Abstract
A reciprocal relationship between insulin sensitivity and glucose tolerance has been reported in some mouse models and humans with isolated changes in growth hormone (GH) production and signaling. To determine if this could be explained in part by tissue-specific changes in insulin sensitivity, hyperinsulinemic-euglycemic clamps were performed in mice with adult-onset, isolated GH deficiency and in mice with elevated endogenous GH levels due to somatotrope-specific loss of IGF-I and insulin receptors. Our results demonstrate that circulating GH levels are negatively correlated with insulin-mediated glucose uptake in muscle but positively correlated with insulin-mediated suppression of hepatic glucose production. A positive relationship was also observed between GH levels and endpoints of hepatic lipid metabolism known to be regulated by insulin. These results suggest hepatic insulin resistance could represent an early metabolic defect in GH deficiency.
Keywords: growth hormone, hepatic glucose production, triglycerides, glucose disposal, hyperinsulinemic-euglycemic clamps
humans with long-term adult-onset growth hormone deficiency (AOGHD) have been reported to have impaired glucose tolerance (5, 27, 32), systemic insulin resistance, and increased hepatic glucose production [HGP; (12, 16, 24)], with a higher prevalence of diabetes (1). However, the direct impact of growth hormone deficiency (GHD) on insulin-mediated glucose homeostasis is not clear cut. AOGHD patients can show reduced (5), normal (12), or elevated (8) insulin levels. A wide spectrum of insulin sensitivity in AOGHD patients may be in part due to the length, severity, multiplicity, and etiology of pituitary deficiencies, leading to variable effects on body composition (1, 9, 14, 30). In fact, insulin sensitivity was shown to be improved in AOGHD subjects compared with age-, sex-, and body mass index (BMI)-matched controls (28). Also, subjects with congenital isolated GHD resulting from an inactivating mutation in the growth hormone-releasing hormone (GHRH) receptor are more insulin sensitive compared with their unaffected relatives despite increased visceral adiposity and glucose intolerance (27, 32). Glucose intolerance, with improved systemic insulin sensitivity [as measured by insulin tolerance tests (ITT)], is also observed in mouse models with GHD (7) and growth hormone (GH) resistance (11, 13). Notably, the opposite metabolic phenotype (improved glucose tolerance with normal/reduced insulin sensitivity) is observed in mice with elevated heterologous (6) or endogenous (10) GH levels.
The disconnect between systemic insulin sensitivity and glucose tolerance observed in mouse model systems and humans with isolated changes in GH production and signaling (6, 7, 10, 13, 15, 22, 27, 32) suggests GH might differentially alter tissue-specific insulin sensitivity and/or change β-cell function/mass. To differentiate between these possibilities, we have performed studies using mice with adult-onset, isolated GHD [AOiGHD (22)] and mice with elevated endogenous GH levels due to somatotrope-specific loss of IGF-I and insulin receptors [HiGH (10)]. In both AOiGHD and HiGH mice the modification in GH levels is selective with concomitant changes in IGF-I, without appreciable alterations in expression/secretion/action of other pituitary hormones (10, 22). In addition, AOiGHD mice are not dwarves but show a modest decrease in lean mass (7), whereas HiGH mice are not giants but show a modest increase in lean mass (10). Therefore, these models represent a unique opportunity to examine the impact of selectively lowering and raising endogenous GH levels while minimizing the impact of GH/IGF-I on body mass. We have reported that alterations in glucose tolerance in these mouse models (7, 10) could not be attributed to changes in glucose-stimulated insulin secretion from islet cultures or changes in β-cell mass (Ref. 7 and Cordoba-Chacon J, Majumdar N, Pokala NK, Gahete MD, Kineman RD, unpublished observations). The current study was designed to determine if changes in circulating GH levels alter tissue-specific insulin actions. Specifically, hyperinsulinemic-euglycemic clamps with double-isotope labeling (2, 3) were performed in male AOiGHD and HiGH mice and their respective controls. Results demonstrate circulating GH levels are negatively correlated with insulin-mediated glucose uptake in muscle but positively correlated with insulin-mediated suppression of HGP.
MATERIALS AND METHODS
Animals and experimental endpoints.
All experiments were approved by the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center Institutional Animal Care and Use Committee (IACUC), with the additional approval of the Vanderbilt University IACUC for the hyperinsulinemic-euglycemic clamp studies. Mice were housed under a 12:12-h light-dark cycle at 22–24°C and provided standard rodent chow diet [Teklad LM-485 Mouse/Rat Sterilizable irradiated diet (3.1 kcal/g; fat: 17% kcal; carbohydrate: 58% kcal; protein: 25% kcal) Teklad diets, Madison, WI] or a low-fat (LF) diet [catalog no. 12450B (3.84 kcal/g; fat: 10% kcal; carbohydrate: 70% kcal; protein: 20% kcal) Research Diets, New Brunswick, NJ] as indicated. AOiGHD was induced at 12 wk by treating mice heterozygous for both the rat GH promoter-driven Cre recombinase transgene and the inducible diphtheria toxin (DT) receptor transgene (rGHp-Cre+/−,iDTR+/−) with DT to selectively destroy somatotropes, leading to a >60% reduction in circulating GH levels, as previously described (22). DT-treated, rGHp-Cre−/−,iDTR+/− littermates (C57Bl/6J background) served as GH-replete controls (22). Hyperinsulinemic-euglycemic clamps were performed 32–34 wk after AOiGHD induction. Male HiGH mice and their littermate controls (C57Bl/6J background) were generated by crossbreeding rGHp-Cre−/−, IGFIRfl/flINSRfl/fl female mice with rGHp-Cre+/−,IGFIRfl/flINSRfl/fl male mice, as previously described (10), and hyperinsulinemic-euglycemic clamps were performed at 20 wk of age.
Hyperinsulinemic-euglycemic clamp.
Mice were implanted with jugular vein and carotid artery catheters as previously described (2). Five to six days postcatheterization, food was withdrawn at 0500, and 3.5 h later 3-[3H]-d-glucose was continuously infused (0.75 μCi/min for 2 min and 0.075 μCi/min for 90 min). Ten minutes before starting the insulin clamp, blood was drawn to determine glucose and insulin. At time 0, 3-[3H]-d-glucose infusion is increased to 0.15 μCi/min, and a saline-washed erythrocyte solution (5.5 μl/min) and an insulin solution (2 mU·kg−1·min−1, 1 μl/min, Humulin Regular U100) were infused continuously. Every 10 min after the insulin infusion started, blood glucose was determined to adjust glucose infusion rate to target euglycemia. Blood was drawn at 80, 90, 100, and 120 min after insulin infusion started to measure plasma 3-[3H]d-glucose specific activity. Plasma insulin was measured at 120 min. At 120 min, a bolus of 13 μCi 2-deoxy-[14C]glucose was infused, and after 25 min the mouse was killed with pentobarbital anesthesia. Blood, heart, vastus lateralis, soleus, gastrocnemius, fat, and liver were collected, weighed, and snap-frozen to determine tissue-specific 2-deoxy-[14C]glucose glucose uptake in form of phosphorylated 2-deoxy-[14C]glucose 6-phosphate and disappearance of plasma 2-deoxy-[14C]glucose, as previously reported (2).
Hepatic lipid analysis.
To determine triglyceride (TG) content in livers, tissue was homogenized in isopropanol (1 ml/100 mg of tissue) and shaken for 45 min to extract the lipids as previously described (26). Hepatic TG levels were determined using reagents and microtiter plate procedures from Wako Diagnostics. To estimate the rate of hepatic very low density lipoprotein (VLDL)-TG production, 4-h-fasted mice were injected with 500 mg/kg Triton WR1339 (tyloxapol; Sigma-Aldrich) to inhibit plasma VLDL clearance. Blood was collected from tail vein nicks at time 0 and frequently up to 4 h after tyloxapol injections to quantify the cumulative TG concentration in plasma using reagents and microtiter plate procedures from Wako Diagnostics.
mRNA isolation and quantitative real-time RT-PCR.
RNA was extracted from livers using the Absolutely RNA Miniprep kit (Agilent Technologies, La Jolla, CA). DNA-free RNA was reverse transcribed using the RevertAid First strand cDNA synthesis kit (Thermo Scientific, Asheville, NC), and cDNA was amplified by quantitative real-time RT-PCR using Brilliant III Ultra-fast SYBR green (Agilent Technologies). Primer sequences are provided in Table 1. mRNA copy number of all transcripts was adjusted by a normalization factor calculated from the mRNA copy number of at least three separate housekeeping genes (β-actin, GAPDH, hypoxanthine guanine phosphoribosyl transferase, and cyclophilin-A mRNA) using GeNorm 3.3 (31).
Table 1.
List of primers for qRT-PCR used in this study
| Gene | Accesion No. | Sequence | Position | Product Size, bp |
|---|---|---|---|---|
| β-Actin | NM_007393.3 | CTGGGACGACATGGAGAAGA | 313 | 205 |
| ACCAGAGGCATACAGGGACA | 517 | |||
| GAPDH | NM_008084.2 | ATGGCCTTCCGTGTTCCTAC | 735 | 104 |
| GCCTGCTTCACCACCTTCTT | 838 | |||
| HPRT | NM_013556 | CAGTCAACGGGGGACATAAA | 471 | 183 |
| AGAGGTCCTTTTCACCAGCAA | 653 | |||
| Cyclophilin A | NM_008907.1 | TGGTCTTTGGGAAGGTGAAAG | 421 | 109 |
| TGTCCACAGTCGGAAATGGT | 529 | |||
| Pck1 | NM_011044.2 | TGGGGTGTTTGTAGGAGCAG | 1496 | 128 |
| CCAGGTATTTGCCGAAGTTG | 1623 | |||
| SREBP1c | XM_006532716.1 | GGAGCCATGGATTGCACATT | 107 | 70 |
| GGCCCGGGAAGTCACTGT | 176 | |||
| AAC | NM_133360.2 | ATCCTGCGAACCTGGATTCT | 6022 | 158 |
| CCCACCAGAGAAACCTCTCC | 6179 | |||
| FASN | NM_007988.3 | TGAGCACACTGCTGGTGAAC | 7162 | 200 |
| CAGGTTCGGAATGCTATCCA | 7361 | |||
| SCD-1 | NM_009127.4 | ATCGCCCCTACGACAAGAAC | 1106 | 137 |
| GTTGATGTGCCAGCGGTACT | 1242 |
qRT-PCR, quantitative real-time RT-PCR; HPRT, hypoxanthine guanine phosphoribosyl transferase; Pck1, phosphoenolpyruvate carboxykinase 1; SREBP1, sterol regulatory element binding transcription factor 1; AAC, acetyl-CoA carboxylase; FASN, fatty acyl synthase; SCD-1, stearoyl-coenzyme A desaturase 1.
Statistical analysis.
Student's t-tests were performed to analyze the effect of genotype. Two-way ANOVA followed by Bonferroni post hoc test was performed to analyze the effect of genotype in tyloxapol-induced VLDL-TG accumulation. P values <0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
RESULTS
AOiGHD mice show improved muscle insulin sensitivity, whereas insulin-induced suppression of HGP is impaired.
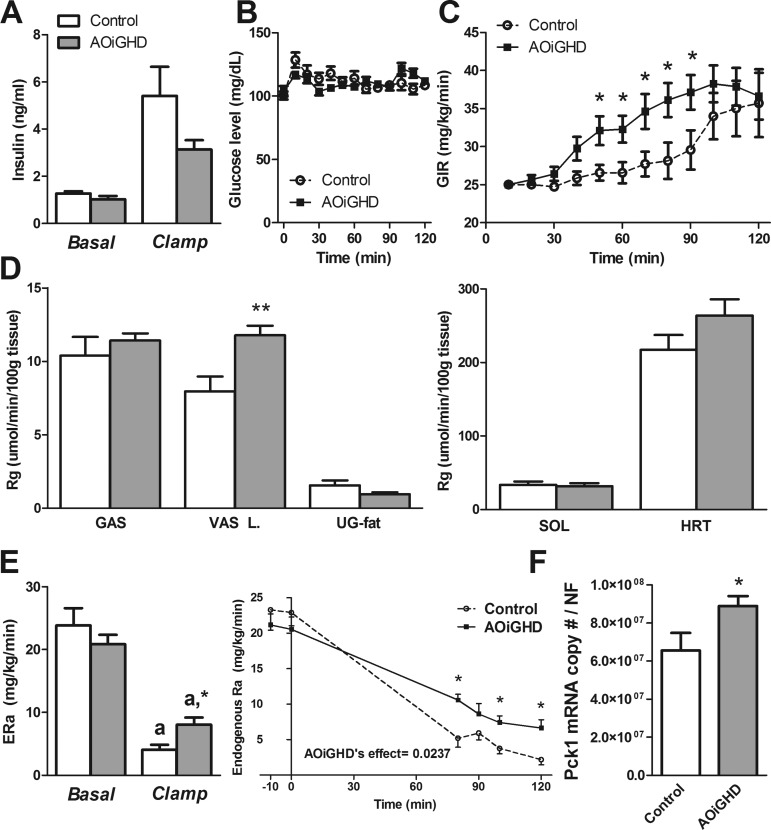
Body weights did not differ between AOiGHD mice and their littermate controls (31.2 ± 0.8 vs. 29.6 ± 0.7 g). Although body composition was not performed in this cohort of mice, we have previously reported AOiGHD mice have reduced lean mass, but no significant change in fat mass, at 40–46 wk of age (28–34 wk GHD) (7). Basal and clamp insulin levels did not differ between AOiGHD (30–32 wk GHD) and control (Fig. 1A) mice. To maintain euglycemia during 50–90 min of the hyperinsulinemic clamp (Fig. 1B), a higher glucose infusion rate (GIR mg·kg−1·min−1) was required for AOiGHD mice compared with GH-replete controls (Fig. 1C). At steady state, the GIR did not differ between groups (mean GIR of 100, 110, and 120 min, controls: 34.90 ± 3.99 vs. AOiGHD: 37.58 ± 2.76 mg·kg−1·min−1). However, the vastus lateralis of AOiGHD mice showed improved insulin-mediated glucose uptake (P < 0.006) compared with controls (Fig. 1D), whereas glucose uptake was not significantly altered in the gastrocnemius, soleus, heart, or fat. Basal HGP [=endogenous glucose production (ERa)] did not differ between AOiGHD and control mice (Fig. 1E). At steady state, insulin infusion suppressed HGP in both groups. However, HGP of AOiGHD remained significantly elevated above controls after the clamp (Fig. 1E). Consistent with this observation, phosphoenolpyruvate carboxykinase 1 (Pck1) expression is elevated in AOiGHD livers (Fig. 1F), suggesting diminished insulin actions in AOiGHD livers.
Fig. 1.
Assessment of tissue-specific insulin actions by hyperinsulinemic-euglycemic clamps in adult-onset, isolated growth hormone deficiency (AOiGHD) and control mice, maintained on a low-fat (LF) diet. A: plasma insulin levels before and after hyperinsulinemic-euglycemic clamp. B and C: blood glucose (B) and glucose infusion ratio (GIR, C) during hyperinsulinemic-euglycemic clamp. D: tissue-specific glucose metabolic index (Rg) of gastrocnemious (GAS), vastus lateralis (VAS L), urogenital fat (UG-fat), soleus (SOL), and heart (HRT). E: endogenous glucose production (ERa) before and after hyperinsulinemic-euglycemic clamp [n = 8–9 mice/group, 30–32 wk of growth hormone deficiency (GHD), LF feeding started at 4 wk of age]. F: hepatic phosphoenolpyruvate carboxykinase 1 (Pck1) expression of AOiGHD and control mice (28 wk of GHD, chow fed, n = 5–14 mice/group). aSignificant difference between clamped and basal state within genotype (E). *P < 0.05 and **P < 0.01, significant difference between control and AOiGHD mice (C, D, E, and F).
HiGH mice show reduced muscle insulin sensitivity, whereas insulin-induced suppression of HGP is enhanced.
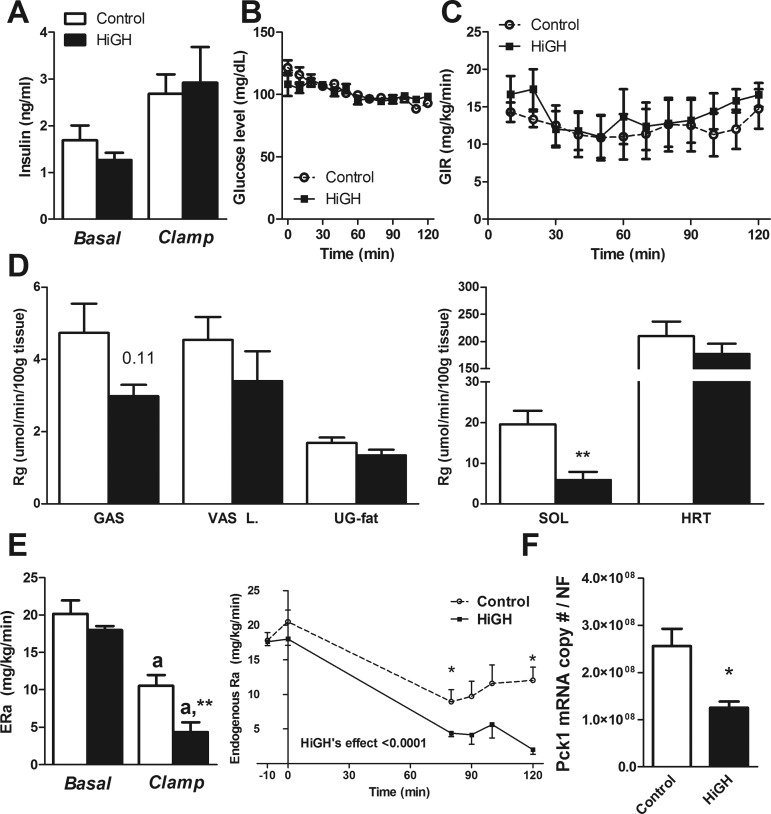
HiGH mice in this study were heavier than littermate controls (27.6 ± 0.7 vs. 32.2 ± 0.7 g). Although body composition was not performed in this cohort of mice, we have previously reported that HiGH mice show increased lean mice, without alterations in absolute fat mass, when measured at 20 wk of age (10). Basal and clamp insulin levels did not differ between HiGH and control mice (Fig. 2A). Euglycemia was maintained during the clamp (Fig. 2B), and GIR did not differ between control and HiGH mice (Fig. 2C). At steady state, the GIR did not differ between groups (mean GIR of 100, 110, and 120 min, controls: 12.67 ± 2.87 vs. HiGH: 15.6 ± 2.02 mg·kg−1·min−1). Glucose uptake was impaired in the soleus (P = 0.009) of HiGH mice and tended to be impaired in the gastrocnemius (P = 0.11), whereas glucose uptake was not altered in the vastus lateralis, heart, or white adipose tissue (Fig. 2D). Basal hepatic glucose production (HGP = ERa) did not differ between HiGH and control mice (Fig. 2E). At steady state, insulin infusion suppressed HGP in both groups. However, insulin suppression of HGP during the clamp was significantly amplified in HiGH mice compared with controls (Fig. 2E). Consistent with this observation, Pck1 expression (Fig. 2F) is reduced in HiGH livers, suggesting enhanced insulin actions in the HiGH livers.
Fig. 2.
Assessment of tissue-specific insulin actions by hyperinsulinemic-euglycemic clamps in mice with elevated endogenous growth hormone levels due to somatotrope-specific loss of IGF-I and insulin receptors (HiGH) and control mice, maintained on a chow diet. A: plasma insulin levels before and after hyperinsulinemic-euglycemic clamp. B and C: blood glucose (B) and GIR (C) during hyperinsulinemic-euglycemic clamp. D: tissue-specific Rg of GAS, VAS L, UG-fat, SOL, and HRT. E: ERa assessed by hyperinsulinemic-euglycemic clamp (n = 6–8 mice/group, 20 wk old). F: Pck1 expression of HiGH and control mice (20 wk old, LF fed, n = 5–7 mice/group). aSignificant difference between clamped and basal state within genotype (E). *P < 0.05 and **P < 0.01, significant difference between control and HiGH mice (D, E, and F).
Additional endpoints supporting a positive relationship between circulating GH levels and hepatic insulin sensitivity.
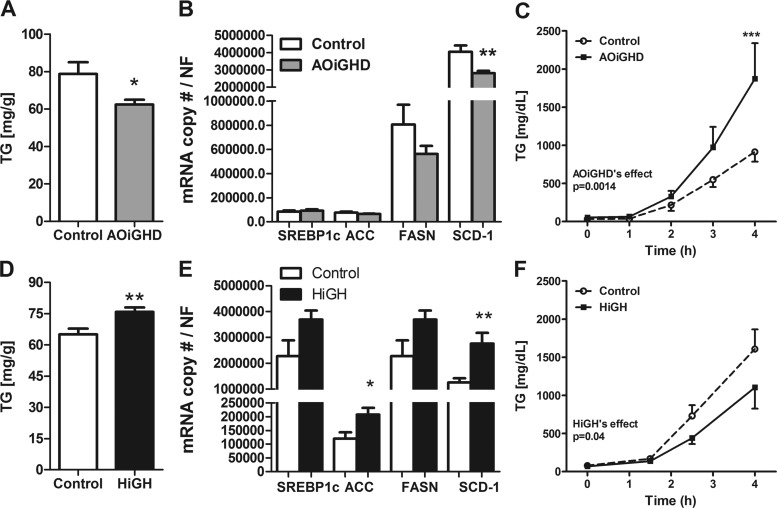
AOiGHD mice displayed reduced hepatic TG content (Fig. 3A), which was associated with a downregulation of SCD-1 gene expression, without significant changes in other lipogenic genes in LF-fed mice (Fig. 3B) and chow-fed mice (data not shown). Reduced hepatic TG content in AOiGHD mice may be due, in part, to an increase in VLDL-TG production based on the fact that circulating TG concentrations were elevated following tyloxapol injection (Fig. 3C).
Fig. 3.
Hepatic lipid content, lipogenic gene expression, and very low density lipoprotein (VLDL)-triglyceride (TG) secretion in AOiGHD and HiGH mice. Liver TG content in AOiGHD (A), HiGH (D), and control [28 wk of GHD, LF fed (A); 32–34 wk old, chow fed (D), n = 7–9 mice/group] mice. mRNA levels of lipogenic genes in livers of AOiGHD (B), HiGH (E), and control [39 wk of GHD, LF fed, n = 12–17 mice/group (B); 20 wk old, LF fed, n = 5–7 mice/group (E)] mice. SREBP1c, sterol regulatory element binging transcription factor 1c; ACC, acetyl-CoA carboxylase; FASN, fatty acyl synthase; SCD-1, stearoyl-coenzyme A desaturase 1. Hepatic VLDL secretion rate assessed by tyloxapol-induced inhibition of VLDL clearance in AOiGHD (C), HiGH (F), and control [29 wk GHD, chow fed, n = 5–9 mice/group (C); 12–14 wk old, chow fed, n = 7 mice/group (F)] mice. *P < 0.05, **P < 0.01, and ***P < 0.0001, significant difference between control and AOiGHD or HiGH mice.
HiGH mice displayed an increase in hepatic TG content (Fig. 3D), and this was associated with an increase in the expression of lipogenic genes in LF-fed mice (Fig. 3E) and chow-fed mice (data not shown). The increase in hepatic lipid accumulation in HiGH mice may be due, in part, to a reduction in hepatic TG secretion, as assessed after tyloxapol injection (Fig. 3F).
DISCUSSION
Using a genetic approach to either modestly decrease (AOiGHD) or increase (HiGH) GH tone, we examined hepatic and peripheral insulin action using a hyperinsulinemic-euglycemic clamp. The regulation of hepatic and peripheral insulin action was differentially regulated by GH. Consistent with prior work (18–20, 23, 25, 29), relative GH excess resulted in mild peripheral insulin resistance, whereas relatively low GH levels modestly improved insulin action. The striking observation was the impact of GH on liver insulin action. A relative excess of GH enhanced hepatic insulin action while relative deficiency of GH impaired hepatic insulin action. Thus, inappropriate GH secretion can have markedly different effects on hepatic and peripheral insulin action.
Hyperinsulinemic-euglycemic clamps revealed male AOiGHD had improved insulin-dependent muscle glucose disposal but impaired insulin-dependent suppression of HGP. We optimized the study design to detect alterations in both hepatic and peripheral insulin action by using a relatively low dose of insulin in the clamps (2 mU insulin·kg−1·min−1). This allowed us to detect an impairment in hepatic insulin action in animals on a LF diet. These results may initially seem at odds with hyperinsulinemic-euglycemic clamp studies performed in AOGHD patients, showing systemic insulin resistance, in addition to increased HGP (12, 16, 24). However, evidence has accumulated suggesting that changes of systemic insulin sensitivity may be secondary to alterations in body composition (increase in visceral adiposity and decrease in lean mass) and not due to GHD per se (14, 30). This is supported by studies showing AOGHD patients can have reduced (5) or normal (12) insulin levels and can even show improved insulin sensitivity compared with BMI-matched controls (28). In the current study, the pituitary defect was induced after sexual maturation and was selective for GH (10, 22). In addition, weight gain was moderated by LF feeding. Therefore, this model system provides an opportunity to more directly explore the impact of adult-onset GHD on tissue-specific insulin actions. Based on our current results, we might speculate that impaired hepatic glucose metabolism observed in AOiGHD mice represents an “early” metabolic defect in GHD, a defect that can be offset by improvement in peripheral insulin sensitivity if caloric intake is in moderation. In fact, we have previously reported that AOiGHD mice fed a LF-diet show normal glucose tolerance [by ip glucose tolerance test (GTT)], but improved insulin tolerance (by ip ITT), compared with GH-replete controls (22). However, in the context of HF feeding, improved insulin tolerance is diminished in AOiGHD mice, and marked glucose intolerance develops after an intraperitoneal bolus of glucose (22). Because we have previously reported that β-cell mass and in vitro glucose-stimulated insulin release is not altered in HF-fed AOiGHD mice (7), the current findings suggest AOiGHD-related impairment of insulin-dependent suppression of HGP may contribute to impaired glucose tolerance observed when caloric intake is in excess (22). Glucose intolerance with improved insulin sensitivity is also observed in 6- to 12-mo-old GH receptor antagonist transgenic mice (13), GHRH knockout mice (7), GH receptor knockout mice (11), and in patients with isolated GHD due to inactivating mutations in the GHRH receptor gene (27, 32). Interestingly, the opposite phenotype (improved glucose tolerance with normal/impaired insulin sensitivity) is observed in HiGH mice (10) and in mice expressing the bovine GH transgene (6). The current clamp results suggest that the improved glucose tolerance observed in HiGH mice is in part due to improved insulin-mediated suppression of HGP, which could serve to offset impaired insulin-stimulated glucose uptake in muscle, thereby maintaining glucose homeostasis.
To our knowledge, the current study represents the first report examining the impact of selective reduction or increase in circulating GH/IGF-I levels on tissue-specific insulin actions as measured by hyperinsulinemic-euglycemic clamps. However, clamps have been performed in other mouse models where GH output or signaling is altered. Specifically, a very recent paper performed clamps in Ames mice (34). In that study, insulin actions are improved in muscle, fat, and liver. However, unlike AOiGHD mice (22), Ames mice are dwarfs and deficient in GH, prolactin, and thyroid-stimulating hormone. In addition, Yakar et al. (35) performed clamps in liver-specific IGF-I knockout (LID) mice, which have elevated GH due to reduced IGF-I negative feedback. In that study, insulin actions were impaired in muscle, fat, and liver. However, unlike HiGH mice (10), LID mice show impaired response to ITT and dyslipidemia. Finally, Vijayakumar et al. (33) performed clamps in muscle-specific GH receptor knockout (mGHRKO) mice made obese by HF feeding. In that study, mGHRKO mice exhibited normal GH and IGF-I levels with improved muscle and liver insulin actions. In all of these models (including the AOiGHD and HiGH mice in the current report), GH is negatively correlated with insulin-mediated muscle glucose uptake. This is consistent with reports showing GH treatment suppresses insulin-induced glucose uptake in muscle in normal human subjects (20). Although the current study was not designed to investigate the cellular mechanism of GH-mediated inhibition of muscle glucose uptake, our current results suggest skeletal muscles are more sensitive than adipose tissue to changes in endogenous GH levels, with respect to insulin-mediated glucose uptake, as suggested by data generated by others (21, 33).
Of note, we observed a differential impact of GH alterations in muscle subtype, insulin-mediated, glucose uptake within and between AOiGHD and HiGH. Multiple factors could contribute to these differences, which include: low dose of insulin during the clamp; age at onset of GH alteration in AOiGHD vs. HiGH mice, which may alter muscle subtype development and maintenance; and type of muscle [oxidative (soleus), glycolytic (vastus lateralis), and mixed (gastronemious)]. Based on the experience of the Vanderbilt Mouse Metabolic Phenotyping Center, the gastrocnemius and soleus have a higher basal glucose uptake, so it is easier to observe a reduction (as observed in HiGH mice), and perhaps harder to observe an increase in insulin-mediated glucose uptake (absent in AOiGHD mice). However, at this time we do not have sufficient data to differentiate between these possibilities, which in fact may not be mutually exclusive.
In sharp contrast to the negative association between circulating GH and muscle insulin sensitivity observed in AOiGHD and HiGH mice, as well as the other mouse models tested in this regard (33, 35), changes in circulating GH/IGF-I were positively associated with insulin's action in the liver. Specifically, insulin acts on the liver to block glucose production and stimulate glycogen and lipid production/storage. In HiGH mice, the increased ability of insulin to suppress HGP, in addition to reduced Pck1, enhanced lipogenic gene expression, reduced VLDL-TG secretion in the absence of reductions in fatty acid supply, and increased hepatic TG content indicates insulin's action on hepatic function may be enhanced (4, 17). The reciprocal changes in these endpoints, observed in AOiGHD mice, coupled with the fact that AOiGHD mice exhibit postprandial hypertriglyceridemia (7), indicate that insulin's actions on the liver may be reduced. Given these studies were performed in mouse models with isolated changes in endogenous GH levels and weight was controlled by diet, we might speculate impaired hepatic function represents an early metabolic defect in AOGHD patients that could serve to promote the progression to diabetes if caloric intake is in excess (1). In addition, the positive effect of GH on liver function may serve to offset the negative effects of GH on muscle insulin sensitivity, serving to control glucose homeostasis when low-dose GH replacement therapy is applied.
In summary, the current results demonstrate that modest alterations in endogenous GH levels differentially impact tissue-specific insulin action, where GH is negatively associated with insulin-dependent glucose uptake in the muscle and positively associated with insulin-dependent regulation of hepatic glucose and lipid production.
GRANTS
This work was supported by the Fundación Alfonso Martin Escudero (to J. Cordoba-Chacon), the “Sara Borrell” program (Grant No. CD11/00276) (to M. D. Gahete), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-059637 and DK-020893 (to O. P. McGuinness), Department of Veterans Affairs, Office of Research and Development Merit Award BX001114, and NIDDK Grant R01-DK-088133 (to R. D. Kineman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.-C., M.D.G., O.P.M., and R.D.K. performed experiments; J.C.-C., M.D.G., and O.P.M. analyzed data; J.C.-C., O.P.M., and R.D.K. interpreted results of experiments; J.C.-C. prepared figures; J.C.-C. and R.D.K. drafted manuscript; J.C.-C., M.D.G., O.P.M., and R.D.K. edited and revised manuscript; J.C.-C., M.D.G., O.P.M., and R.D.K. approved final version of manuscript; R.D.K. conception and design of research.
ACKNOWLEDGMENTS
We thank Neena Majumdar for technical assistance.
REFERENCES
- 1.Abs R, Mattsson AF, Thunander M, Verhelst J, Goth MI, Wilton P, Koltowska-Haggstrom M, Luger A. Prevalence of diabetes mellitus in 6050 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. Eur J Endocrinol 168: 297–305, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Ayala JE, Bracy DP, Malabanan C, James FD, Ansari T, Fueger PT, McGuinness OP, Wasserman DH. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp 16: 3188, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56: 952–964, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Beshyah SA, Gelding SV, Andres C, Johnston DG, Gray IP. Beta-cell function in hypopituitary adults before and during growth hormone treatment. Clin Sci (Lond) 89: 321–328, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Boparai RK, Arum O, Khardori R, Bartke A. Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol Chem 391: 1149–1155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordoba-Chacon J, Gahete MD, Pokala NK, Geldermann D, Alba M, Salvatori R, Luque RM, Kineman RD. Long- but not short-term adult-onset, isolated GH deficiency in male mice leads to deterioration of beta-cell function, which cannot be accounted for by changes in β-cell mass. Endocrinology 155: 726–735, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuneo RC, Salomon F, McGauley GA, Sonksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf) 37: 387–397, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cuneo RC, Salomon F, Sonksen PH. The syndrome of growth hormone deficiency in adults. In: Growth Hormone in Adults: Physiological and Clinical Aspects, edited by Juul A, Jorgensen JOL. Cambridge, UK: Cambridge Univ Press, 2000, p. 125–152. [Google Scholar]
- 10.Gahete MD, Cordoba-Chacon J, Anadumaka CV, Lin Q, Bruning JC, Kahn CR, Luque RM, Kineman RD. Elevated GH/IGF-I, due to somatotrope-specific loss of both IGF-I and insulin receptors, alters glucose homeostasis and insulin sensitivity in a diet-dependent manner. Endocrinology 152: 4825–4837, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Lu Y, Houle D, Robertson K, Tang Z, Kopchick JJ, Liu YL, Liu JL. Pancreatic islet-specific expression of an insulin-like growth factor-I transgene compensates islet cell growth in growth hormone receptor gene-deficient mice. Endocrinology 146: 2602–2609, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hew FL, Koschmann M, Christopher M, Rantzau C, Vaag A, Ward G, Beck-Nielsen H, Alford F. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. J Clin Endocrinol Metab 81: 555–564, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Householder LA, Yigit Suer O, Funk K, Black K, Kopchick JJ, Berryman DE. Reduction of growth hormone (GH) action in GH antagonist mice results in a dwarf and obese phenotype with impaired glucose metabolism. In: 16th Int Congr Endocrinol and 96th Endocr Soc Ann Mett and Expo. Chicago, IL, 2014, p. Abstract SUN-0631. [Google Scholar]
- 14.Hwu CM, Kwok CF, Lai TY, Shih KC, Lee TS, Hsiao LC, Lee SH, Fang VS, Ho LT. Growth hormone (GH) replacement reduces total body fat and normalizes insulin sensitivity in GH-deficient adults: a report of one-year clinical experience. J Clin Endocrinol Metab 82: 3285–3292, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Jara A, Benner CM, Sim D, Liu X, List EO, Householder LA, Berryman DE, Kopchick JJ. Elevated systolic blood pressure in male GH transgenic mice is age dependent. Endocrinology 155: 975–986, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson JO, Fowelin J, Landin K, Lager I, Bengtsson BA. Growth hormone-deficient adults are insulin-resistant. Metabolism 44: 1126–1129, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Kamagate A, Dong HH. FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 7: 3162–3170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, Jorgensen JO. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab 292: E920–E927, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Krusenstjerna-Hafstrom T, Clasen BF, Moller N, Jessen N, Pedersen SB, Christiansen JS, Jorgensen JO. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab 96: 2548–2557, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Krusenstjerna-Hafstrom T, Madsen M, Vendelbo MH, Pedersen SB, Christiansen JS, Moller N, Jessen N, Jorgensen JO. Insulin and GH signaling in human skeletal muscle in vivo following exogenous GH exposure: impact of an oral glucose load. PLoS One 6: e19392, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 27: 524–535, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luque RM, Lin Q, Cordoba-Chacon J, Subbaiah PV, Buch T, Waisman A, Vankelecom H, Kineman RD. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One 6: e15767, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacGorman LR, Rizza RA, Gerich JE. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab 53: 556–559, 1981. [DOI] [PubMed] [Google Scholar]
- 24.McConnell EM, Atkinson AB, Ennis C, Hadden DR, McCance DR, Sheridan B, Bell PM. The effects on insulin action in adult hypopituitarism of recombinant human GH therapy individually titrated for six months. J Clin Endocrinol Metab 86: 5342–5347, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30: 152–177, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Morton NM, Densmore V, Wamil M, Ramage L, Nichol K, Bunger L, Seckl JR, Kenyon CJ. A polygenic model of the metabolic syndrome with reduced circulating and intra-adipose glucocorticoid action. Diabetes 54: 3371–3378, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira CR, Salvatori R, Barreto-Filho JA, Rocha IE, Mari A, Pereira RM, Campos VC, Menezes M, Gomes E, Meneguz-Moreno RA, Araujo VP, Leite NT, Nascimento-Junior AC, Farias MI, Viscente TA, Araujo RD, Melo EV, Aguiar-Oliveira MH. Insulin sensitivity and beta-cell function in adults with lifetime, untreated isolated growth hormone deficiency. J Clin Endocrinol Metab 97: 1013–1019, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Riedl M, Ludvik B, Pacini G, Clodi M, Kotzmann H, Wagner O, Kautzky-Willer A, Prager R, Luger A. The increased insulin sensitivity in growth hormone-deficient adults is reduced by growth hormone replacement therapy. Eur J Clin Invest 30: 771–778, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31: 663–669, 1982. [DOI] [PubMed] [Google Scholar]
- 30.Salomon F, Cuneo R, Sonksen PH. Insulin sensitivity and insulin resistance in growth-hormone-deficient adults. Horm Res 40: 34–36, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicente TA, Rocha IE, Salvatori R, Oliveira CR, Pereira RM, Souza AH, Campos VC, Santos EG, Diniz RD, Valenca EH, Epitacio-Pereira CC, Oliveira MC, Mari A, Aguiar-Oliveira MH. Lifetime congenital isolated GH deficiency does not protect from the development of diabetes. Endocr Connect 2: 112–117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayakumar A, Wu Y, Sun H, Li X, Jeddy Z, Liu C, Schwartz GJ, Yakar S, LeRoith D. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes 61: 94–103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesenborn DS, Ayala JE, King E, Masternak MM. Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr) 36: 9709, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest 113: 96–105, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]