identified.
Interpretation
The meta-analysis showed that preventive therapy reduced incident tuberculosis in HIV-infected adults with positive tuberculin skin tests (TST), but not in those with negative TSTs.(2) However, the placebo-controlled trials included in the meta-analysis were all from the pre-ART era, or were done in areas prior to ART access. Determining the efficacy and safety of tuberculosis preventive therapy in people on ART is important as ART also reduces the risk of tuberculosis, toxicity of preventive therapy may be enhanced, and because 25.9 million of the estimated 35.3 million living with HIV in 2012 qualify for ART according to 2013 WHO guidelines.(35). Three subsequent trials compared longer duration IPT with other preventive therapy regimens.(8, 9, 20) The three trials of longer duration IPT were done in ART-naïve individuals (except for 2% of participants in the BOTUSA study(8)), with participants starting ART according to clinical need during follow up. Thirty six months IPT was more effective than 6 months in the BOTUSA study, with the benefit limited to those who were TST positive(8), but a benefit was not shown in the other two trials with smaller sample sizes(9, 20). In the BOTUSA study ART for a year reduced tuberculosis incidence by 50%. A cluster randomized trial found that improved tuberculosis screening and IPT reduced incident tuberculosis, which was independent of the effects of concomitant ART at baseline.(34)
Our study showed that twelve months of IPT reduced the incidence of tuberculosis in individuals on ART, was easy to implement in an ART clinic, and seemed well tolerated. Unlike other studies, we found that participants on ART with negative TSTs or IGRA appeared to benefit more from IPT than test positive participants. The important public health message from our study is that, until there is a more predictive test for latent tuberculosis infection or a multivariate algorithm that predicts benefit in the context of ART, IPT should be recommended to all patients receiving ART in moderate or high incidence areas regardless of TST status. Future studies should explore improved diagnostics for latent tuberculosis infection, longer duration of IPT or more sterilizing preventive therapy regimens, in people on ART.
Supplementary Material
Acknowledgements
We thank individuals and staff at the Ubuntu Clinic in Khayelitsha as well as the ART-IPT study core team. Special thanks to Drs Judith Mwansa, Elizabeth Du Toit, Funeka Bango and Jan Kuehne, Srs Pat Mtiya, Nonini Rini, Nompumelelo Mantangana, Lindiwe Kotelana, Joyce Sani, Sr Bulelwa Sekosona, and Estelle Kastoor, Ms Raylene Titus, Glynnis Lawrence, Vuyokazi Ntshakaza, Marcel Adams, Pam Mandindi, Rhoda Paulse, Pheliwe Ranuga, and Mr Mandla Oliphant and Mr Khanya Mkoko. We also thank the Provincial Government of the Western Cape and City of Cape Town Health Department for integrating the ART-IPT study into clinic data and patient systems, especially Drs Virginia Azevedo and Gio Perez. The Treatment Action Campaign of Khayelitsha is thanked for providing invaluable waiting-room patient literacy and advocacy. Members of our data safety and monitoring board are thanked for their contribution(Professors Gavin Churchyard, Jonathan Sterne and Bill Burman).
This work was supported in part by the Department of Health of South Africa, the Wellcome Trust and Médecins sans Frontières who provided additional staff to Ubuntu Clinic. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MXR and RJW were supported by the Wellcome Trust (084323,084670,088316) and the European and Developing Countries Clinical Trials Partnership (EDCTP). MXR was additionally supported by a Hasso Plattner Fellowship from the Institute of Infectious Diseases and Molecular Medicine at the University of Cape Town, South Africa. RJW was additionally supported by the European Union (SANTE/2005/105-061-102) and EDCTP (IP.07.32080.002). No significant disclosures for JRG, GM, AB, GVC, SM, EG and RG. No funding bodies played any role in the design, writing or decision to publish this manuscript.
Footnotes
Competing Interests
No authors have any competing interests.
References
- 1.WHO . WHO 2011 Report: Global Tuberculosis Control. World Health Organization; Geneva: [Access date: 15 September 2012]. 2011. URL: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database of Systematic Reviews. 2010;20(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suthar AB, Lawn SD, Del Amo J, Getahun H, Dye C, Sculier D, et al. Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med. 2012 Jul;9(7):e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenner L, Forster M, Boulle A, Phiri S, Braitstein P, Lewden C, et al. Tuberculosis in HIV programmes in lower-income countries: practices and risk factors. Int J Tuberc Lung Dis. 2011 May;15(5):620–7. doi: 10.5588/ijtld.10.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009 Mar 13;23(5):631–6. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007 Jul 11;21(11):1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 May 7;377(9777):1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan S, Menon PA, Gopalan N, Perumal V, Santhanakrishnan RK, Ramachandran R, et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized clinical trial. PLoS One. 2012;7(12):e47400. doi: 10.1371/journal.pone.0047400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen X, Wang JS, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002 Jan;57(11):799–804. doi: 10.1007/s00228-001-0396-3. [DOI] [PubMed] [Google Scholar]
- 11.Rangaka MX, Wilkinson RJ, Glynn JR, Boulle A, van Cutsem G, Goliath R, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012 Dec;55(12):1698–706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangaka MX, Gideon HP, Wilkinson KA, Pai M, Mwansa-Kambafwile J, Maartens G, et al. Interferon release does not add discriminatory value to smear-negative HIV-tuberculosis algorithms. Eur Respir J. 2012 Jan;39(1):163–71. doi: 10.1183/09031936.00058911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986 Sep;42(3):507–19. [PubMed] [Google Scholar]
- 15.Lakatos E, Lan KK. A comparison of sample size methods for the logrank statistic. Stat Med. 1992 Jan 30;11(2):179–91. doi: 10.1002/sim.4780110205. [DOI] [PubMed] [Google Scholar]
- 16.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 17.Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, Porter JDH, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001 Jan 26;15(2):215–22. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JL, Okwera A, Hom DL, Mayanja H, Mutuluuza Kityo C, Nsubuga P, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001 Nov 9;15(16):2137–47. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 19.Stop TB. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Stop TB Department. World Health Organization; Geneva: [Access Date: 7 December 2011]. 2011. URL: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. [Google Scholar]
- 20.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011 Jul 7;365(1):11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007 Mar 1;175(5):514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson KA, Kon OM, Newton SM, Meintjes G, Davidson RN, Pasvol G, et al. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis. 2006 Feb 1;193(3):354–9. doi: 10.1086/499311. [DOI] [PubMed] [Google Scholar]
- 23.Kirenga BJ, Worodria W, Massinga-Loembe M, Nalwoga T, Manabe YC, Kestens L, et al. Tuberculin skin test conversion among HIV patients on antiretroviral therapy in Uganda. Int J Tuberc Lung Dis. 2013 Mar;17(3):336–41. doi: 10.5588/ijtld.12.0298. [DOI] [PubMed] [Google Scholar]
- 24.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012 Jan;67(1):62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 25.Pai M, O’Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 2007 Jun;4(6):e208. doi: 10.1371/journal.pmed.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009 Dec 1;200(11):1736–45. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 27.Fisk TL, Hon HM, Lennox JL, Fordham von Reyn C, Horsburgh CR., Jr. Detection of latent tuberculosis among HIV-infected patients after initiation of highly active antiretroviral therapy. AIDS. 2003 May 2;17(7):1102–4. doi: 10.1097/00002030-200305020-00027. [DOI] [PubMed] [Google Scholar]
- 28.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998 Jul;158(1):157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 29.Boulle A, Zweigental V, Hildebrand K, Magwaca N, Coetzee D. Incidence of tuberculosis pre- and post-ART in a setting of high tuberculosis-HIV comorbidity; Paper presented at: 15th International AIDS Conference; Bangkok, Thailand. July 11-16, 2004; 2004. Abstract MoPeB 3239.; 2004. [Google Scholar]
- 30.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006 Aug 1;20(12):1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 31.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010 Jun 1;24(9):1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010 Feb 20;24(4):563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 33.Brinkhof MW, Egger M, Boulle A, May M, Hosseinipour M, Sprinz E, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007 Dec 1;45(11):1518–21. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, King BS, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013 Oct;13(10):852–8. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. World Health Organization [Access Date: 13 January 2014];Global update on HIV treatment 2013: results, impact and opportunities: WHO report in partnership with UNICEF and UNAIDS. 2013 URL: http://www.who.int/hiv/pub/progressreports/update2013/en/ cited; Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.