Abstract
Sphingosine 1-phosphate (S1P) is a powerful regulator of platelet formation. Enzymes generating S1P include sphingosine kinase 1. The present study thus explored the role of sphingosine kinase 1 in platelet formation and function. Activation-dependent platelet integrin αIIbβ3 activation and secretion of platelets lacking functional sphingosine kinase 1 (sphk1−/−) and of wild-type platelets (sphk1+/+) were determined utilizing flow cytometry and chronolume luciferin assay. Cytosolic Ca2+ activity ([Ca2+]i) and aggregation were measured using fura-2 fluorescence and aggregometry, respectively. In vitro platelet adhesion and thrombus formation were evaluated using a flow chamber with shear rates of 1,700 s−1. Activation-dependent increase of [Ca2+]i, degranulation (release of alpha and dense granules), integrin αIIbβ3 activation, and aggregation were all significantly increased in sphk1−/− platelets compared with sphk1+/+ platelets. Moreover, while platelet adhesion and thrombus formation under arterial shear rates were significantly augmented in Sphk1-deficient platelets, bleeding time and blood count were unaffected in sphk1−/− mice. In conclusion, sphingosine kinase 1 is a powerful negative regulator of platelet function counteracting degranulation, aggregation, and thrombus formation.
Keywords: sphingosine kinase 1, platelet activation, [Ca2+]i, thrombus formation
platelets accomplish primary hemostasis following vascular injury and by the same token contribute to the development of acute thrombotic occlusion with subsequent disruption of tissue perfusion leading, e.g., to myocardial infarction and ischemic stroke (15, 40). Platelets further participate in the pathopysiology of a wide variety of further clinical disorders, such as cancer (22), inflammation (7, 47), and host-pathogen interaction (28, 47). Stimulators of platelets include subendothelial collagen, adenosine diphosphate (ADP), thromboxane A2 (TXA2), thrombin, or collagen-related peptide (CRP) (8, 30). Activation of platelets leads to degranulation, aggregation, thrombus formation, and thus vascular occlusion (9, 10). Thrombin and CRP trigger platelet integrin αIIbβ3 activation and degranulation by increasing intracellular calcium concentration ([Ca2+]i) (27), which finally results in cytoskeletal reorganization (13).
In mast cells, intracellular Ca2+ is rapidly and transiently increased by spingosine kinase 1 (31). Furthermore, sphingosine 1-phosphate (S1P) mobilizes intracellular Ca2+ in mast cells in an inositol triphosphate (IP3)-dependent manner (29). In other cell types, sphingosine kinases regulate [Ca2+]i by influencing voltage-gated Ca2+ channels (6) or by activating the store-operated calcium entry (21). Sphingosine kinases and sphingosine 1-phosphate (S1P) further regulate platelet formation by megakaryopoiesis (19). They are effective by upregulating Src family kinases in megakaryocytes (55). Megakyocytes express the S1P multifunctional receptor S1pr1, and S1P signaling is an important prerequisite for proper thrombopoesis (54). Thus S1pr1-deficient megakaryocytes showed impaired migration of proplatelet extrusions in the circulatory compartment and impaired proplatelet shedding. Beyond that hematopoetic-specific knockout of S1pr1 leads to severe thrombocytopenia (19).
In other cell types, S1P participates in the regulation of cell migration, proliferation, and cell adhesion (25, 37), as well as angiogenesis, atherosclerosis, and inflammation (11, 23, 37, 43). Furthermore, S1P is heavily produced and secreted from activated platelets (53). In human platelets, S1P release after thrombin stimulation is mainly dependent on thromboxane formation (48).
Besides its described effect on megakaryopoiesis and proplatelet formation, the effect of S1P on platelet function is discussed controversially. On the one hand, S1P and the related compound sphingosylphosphorylcholine were described as inhibiting factors of platelet function and aggregation (3, 35, 51). On the other hand, S1P was described as activator of platelet aggregation, shape change, and calcium influx (38, 53).
Enzymes generating the bioactive sphingolipid S1P include sphingosine kinase 1 and sphingosine kinase 2 (18, 34), whereby the two isoforms seem to have overlapping functions since inactivation of neither gene product elicits phenotypical changes. Interestingly, sphingosine kinase 2 is mainly located in the nucleus of megakaryocytes (55), whereas sphingosine kinase 1 seems to be located in the cytoplasm and the inner leaflet of the plasma membrane (2). While both isoforms are expressed in platelets, sphingosine kinase 1 seems to be the most important isoform in platelets (48). Furthermore, spingosine kinase 1 is able to disrupt the cyooxygenase-2 (COX-2) signaling, thus decreasing PGI2 and increasing thromboxane formation (14).
Despite the strong impact of sphingosine kinase 1 and its metabolite S1P on platelet function and signaling, the role of sphingosine kinase 1 in the regulation of platelet function is still ill defined. The present study thus explored the role of sphingosine kinase 1 in platelet activation and Ca2+ signaling. To this end platelets have been isolated from gene-targeted mice lacking functional sphingosine kinase 1 (sphk1−/−) and from their wild-type littermates (sphk1+/+).
MATERIALS AND METHODS
Chemicals and antibodies.
Platelets were activated using collagen-related peptide (Richard Farndale, University of Cambridge, Cambridge, UK) or thrombin (Roche). Fibrinogen (Enzyme Research Laboratories) was used for aggregation studies. Sphingosine kinase 1 inhibitor SK1-I was purchased from Enzo Lifescience. Fluorophore-labeled antibodies for P-selectin (Wug.E9-FITC) and activated integrin αIIbβ3 (JON/A-PE) as well as the corresponding isotype-matched control antibodies (Rat IgG FITC and Rat IgG PE) were purchased from Emfret Analytics. Fluorophore-labeled antibody for CD45 was purchased from BD Pharmingen and used for analysis of platelet-leukocyte coaggregation in flow cytometry.
Mice.
Sphingosine kinase 1 knockout mice (sphk1−/−) and their wild-type littermates (sphk1+/+) were generated and bred on a C57BL/6 background as described elsewhere (2). Animals were genotyped by PCR. For experiments, both male and female mice were selected. All animal experiments were conducted according to the German law for the welfare of animals and were approved by local authorities. The investigation conforms to the Directive 2010/63/EU of the European Parliament.
Preparation of mouse platelets.
Platelets were obtained from 10- to 12-wk-old sphk1−/− and sphk1+/+ mice of either sex as described previously (7). The mice were anesthetized with isoflurane, and blood was drawn from the retroorbital plexus into anticoagulated tubes. Blood parameters were analyzed with pocH-100iv automatic hematology analyzer (Sysmex). Platelet-rich plasma (PRP) was obtained by centrifugation at 260 g for 5 min. Afterwards, PRP was centrifuged at 640 g for 5 min to pellet the platelets. Where necessary apyrase (0.02 U/ml; Sigma-Aldrich) and prostaglandin I2 (0.5 μM; Calbiochem) were added to the PRP to prevent activation of platelets during isolation. After two washing steps, the pellet of washed platelets was resuspended in modified Tyrode-HEPES buffer (pH 7.4, supplemented with 1 mM CaCl2).
Blood parameters.
For examination of the blood parameters, 100 μl of blood from sphk1+/+ and sphk1−/− were drawn and collected in heparinized tubes. Afterwards, analysis was done with a KX21-N automatic hematology analyzer (Sysmex).
Cytosolic Ca2+ concentration.
Washed murine platelets were suspended in Tyrode buffer without calcium and loaded with 5 μM fura-2 acetoxymethylester (Invitrogen) in the presence of 0.2 μg/ml Pluronic F-127 (Biotium) at 37°C for 30 min as described previously (9). Loaded platelets, washed once and resuspended in Tyrode buffer containing 1 mM Ca2+, were activated with the indicated agonists. Calcium responses were measured under stirring with a spectrofluorimeter (LS 55; PerkinElmer) at alternate excitation wavelengths of 340 and 380 nm (37°C). The 340/380 nm ratio values were converted into nanomolar concentrations of [Ca2+] by lysis with Triton X-100 (Sigma-Aldrich) and a surplus of EGTA. Where indicated 0.5 mM EGTA (Roth) or 50 μM 2-APB (Sigma-Aldrich) were added to the Tyrode buffer before the Ca2+ measurement.
ATP release.
ATP release was determined to study secretion of dense granules as described previously (10). To this end, platelets were treated with different agonist concentrations. For determination of ATP release, the isolated platelets were adjusted to a concentration of 250 × 106 platelets/μl. After calibration of one sample with the ATP standard (ChronoLog, Havertown, PA), the ATP concentration was determined utilizing the ChronoLume luciferin assay (ChronoLog) on a luminoaggregometer (model 700; ChronoLog) according to the manufacturer's protocol. Where indicated the platelets were pretreated for 30 min with the sphingosine kinase 1 inhibitor SK1 (10 μM).
Flow cytometry.
Two-color analysis of mouse platelet activation and platelet-leukocyte aggregates (PLA) was conducted using fluorophore-labeled antibodies for expression of P-selectin (Wug.E9-FITC), activated integrin αIIbβ3 (JON/A-PE), and CD45 (APC) as described previously (33). Heparinized whole blood was diluted 1:20 in modified Tyrode buffer and washed twice. After addition of 1 mM CaCl2, blood samples were mixed with antibodies and subsequently stimulated with agonists for 15 min at room temperature. The reaction was stopped by addition of PBS supplemented with calcium, and samples were immediately analyzed on a FACSCalibur flow cytometer (BD Biosciences). After a number of 10,000 collected events, the measurement was stopped and the analysis was done with the CellQuest Pro software (BD Biosciences).
Platelet aggregometry.
Aggregation of isolated platelets at a concentration of 250 × 106 platelets/μl in Tyrode buffer pH 7.4 was estimated from light transmission determined with a luminoaggregometer (model 700; ChronoLog). After the measurement was adjusted according to the manufacturer's protocol, platelets were activated with thrombin or CRP at the indicated concentrations for 10 min at 37°C and a stir speed of 1,000 rpm. Analysis was performed with the Aggrolink8 software (ChronoLog).
Flow chamber.
Heparinized mouse whole blood was diluted 1:3 in modified Tyrode buffer (2 mM CaCl2) and perfused through a transparent flow chamber (slit depth: 50 μm) over a collagen-coated surface (200 μg/ml) with high (1,700 s−1) shear rates for 5 min as described previously (10). After perfusion the chamber was rinsed for 5 min by perfusion with Tyrode buffer and pictures were taken from five to six different microscopic areas (using optical objectives ×20 and ×40; Carl Zeiss). Analysis was done with AxioVision software (Carl Zeiss), and the mean percent value of the covered area was determined. Where indicated the platelets were pretreated for 30 min with the sphingosine kinase 1 inhibitor SK1 (10 μM).
Bleeding time.
Mice were anesthetized, and a 3-mm segment of the tail tip was removed with a scalpel. Tail bleeding was monitored by gentle absorption of the blood with filter paper at 20-s intervals without making contact with the wound site. When no blood was observed on the paper, bleeding was considered to have ceased. Experiments were stopped after 20 min.
Statistical analysis.
Data are provided as means ± SE; n represents the number of experiments. All data were tested for significance using paired or unpaired Student t-test and one-way ANOVA with Dunnett's post hoc test. Results with P < 0.05 were considered statistically significant.
RESULTS
To explore the role of sphingosine kinase 1 in the regulation of platelet function, platelets were isolated from gene-targeted mice lacking functional sphingosine kinase 1 (sphk1−/−) and their respective wild-type littermates (sphk1+/+). Blood platelet counts and mean platelet volume (MPV) were similar in sphk1+/+ and sphk1−/− mice (Table 1) indicating that sphingosine kinase 1 is not essential for platelet generation. Other hematological parameters were again similar in sphk1+/+ and sphk1−/− mice (Table 1).
Table 1.
Blood count of sphk1−/− and sphk1+/+ mice
| sphk1+/+ | sphk1−/− | |
|---|---|---|
| Platelets, ×103/μl | 844.67 ± 41.04 | 799.14 ± 65.86 |
| MPV, fl | 6.33 ± 0.04 | 6.38 ± 0.05 |
| RBC, ×106/μl | 8.52 ± 0.19 | 8.41 ± 0.70 |
| HGB, g/dl | 5.34 ± 1.61 | 4.03 ± 0.26 |
| HCT, % | 45.06 ± 0.97 | 46.08 ± 0.87 |
| MCV, fl | 52.89 ± 0.19 | 53.11 ± 0.28 |
Arithmetic means ± SE (n = 12) of platelet count (Platelets), mean platelet volume (MPV), red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), and mean RBC volume (MCV).
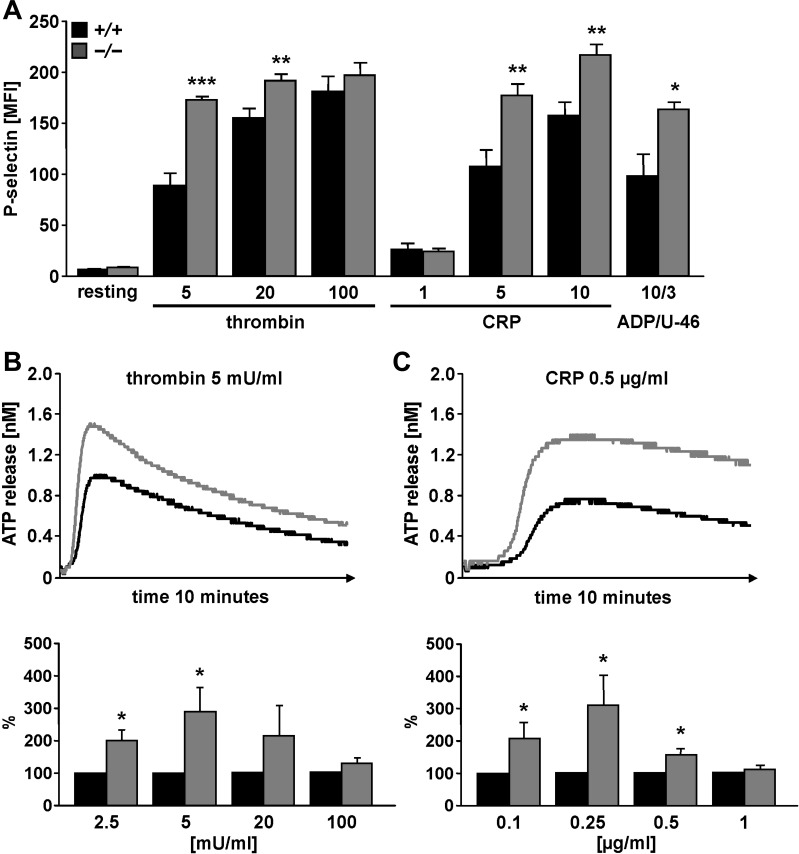
To examine the sphingosine kinase 1-dependent degranulation of platelet alpha and dense granules in sphk1+/+ and sphk1−/− mice, activation-dependent P-selectin exposure (alpha granules) and ATP release (dense granules) were determined using flow cytometry and luminescence (Fig. 1). As a result, stimulation of platelets with either CRP (5 or 10 μg/ml), thrombin (5 or 20 mU/ml), or 10 μM ADP/3 μM U-46619 was followed by a marked increase of P-selectin abundance at the platelet surface of both genotypes, an effect, however, significantly higher in sphk1−/− platelets than in sphk1+/+ platelets (Fig. 1A). Platelet activation with thrombin (2.5 and 5 mU/ml) and CRP (0.1, 0.25, and 0.5 μg/ml) further triggered ATP release, an effect again significantly more pronounced in sphk1−/− platelets than in sphk1+/+ platelets (Fig. 1, B and C).
Fig. 1.
Agonist-induced granule release in platelets from sphk1−/− and sphk1+/+ mice. A: arithmetic means ± SE (n = 6) of P-selectin exposure determined by flow cytometry in platelets from sphk1+/+ (black bars) and sphk1−/− (grey bars) mice in response to collagen-related peptide (CRP; μg/ml), thrombin (mU/ml), or ADP/U-46 (μM) at the indicated concentrations. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant difference from sphk1+/+ platelets. B: thrombin-dependent ATP release from sphk1+/+ and sphk1−/− platelets. Original tracings (top) and arithmetic means ± SE (n = 8, bottom) illustrating the ATP concentration in the supernatant following stimulation of platelets from either sphk1+/+ (black) or sphk1−/− (grey) mice with thrombin at the indicated concentrations. *P < 0.05, statistically significant difference from sphk1+/+ platelets. C: CRP-dependent ATP release from sphk1+/+ and sphk1−/− platelets. Original tracings (top) and arithmetic means ± SE (n = 8, bottom) illustrating ATP concentration in the supernatant following stimulation of platelets from either sphk1+/+ (black) or sphk1−/− (grey) mice with CRP at the indicated concentrations. *P < 0.05, statistically significant difference from sphk1+/+ platelets.
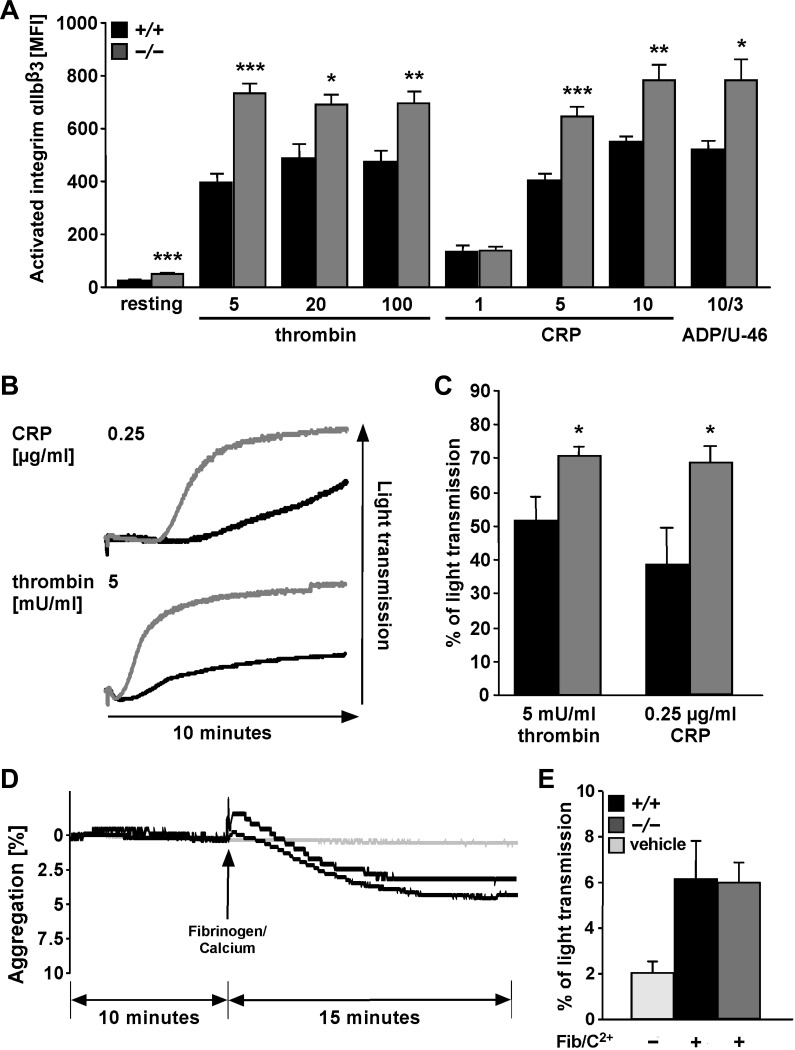
A further hallmark of activated platelets is the increased integrin αIIbβ3 activation. Thus integrin αIIbβ3 activation was determined utilizing antibodies in flow cytometry. The treatment of platelets with either CRP (5 or 10 μg/ml CRP), thrombin (5, 20, or 100 mU/ml), or 10 μM ADP/3 μM U-46619 was followed by activation of integrin αIIbβ3, an effect again significantly higher in sphk1−/− platelets than in sphk1+/+ platelets (Fig. 2A). Following activation with low dose of CRP (0.25 μg/ml) or thrombin (5 mU/ml), platelet aggregation was significantly higher in sphk1−/− platelets than in sphk1+/+ platelets (Fig. 2, B and C). In view of a significantly stronger integrin αIIbβ3 activation in resting platelets (Fig. 2A), the spontaneous aggregation of sphk1−/− and sphk1+/+ platelets was examined. As shown in Fig. 2, D and E, there was no significant difference between sphk1−/− and sphk1+/+ platelets before and after fibrinogen/Ca2+ induction (Fig. 2, D and E). In addition, platelets from sphk1−/− mice did not show spontaneous or activation-dependent formation of platelet-leukocyte aggregates (data not shown).
Fig. 2.
Agonist-induced activation of integrin αIIbβ3 and aggregation in platelets from sphk1−/− and sphk1+/+ mice. A: arithmetic means ± SE (n = 6) of activated integrin αIIbβ3 determined by flow cytometry in platelets from sphk1+/+ (black bars) and sphk1−/− (grey bars) mice in response to CRP (μg/ml), thrombin (mU/ml), or ADP/U-46 (μM) at the indicated concentrations. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant difference from sphk1+/+ platelets. B: representative tracings of aggregometry after stimulation of platelets from either sphk1+/+ (black) or sphk1−/− (grey) mice with 0.25 μg/ml CRP (top) or 5 mU/ml thrombin (bottom). C: arithmetic means ± SE (n = 7) of aggregometry after stimulation of platelets from either sphk1+/+ (black bars) or sphk1−/− (gray bars) mice with either 0.25 μg/ml CRP or 5 mU/ml thrombin. *P < 0.05, statistically significant difference from sphk1+/+ platelets. D: representative tracings of spontaneous platelet aggregation before and after addition of fibrinogen (100 μg/ml)/Ca2+ (1 mM) in platelets from sphk1+/+ (black line) and sphk1−/− (dark grey line) mice or the appropriate vehicle control (light grey line). E: arithmetic means ± SE (n = 6) of spontaneous platelet aggregation in the presence of fibrinogen (100 μg/ml)/Ca2+ (1 mM) in platelets from sphk1+/+ (black bar) and sphk1−/− (dark grey bar) mice or the appropriate vehicle control (light grey bar).
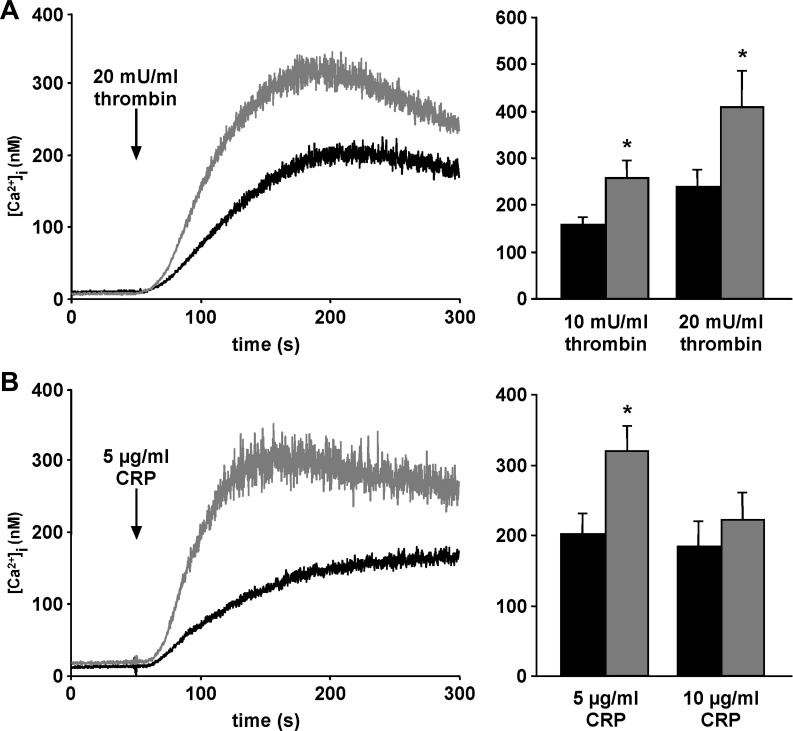
As the increase of [Ca2+]i is expected to stimulate platelet degranulation and integrin αIIbβ3 activation, the [Ca2+]i was estimated. As illustrated in Fig. 3, the treatment of platelets with either thrombin (10 or 20 mU/ml; Fig. 3A) or CRP (5 or 10 μg/ml CRP; Fig. 3B) was followed by a marked increase of [Ca2+]i in platelets of both genotypes, an effect, however, again significantly higher in sphk1−/− platelets than in sphk1+/+ platelets (Fig. 3). Interestingly, the inhibition of the extracellular calcium influx via EGTA or 2-APB treatment abolished these increased [Ca2+]i and no activation-dependent difference between sphk1−/− and sphk1+/+ platelets was detectable (data not shown).
Fig. 3.
Agonist-induced Ca2+ influx in platelets from sphk1−/− and sphk1+/+ mice. A: representative tracings (left) of fura-2-fluorescence reflecting cytosolic Ca2+ concentration ([Ca2+]i) and arithmetic means of maximal Δ[Ca2+]i ± SE (n = 6, right) of sphk1+/+ (black) and sphk1−/− (grey) platelets before and following stimulation with thrombin (10 and 20 mU/ml). *P < 0.05, statistically significant difference from sphk1+/+ platelets. B: representative tracings (left) of fura-2-fluorescence reflecting cytosolic Ca2+ concentration [Ca2+]i and arithmetic means of maximal Δ[Ca2+]i ± SE (n = 6, right) of sphk1+/+ (black) and sphk1−/− (grey) platelets before and following stimulation with CRP (5 and 10 μg/ml). *P < 0.05, statistically significant difference from sphk1+/+ platelets.
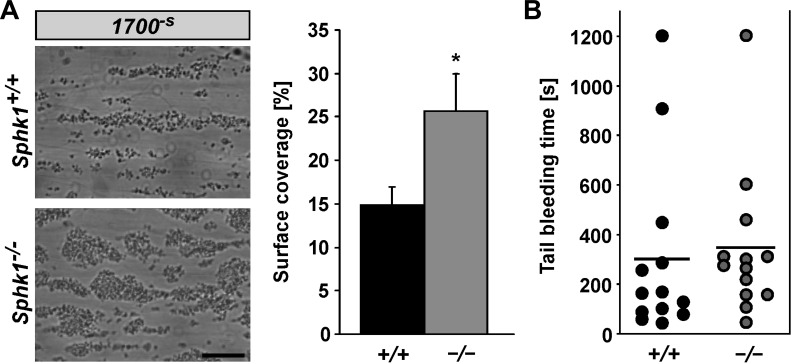
To elucidate the relevance of sphingosine kinase 1 for pathologic thrombus formation, in vitro platelet adhesion to collagen-coated surfaces and thrombus formation under flow at high wall shear rates (1,700 s−1) were analyzed. As shown in Fig. 4A, sphk1−/− platelets formed significantly more in vitro thrombi than sphk1+/+ platelets.
Fig. 4.
Thrombus formation and tail bleeding time of platelets from sphk1−/− and sphk1+/+ mice. A: original phase contrast images (left) of surface coverage by adherent platelets and arithmetic means ± SE (n = 6, right) following perfusion of whole blood from sphk1+/+ (black bar) and sphk1−/− (grey bar) mice over a collagen-coated surface for 5 min at high (1,700−s) arterial shear rates. Scale bar equals 50 μm. *P < 0.05, statistically significant difference from sphk1+/+ platelets. B: arithmetic means ± SE (n = 14) of tail bleeding time measured after amputating the tail tip of sphk1+/+ (black dots) and sphk1−/− (grey dots) mice. Each dot represents 1 individual; black bar represents the mean value.
Although platelets from sphk1−/− mice showed a significantly increased activation-dependent ATP release and in vitro thrombus formation compared with their wild-type littermates, there were no significant effects on ATP release and in vitro thrombus formation by pharmacological inhibition via the sphingosine kinase 1 inhibitor SK1-I in wild-type mouse platelets (data not shown).
Despite the differences in degranulation, aggregation, extracellular Ca2+ influx, and in vitro thrombus formation, the tail bleeding time was not significantly different between sphk1−/− (344 ± 77 s, n = 14) and sphk1+/+ (296 ± 92 s, n = 14) mice (Fig. 4B), suggesting that alteration of sphingosine kinase 1 is able to modulate in vitro thrombus formation and therefore thrombosis without altering this response to wounding.
DISCUSSION
The present study discloses a novel, powerful regulator of platelet activation and function. Lack of sphingosine kinase 1 augments Ca2+ entry into platelets and subsequent activation-dependent platelet degranulation, integrin αIIbβ3 activation, aggregation, and in vitro thrombus formation. Thus sphingosine kinase 1 downregulates Ca2+ entry and Ca2+-sensitive platelet functions following activation with thrombin or CRP.
The present study does not address the exact mechanism involved in sphingosine kinase 1-dependent Ca2+ entry and Ca2+-dependent platelet function. In theory, sphingosine kinase 1 could be effective by decreasing the concentration of the substrate sphingosine and its metabolites by increasing the concentration of the product S1P (45) or by direct interaction with signaling molecules and kinases. The substances could be effective by modifying either Ca2+ entry or Ca2+ extrusion. Besides its effect on sphingosine and S1P formation, sphingosine kinase 1 can affect cell functions and [Ca2+]i by direct target interaction (6, 12).
The substrate sphingosine may be converted by ceramide synthase(s) to ceramide (45), which is known to modify Ca2+ entry and Ca2+-sensitive cellular functions (32, 41). Ceramide contributes to the formation of lipid rafts, cholesterol-enriched membrane microdomains that provide a platform for signaling pathways, such as redox reactions (24). Ceramide increases the curvature and the bending rigidity of the membrane (16, 20) and thus modifies the membrane properties and degranulation of cells. However, platelets cannot accomplish de novo synthesis of ceramide (46) and thus depend on sphingomyelinase for ceramide production. Accordingly, activation-dependent ceramide formation, ceramide-dependent membrane scrambling, thrombin generation, and in vitro thrombus formation are significantly decreased in platelets from acid sphingomyelinase-deficient mice (33).
S1P regulates a wide variety of cellular functions, such as adhesion, migration, survival, and proliferation (25, 37), all functions similarly regulated by Ca2+ entry (26). According to the present study, activation-dependent Ca2+ influx after thrombin and CRP stimulation are significantly increased in platelets of sphingosine kinase 1-deficient mice. In mast cells, sphingosine kinase 1 activation via the IgE receptor results in fast and transient intracellular Ca2+ increase (31). Sphingosine kinase 1 regulates voltage-gated Ca2+ channels as well as store-operated calcium entry (6, 21) and S1P intracellular Ca2+ mobilization in RBL-2H3 mast cells (29), which leads to an increased intracellular Ca2+ level.
In sphingosine kinase 1-deficient platelets, the Sphk2 isoform could in theory produce S1P thereby elevating intracellular Ca2+ in sphk1−/− platelets. Megakaryocytes do express Sphk2, which predominantly localizes to the nucleus (55). Sphingosine kinase activity in mature platelets is restricted to the cell membrane and the cytosol (5). Thus sphingosine kinase 2 and sphingosine kinase 1 presumably serve different functions. Clearly, additional experimentation is required to fully elucidate the putative role of sphingosine kinase 2 in the regulation of platelet function.
Interestingly, lack of sphingosine kinase 1 did not appreciably modify platelet count, indicating that platelet formation does not require this enzyme. Instead sphingosine kinase 2 is required for megakaryopoeisis (55). S1P participates in the regulation of platelet formation (19). S1P is generated by both sphingosine kinase 1 and sphingosine kinase 2 (18, 34). The S1P formation by sphingosine kinase 2 may be sufficient for adequate platelet formation but not for the regulation of platelet Ca2+ entry and Ca2+-sensitive platelet functions.
The importance of S1P as a signaling molecule in the regulation of blood flow, coagulation and inflammation is underscored by the high serum S1P levels in a low-micromolar range, whereas in tissues S1P approaches nanomolar concentrations (39). Among blood cells, S1P is mainly stored in platelets, which lack the S1P-degrading enzyme S1P lyase (48). S1P could be released after platelet activation (38). Thus serum S1P may be mainly released by platelets (36, 52). Since in sphingosine kinase 1 knockout mice the serum S1P levels but not the S1P levels in other tissues were dramatically decreased (2), it is tempting to speculate that the sphingosine kinase 1 isoform is mainly responsible for S1P production in platelets and that sphk1−/− platelets release smaller amounts of S1P than sphk1+/+ platelets. Again, further experiments are needed to fully elucidate the origin of serum S1P in sphk1−/− and sphk1+/+ mice.
Sphingosine kinase 1 disrupts COX-2 signaling and positively regulates microsomal PGE synthase 1 (mPGES-1) (14). Since sphingosine kinase 1 increases PGE2 and decreases TXA2, it modifies platelet activity by influencing the balance between PGE2 and TXA2 concentration and production.
Hemostasis and thrombosis further depend strongly on the MAP kinase pathway (1, 4), which is activated by sphingosine kinase in endothelial cells (44). In neutrophils, sphingosine kinase 1 is a negative regulator of JNK activity, thus blunting inflammation and tissue injury (12). Sphingosine kinase 1 may play a similar role in platelets and/or megakaryocytes. According to the present observations, sphingosine kinase 1 participates in the regulation of platelet secretion, which plays a decisive role in vascular inflammation and atherogenesis (7, 15). Interestingly, the observed effects on platelet secretion were only apparent at low-dose stimulation with thrombin and CRP. Increasing the agonist concentration dissipated the observed differences in platelet secretion (P-selectin expression and ATP release) and integrin αIIbβ3 activation in sphk1−/− and sphk1+/+ platelets, indicating that sphingosine kinase 1 inhibits the sensitivity of platelets to low-dose activation whereas platelet function after high-dose stimulation is not affected. These results indicate that higher agonist concentrations are able to activate alternative signaling pathways in platelets and thus bypass the sphingosine kinase 1-dependent desensitivation of platelet activation. Therefore, high agonist concentrations can abolish the observed inhibitory effect of sphingosine kinase 1 in platelet activation. Finally, sphingosine kinase 1 seems to decrease the sensitivity of platelets to low-dose stimulators of Ca2+ entry and therefore to platelet activation.
Platelets contacting thrombogenic subendothelial collagen are recruited to the injured vessel wall (9). Following translocation of the phosphatidylserine (PS)-exposing membrane, blebs and microvesicles stimulate the coagulation process by triggering assembly and activation of tenase and prothrombinase complex (17). The conversion of activated platelets involves biochemical and morphologic changes similar to those observed in apoptotic cells (42).
Platelets with impaired sphingosine kinase 1 may be particularly prone to thrombus formation under high shear stress, which is a function of platelet degranulation (9). In this context, it is interesting that the tail bleeding time is unaffected in sphk1−/− mice compared with sphk1+/+ mice. As described for the phosphoinositide 3-kinase/Akt pathway, the thrombus formation and the hemostatic plug formation after transection of the tail tip could show different results due to the divergent flow rates in both thrombus formation models (49, 50). Our result thus indicate that sphingosine kinase 1-dependent defects in collagen-mediated in vitro thrombus formation require higher shear rates than those that appear in the small tail arteries and veins. Sphingosine kinase 1 deficiency may thus predispose to thrombotic complications, such as ischemic stroke or atherosclerosis, by increasing activation-dependent Ca2+ signaling, platelet degranulation, aggregation, and thrombus formation under high arterial shear rates. Conversely, stimulation of sphingosine kinase 1 activity may be a therapeutic option to decrease the susceptibility to thrombotic complications.
In conclusion, the present observations identify sphingosine kinase 1 as a novel negative regulator of platelet Ca2+ signaling, platelet degranulation, platelet aggregation, and thrombus formation. Thus stimulation of sphingosine kinase 1 may protect against thrombosis.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft–Klinische Forschergruppe (DFG-KFO 274) “Platelets-Molecular Mechanisms and Translational Implications,” Fortüne (2133-0-0), and DFG (BO 3786/1-1) Research Grant (to O. Borst), as well as the Tuebingen Platelet Investigative Consortium (TuePIC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.M., E.S., B.W., A.F., M.C., D.R., S.V., S.M.H., K.M., and T.G. performed experiments; P.M., E.S., B.W., A.F., M.C., D.R., S.V., S.M.H., K.M., P.S., and T.G. analyzed data; P.M., E.S., B.W., A.F., M.C., K.M., P.S., and T.G. interpreted results of experiments; P.M. and O.B. prepared figures; P.M., E.S., B.W., A.F., M.C., D.R., S.V., S.M.H., K.M., P.S., T.G., M.G., O.B., and F.L. approved final version of manuscript; M.G., O.B., and F.L. conception and design of research; F.L. drafted manuscript.
ACKNOWLEDGMENTS
We thank Efi Faber for providing outstanding technical assistance and acknowledge the meticulous preparation of the manuscript by Tanja Loch.
REFERENCES
- 1.Adam F, Kauskot A, Rosa JP, Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost 6: 2007–2016, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Altmann C, Meyer Zu Heringdorf D, Boyukbas D, Haude M., Jakobs KH, Michel MC. Sphingosylphosphorylcholine, a naturally occurring lipid mediator, inhibits human platelet function. Br J Pharmacol 138: 435–444, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslan JE, Baker SM, Loren CP, Haley KM, Itakura A, Pang J, Greenberg DL, David LL, Manser E, Chernoff J, McCarty OJ. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am J Physiol Cell Physiol 305: C519–C528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banno Y, Kato M, Hara A, Nozawa Y. Evidence for the presence of multiple forms of Sph kinase in human platelets. Biochem J 335: 301–304, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom T, Bergelin N, Slotte JP, Tornquist K. Sphingosine kinase regulates voltage operated calcium channels in GH4C1 rat pituitary cells. Cell Signal 18: 1366–1375, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Borst O, Munzer P, Gatidis S, Schmidt EM, Schonberger T, Schmid E, Towhid ST, Stellos K, Seizer P, May AE, Lang F, Gawaz M. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ Res 111: 1297–1307, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Borst O, Munzer P, Schmid E, Schmidt EM, Russo A, Walker B, Yang W, Leibrock C, Szteyn K, Schmidt S, Elvers M, Faggio C, Shumilina E, Kuro-o M, Gawaz M, Lang F. 1,25(OH)2 vitamin D3-dependent inhibition of platelet Ca2+ signaling and thrombus formation in klotho-deficient mice. FASEB J 28: 2108–2119, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Borst O, Schmidt EM, Munzer P, Schonberger T, Towhid ST, Elvers M, Leibrock C, Schmid E, Eylenstein A, Kuhl D, May AE, Gawaz M, Lang F. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 119: 251–261, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Borst O, Walker B, Munzer P, Russo A, Schmid E, Faggio C, Bigalke B, Laufer S, Gawaz M, Lang F. Skepinone-L, a novel potent and highly selective inhibitor of p38 MAP kinase, effectively impairs platelet activation and thrombus formation. Cell Physiol Biochem 31: 914–924, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Carr JM, Mahalingam S, Bonder CS, Pitson SM. Sphingosine kinase 1 in viral infections. Rev Med Virol 23: 73–84, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Di A, Kawamura T, Gao XP, Tang H, Berdyshev E, Vogel SM, Zhao YY, Sharma T, Bachmaier K, Xu J, Malik AB. A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J Biol Chem 285: 15848–15857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falet H, Chang G, Brohard-Bohn B, Rendu F, Hartwig JH. Integrin αIIbβ3 signals lead cofilin to accelerate platelet actin dynamics. Am J Physiol Cell Physiol 289: C819–C825, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Furuya H, Wada M, Shimizu Y, Yamada PM, Hannun YA, Obeid LM, Kawamori T. Effect of sphingosine kinase 1 inhibition on blood pressure. FASEB J 27: 656–664, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 115: 3378–3384, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goni FM, Alonso A. Membrane fusion induced by phospholipase C and sphingomyelinases. Biosci Rep 20: 443–463, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost 88: 186–193, 2002. [PubMed] [Google Scholar]
- 18.Heffernan-Stroud LA, Obeid LM. Sphingosine kinase 1 in cancer. Adv Cancer Res 117: 201–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hla T, Galvani S, Rafii S, Nachman R. S1P and the birth of platelets. J Exp Med 209: 2137–2140, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holopainen JM, Angelova MI, Kinnunen PK. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys J 78: 830–838, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopson KP, Truelove J, Chun J, Wang Y, Waeber C. S1P activates store-operated calcium entry via receptor- and nonreceptor-mediated pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol 300: C919–C926, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 30: 2362–2367, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P, Smith AD, Li R, Rao JN, Liu L, Donahue JM, Wang JY, Turner DJ. Sphingosine kinase 1 overexpression stimulates intestinal epithelial cell proliferation through increased c-Myc translation. Am J Physiol Cell Physiol 304: C1187–C1197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin S, Zhou F, Katirai F, Li PL. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid Redox Signal 15: 1043–1083, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: a decade of progress. Biochim Biophys Acta 1831: 203–212, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang F, Gawaz M, Borst O. The serum- & glucocorticoid-inducible kinase in the regulation of platelet function. Acta Physiol (Oxf) 2014 Jun 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Lang F, Munzer P, Gawaz M, Borst O. Regulation of STIM1/Orai1-dependent Ca2+ signalling in platelets. Thromb Haemost 110: 925–930, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Lang PA, Contaldo C, Georgiev P, El Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Lohning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM, Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 14: 756–761, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Park CS, Lee YM, Suk HY, Clemons TC, Choi OH. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium 38: 581–592, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 30: 2341–2349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem 277: 17255–17262, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Mihai R, Lai T, Schofield G, Farndon JR. C2-Ceramide increases cytoplasmic calcium concentrations in human parathyroid cells. Biochem Biophys Res Commun 268: 636–641, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Munzer P, Borst O, Walker B, Schmid E, Feijge MA, Cosemans JM, Chatterjee M, Schmidt EM, Schmidt S, Towhid ST, Leibrock C, Elvers M, Schaller M, Seizer P, Ferlinz K, May AE, Gulbins E, Heemskerk JW, Gawaz M, Lang F. Acid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formation. Arterioscler Thromb Vasc Biol 34: 61–71, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J 280: 5317–5336, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Nugent D, Xu Y. Sphingosine-1-phosphate: characterization of its inhibition of platelet aggregation. Platelets 11: 226–232, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ono Y, Kurano M, Ohkawa R, Yokota H, Igarashi K, Aoki J, Tozuka M, Yatomi Y. Sphingosine 1-phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1-phosphate concentration. Lipids Health Dis 12: 20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta 1831: 157–166, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randriamboavonjy V, Badenhoop K, Schmidt H, Geisslinger G, Fisslthaler B, Fleming I. The S1P(2) receptor expressed in human platelets is linked to the RhoA-Rho kinase pathway and is down regulated in type 2 diabetes. Basic Res Cardiol 104: 333–340, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Rauch BH. Sphingosine 1-phosphate as a link between blood coagulation and inflammation. Cell Physiol Biochem 34: 185–196, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Ruggeri ZM. Platelets in atherothrombosis. Nat Med 8: 1227–1234, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Samapati R, Yang Y, Yin J, Stoerger C, Arenz C, Dietrich A, Gudermann T, Adam D, Wu S, Freichel M, Flockerzi V, Uhlig S, Kuebler WM. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am J Respir Crit Care Med 185: 160–170, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O'Reilly LA, Henley KJ, Ono A, Hsiao S, Willcox A, Roberts AW, Huang DC, Salem HH, Kile BT, Jackson SP. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 114: 663–666, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Schwalm S, Pfeilschifter J, Huwiler A. Sphingosine-1-phosphate: a Janus-faced mediator of fibrotic diseases. Biochim Biophys Acta 1831: 239–250, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol 22: 7758–7768, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siow D, Wattenberg B. The compartmentalization and translocation of the sphingosine kinases: mechanisms and functions in cell signaling and sphingolipid metabolism. Crit Rev Biochem Mol Biol 46: 365–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tani M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res 46: 2458–2467, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler Thromb Vasc Biol 30: 2357–2361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulrych T, Bohm A, Polzin A, Daum G, Nusing RM, Geisslinger G, Hohlfeld T, Schror K, Rauch BH. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J Thromb Haemost 9: 790–798, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe N, Nakajima H, Suzuki H, Oda A, Matsubara Y, Moroi M, Terauchi Y, Kadowaki T, Suzuki H, Koyasu S, Ikeda Y, Handa M. Functional phenotype of phosphoinositide 3-kinase p85alpha-null platelets characterized by an impaired response to GP VI stimulation. Blood 102: 541–548, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest 113: 441–450, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, Zhu K, Hong G, Wu W, Baudhuin LM, Xiao Y, Damron DS. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat Cell Biol 2: 261–267, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem 121: 969–973, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood 86: 193–202, 1995. [PubMed] [Google Scholar]
- 54.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Bruhl ML, Tirniceriu A, Gaertner F, Proia RL, Graf T, Bolz SS, Montanez E, Prinz M, Muller A, von Baumgarten L, Billich A, Sixt M, Fassler R, von Andrian UH, Junt T, Massberg S. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med 209: 2165–2181, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Urtz N, Gaertner F, Legate KR, Petzold T, Lorenz M, Mazharian A, Watson SP, Massberg S. Sphingosine kinase 2 (Sphk2) regulates platelet biogenesis by providing intracellular sphingosine 1-phosphate (S1P). Blood 122: 791–802, 2013. [DOI] [PubMed] [Google Scholar]