Abstract
Operant conditioning of a spinal cord reflex can improve locomotion in rats and humans with incomplete spinal cord injury. This study examined the persistence of its beneficial effects. In rats in which a right lateral column contusion injury had produced asymmetric locomotion, up-conditioning of the right soleus H-reflex eliminated the asymmetry while down-conditioning had no effect. After the 50-day conditioning period ended, the H-reflex was monitored for 100 [±9 (SD)] (range 79–108) more days and locomotion was then reevaluated. After conditioning ended in up-conditioned rats, the H-reflex continued to increase, and locomotion continued to improve. In down-conditioned rats, the H-reflex decrease gradually disappeared after conditioning ended, and locomotion at the end of data collection remained as impaired as it had been before and immediately after down-conditioning. The persistence (and further progression) of H-reflex increase but not H-reflex decrease in these spinal cord-injured rats is consistent with the fact that up-conditioning improved their locomotion while down-conditioning did not. That is, even after up-conditioning ended, the up-conditioned H-reflex pathway remained adaptive because it improved locomotion. The persistence and further enhancement of the locomotor improvement indicates that spinal reflex conditioning protocols might supplement current therapies and enhance neurorehabilitation. They may be especially useful when significant spinal cord regeneration becomes possible and precise methods for retraining the regenerated spinal cord are needed.
Keywords: H-reflex conditioning, spinal cord plasticity, motor control, spinal cord injury, locomotion, rehabilitation, learning, memory
over the past 25 years, extensive anatomical and physiological studies in nonhuman primates and rodents have shown that operant conditioning of the H-reflex creates a hierarchy of spinal and supraspinal plasticity. The spinal plasticity, which is directly responsible for the change in H-reflex size, is induced and maintained by plasticity in the brain, which acts through the corticospinal tract (CST) (see for review Thompson and Wolpaw 2014; Wolpaw 2010; Wolpaw and Chen 2009).
H-reflex conditioning can improve locomotion after partial spinal cord injuries. In rats with right lateral column (LC) injury, the step cycle is asymmetric [i.e., the time from right foot contact (RFC) to left foot contact (LFC) is shorter than the time from LFC to RFC because of weakened right stance (i.e., reduced right extensor EMG activity)] (Chen et al. 2006). In these rats, up-conditioning of the right soleus H-reflex increases the right soleus locomotor burst and thereby strengthens right stance and restores step cycle symmetry (Chen et al. 2006, 2014). In humans with spastic gait disorders due to incomplete spinal cord injury, down-conditioning of the soleus H-reflex can improve walking speed and symmetry (Manella et al. 2013; Thompson et al. 2013). The gains are evident both in formal laboratory evaluations and in the daily lives of the subjects. These results suggest that reflex conditioning protocols can provide a valuable new approach to neurorehabilitation that could supplement standard methods and augment restoration of function.
In a recent study (Chen et al. 2014), rats with spinal cord contusion injuries that had weakened the right stance phase of locomotion and caused limping underwent up-conditioning or down-conditioning of the right soleus H-reflex over a 50-day conditioning period. Up-conditioning gradually increased the H-reflex, strengthened right stance, and improved locomotor speed and symmetry. Down-conditioning gradually decreased the H-reflex but did not further impair locomotion. The present study examined the long-term persistence of H-reflex conditioning and its locomotor effects in a representative subset of these rats. H-reflex size was monitored for 79–108 days after up- or down-conditioning ended, and locomotion was evaluated again at the end of this period.
The results address a question that is crucial for the therapeutic application of reflex conditioning protocols: do the benefits of H-reflex up-conditioning persist after conditioning ends? That is, does the conditioned change in H-reflex size persist, and, most important, does its beneficial effect on locomotion persist? The results are clear and encouraging. They provide further support for the potential clinical value of reflex conditioning protocols. Furthermore, they offer further insight into the role of spinal cord plasticity in the acquisition and maintenance of motor behaviors.
METHODS
Subjects were nine young adult female Sprague-Dawley rats weighing 210–286 g [mean 244 (±31 SD) g] at the beginning of the study. These rats were a subset of rats previously studied in an evaluation of the impact of H-reflex conditioning on locomotor kinematics in spinal cord-injured rats (Chen et al. 2014). In this subset, the H-reflex was monitored for up to 108 days (1 for 79 days, 4 for 100 days, 3 for 105 days, and 1 for 108 days) after H-reflex up-conditioning (4 rats) or down-conditioning (5 rats) ended, and locomotor EMG activity and kinematics were then reevaluated. All procedures were in conformance with the Guide for the Care and Use of Laboratory Animals (National Academies Press Washington, 2011) and had been reviewed and approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. The methods for spinal cord injury, electrode implantation, treadmill locomotion, H-reflex conditioning, histological evaluation, and data analysis have been fully described elsewhere (Chen et al. 2005, 2006, 2014) and are summarized here. Figure 1 shows the study protocol.
Fig. 1.
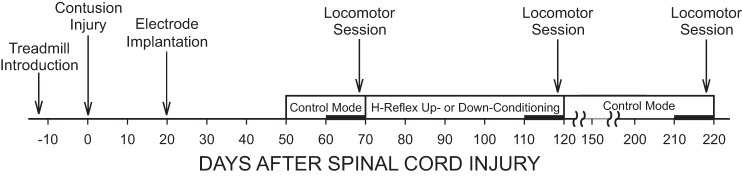
Study protocol. After learning to walk on the treadmill, each rat was subjected under anesthesia to a midthoracic contusion injury of the right lateral column (LC) at T9 and was implanted with soleus EMG electrodes and nerve cuff stimulating electrodes 20 days later. At least 56 days after the injury, each rat was exposed to the control mode (i.e., the right soleus H-reflex was simply measured) for 20 days; the H-reflex up- or down-conditioning mode [i.e., reward occurred when the H-reflex was greater (up-conditioning) or smaller (down-conditioning) than a criterion] for 50 days; and the control mode for 79–108 more days. Soleus locomotor EMG, H-reflexes, and kinematics were assessed before and immediately after conditioning and at the end of data collection. The data from days 60–70, 110–120, and 210–220 (indicated by heavy lines) were used to determine initial (i.e., control) H-reflex size, H-reflex size at the end of up- or down-conditioning, and H-reflex size at the end of data collection (i.e., final H-reflex size), respectively.
Lateral column contusion.
Under general anesthesia (ketamine HCl 80 mg/kg ip and xylazine 10 mg/kg ip), each rat received a calibrated contusion injury to the right LC of the spinal cord at T9 [Infinite Horizon (IH) Spinal Cord Impactor, Precision Systems and Instrumentation]. After a partial dorsal laminectomy with minimal disturbance of the dural envelope, the rat was mounted on the impactor with the nearby dorsal processes fixed and the injury (force setting of 200 kdyn) was performed by a 1-mm impactor tip placed on the dorsal surface of the spinal cord halfway from the midline to the right lateral edge. The site was then rinsed with normal saline and covered with Durafilm to minimize adhesions, and the muscle and skin were sutured in layers. Care in the immediate postinjury days included analgesia, antibiotics, bladder expression, and high-calorie dietary supplementation as described previously (Chen and Wolpaw 1997, 2002). In all rats, bladder function returned in 2–5 days and the BBB score (Basso et al. 1995) was ≥20 by 12 days.
Electrode implantation.
At least 20 days after the injury, each rat was implanted under the same general anesthesia with chronic stimulating and recording electrodes as previously described (Chen et al. 2001, 2002; Chen and Wolpaw 1995). To evoke the right soleus H-reflex, a silicone rubber nerve-stimulating cuff was placed on the posterior tibial nerve just above the triceps surae branches. To record EMG activity, a pair of fine-wire EMG electrodes was placed in the right soleus muscle. The wires from the nerve cuff and the muscle passed subcutaneously to a connector plug on the skull. In addition, small (2 mm) dots were tattooed bilaterally on the skin over the lateral aspects of the knee, hip (i.e., trochanter major), and iliac crests at the fifth lumbar vertebra to guide placement of markers during locomotor sessions (see below). After surgery, the rat was kept warm and received an analgesic (Demerol 0.2 mg im). It was returned to its cage and allowed to eat and drink freely. Care in the succeeding days included analgesia, antibiotics, and high-calorie dietary supplementation as described previously (Chen et al. 1996, 1999, 2014; Chen and Wolpaw 2002).
Soleus H-reflex monitoring and conditioning.
Data collection began >30 days after implantation surgery [i.e., ≥56 days (range 56–93 days) after spinal cord injury] and continued 24 h/day, 7 days/wk for 150–179 days (Fig. 1), during which the rat lived in a standard rat cage with a 40-cm flexible cable attached to the skull plug. The cable allowed the rat to move freely about the cage and conveyed the wires from the electrodes to a commutator above the cage and thence to EMG amplifiers and a stimulus isolation unit. The rat had continuous access to water and food, except that during H-reflex conditioning it received most of its food through the task described below. Animal well-being was assessed several times per day, and body weight was measured every week. Laboratory lights were lowered from 2100 to 0600 daily.
A computer system monitored right soleus EMG activity (gain 1,000, band pass 100–1,000 Hz, sampling rate 5,000 Hz) continuously 24 h/day, 7 days/wk, throughout the study. Whenever the absolute value (equivalent to the full-wave rectified value) of EMG activity stayed in a specified range for a randomly varying 2.3- to 2.7-s period, the computer elicited an H-reflex. The nerve cuff stimulus amplitude and duration were initially set to produce a maximum H-reflex (as well as an M response that was typically just above threshold). Pulse duration remained fixed (usually 0.5 ms), and pulse amplitude was automatically adjusted after each trial to maintain the M response [i.e., average amplitude of EMG activity in the M-response interval (typically 1.5–4.0 ms after nerve stimulation)] at a target size. This ensured that the effective stimulus strength was constant throughout (Chen and Wolpaw 1995; Wolpaw 1987). H-reflex size was defined as average EMG amplitude in the H-reflex interval (typically 5.5–10.0 ms after stimulation) minus average background EMG amplitude at the time of stimulation and was expressed in units of average background EMG amplitude.
In the control mode, the computer simply digitized and stored the absolute value of soleus EMG activity for 100 ms after the stimulus. After 10–21 days in this mode, the rat was exposed to H-reflex up-conditioning (HRup rats) or down-conditioning (HRdown rats) for 50 days. In the up- or down-conditioning mode, the computer gave a reward (i.e., a 20-mg food pellet) 200 ms after nerve stimulation whenever soleus EMG activity in the H-reflex interval was above (up-conditioning) or below (down-conditioning) a criterion. The criterion was adjusted as needed each day so that the rat obtained an adequate amount of food (e.g., ∼700 reward pellets/day for a 350-g rat). After the 50-day conditioning period, the rat returned to the control mode for 79–108 more days (Fig. 1).
Locomotor data collection.
Before the contusion injury, each rat learned to walk on a motor-driven treadmill at 9–12 m/min (Chen et al. 2005, 2006). Briefly, each rat learned in one or two training sessions to walk quadrupedally on a motor-driven treadmill (1 session per day; 10–20 min walking per session). During this training, the rat was motivated primarily by food reward (bread or cereal). In a few rats, this was supplemented early in training by a weak (0.69 mA, 0.2 s) electric stimulus from a metal grid just behind the posterior end of the treadmill. This minimal aversive stimulus caused no vocalization or other evidence of significant distress and was administered only once or twice per rat. These training sessions were effective: when the rats were placed on the treadmill for actual locomotor data collection later on, they typically walked immediately.
Locomotor data were subsequently collected from each rat in three treadmill sessions (Fig. 1): one session near the end of the control-mode exposure just before H-reflex up- or down-conditioning began; one session at the end of conditioning; and a final session 79–108 days after the end of conditioning. Prior to each locomotor session, each rat was shaved and, for each leg, 3-mm reflective adhesive markers were placed on the lateral aspects of the fifth metatarsophalangeal joint, the ankle joint (i.e., lateral malleolus), the knee joint, the hip joint (i.e., trochanter major), the iliac crest at the fifth lumbar vertebra, and the midpoint between the ankle and knee joints to enable later analysis of locomotor kinematics. In each rat, treadmill speed was the same for all three sessions. During locomotion, soleus EMG activity was continuously recorded (bandpass 100–1,000 Hz), digitized (4,000 Hz), and stored. In addition, locomotor kinematics were recorded bilaterally with a three-dimensional (3D) video data collection and analysis system (100 frames/s) (Vicon Motion Systems). Data were collected under two conditions. One was undisturbed locomotion. In the other, the soleus H-reflex was elicited just after the middle of the stance phase (i.e., the “locomotor H-reflex”) as described by Chen et al. (2005, 2006, 2014). Approximately 5 min (i.e., ∼500 step cycles) of data was collected under each condition. At the end of each session, the femur and tibia lengths in each leg were measured externally.
The EMG activity recorded during undisturbed locomotion was rectified, low-pass filtered (by a 50-ms running average), and used to calculate soleus burst amplitudes (i.e., EMG area between burst onset and offset) (Chen et al. 2005, 2006). The concurrent 3D locomotor kinematic data were analyzed with the Vicon Motus software (Vicon Motion Systems) to determine step cycle duration (i.e., average time between successive right foot contacts); step cycle length [i.e., the distance covered by a complete step cycle, calculated as treadmill speed (in m/s) multiplied by step cycle duration (in s)]; step cycle symmetry, defined as the ratio of the time from RFC to LFC to the time from LFC to RFC (i.e., a ratio of 1.00 indicates that the right/left timing of the step cycle is symmetric); and the average right and left ankle, knee, and hip joint angles and hip heights over the step cycle (see Chen et al. 2014 Fig. 1B for illustration). To minimize the error caused by movement of the knee joint skin marker in relation to the joint over the course of the step cycle, the knee position was calculated with the “virtual knee setup” of the Vicon Motus software (Vicon Motion System). This method is based on the lengths of femur and tibia and the coordinates of the ankle joint, the knee joint, and the midpoint between the ankle and knee joints (Metz et al. 1998). In addition, to further minimize the impact on the data of skin marker movements as the joints flexed and extended over the step cycle, joint angles were calculated as average values for the entire step cycle.
As for the H-reflexes elicited in the conditioning protocol (i.e., “protocol H-reflexes”), locomotor H-reflex sizes were calculated as average absolute value of EMG activity in the H-reflex interval minus average absolute value of background EMG activity at the time of stimulation and were expressed in units of average absolute value of background EMG activity (Chen et al. 2005). (If H-reflex conditioning affected locomotor burst amplitude, it affected the level of the background EMG activity when the locomotor H-reflex was elicited. Nevertheless, the typically wide intertrial variation in burst amplitude enabled off-line analysis to compare pre- and postconditioning locomotor H-reflexes elicited at the same average level of background EMG by comparing pre- and postconditioning trials for which background EMG activity fell into the same range.)
Perfusion, postmortem examination, and lesion verification.
At the end of data collection, each rat was anesthetized and perfused through the heart for postmortem examination and lesion verification. The nerve cuff, EMG electrodes, and tibial nerve were examined, and the right and left soleus muscles were removed and weighed. The femur and tibia were exposed, and their lengths were measured and used to refine the femur and tibia lengths for the kinematic analysis (see Locomotor data collection). The spinal cord was removed, and blocks including the lesion were embedded in paraffin. Transverse 20-μm-thick serial sections from these blocks were processed and used to define the location and size of the contusion injury (Chen et al. 2002; Chen and Wolpaw 1997, 2002).
Statistical analysis.
The data consist of H-reflex sizes, locomotor EMG activity, and locomotor kinematics. They fall into five categories: 1) H-reflex sizes measured throughout the day whenever the rats satisfied the background EMG criteria (i.e., referred to as “protocol H-reflexes”); 2) H-reflex sizes measured during the stance phase of locomotion (i.e., referred to as “locomotor H-reflexes”); 3) soleus locomotor EMG burst amplitudes; 4) ankle, knee, and hip joint angles and hip heights during locomotion; and 5) putative key locomotor features (i.e., step cycle length and step cycle right/left timing symmetry). The purpose of this study was to determine whether the effects of up- or down-conditioning on H-reflexes and locomotion in the spinal cord-injured rats persisted after conditioning ended. To do this, we compared the initial data, the data at the end of conditioning, and the data at the end of data collection (i.e., the final data) by ANOVA. If a statistically significant difference was detected, pairwise multiple comparisons were performed by Tukey test.
RESULTS
All the rats remained healthy and active throughout data collection. Their weights increased from 210–286 [244 (±31 SD)] g at injury, to 258–327 [285 (±23)] g at implantation surgery, to 295–443 [360 (±40)] g at perfusion. Soleus muscle weights (in % body wt) averaged 0.053(±0.007)% for the right and 0.051(±0.007)% for the left, and they did not differ significantly from each other (P = 0.34 by paired t-test) or from those of normal rats (Chen et al. 1996, 1999, 2001, 2002, 2005, 2006; Chen and Wolpaw 1995, 1997, 2002). Nerve cuff examination revealed the expected connective tissue investment of the wires and good preservation of the nerve within the cuff.
Histology.
As illustrated in Chen et al. (2014), the T9 contusion injury was confined largely or totally to the right LC. On average, 37(±16 SD)% (range 12–69%) of the right LC remained. Other white matter tracts were fully or almost fully intact. One hundred percent of the left LC, the left dorsal column CST, the left dorsal column ascending tract (DA), and the right and left ventral columns (VC), 94(±10)% of the right CST, and 90(±23)% of the right DA remained intact.
Protocol H-reflexes.
Soleus H-reflex conditioning was successful in each of the rats [i.e., protocol H-reflex size change ≥20% in the correct direction (Chen and Wolpaw 1995, 1997, 2002; Wolpaw et al. 1993)]. In the HRup rats, the protocol H-reflex size averaged 238(±50 SE, ±100 SD)% of initial size for the last 10 days of up-conditioning and 394(±101, ±201)% for the final 10 days of data collection (P = 0.033, ANOVA). The final H-reflexes were significantly increased from their initial values (P = 0.027, Tukey test). In the HRdown rats, the protocol H-reflex at the end of down-conditioning averaged 50(±10, ±23)% of initial size, and the final protocol H-reflex averaged 91(±16, ±36)% (P = 0.016, ANOVA). The final H-reflexes did not differ significantly from their initial values (P = 0.82, Tukey test).
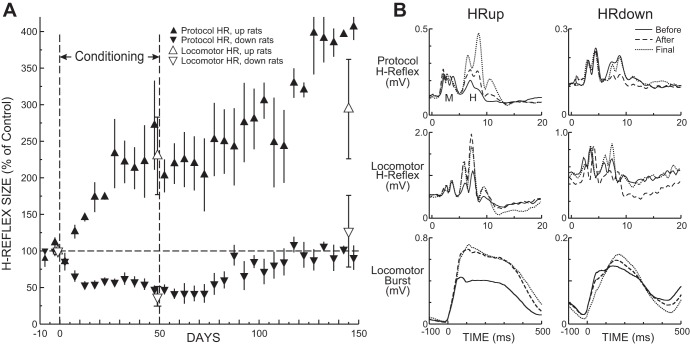
In Fig. 2A, the average (±SE) 5-day values of the protocol H-reflex in HRup and HRdown rats throughout data collection are shown. In the HRup rats, the H-reflex rises gradually over the 50 days of up-conditioning and continues to rise during the subsequent reexposure to the control mode. In the HRdown rats, the H-reflex decreases over the 50 days of down-conditioning and then gradually returns to near its initial value over the subsequent reexposure to the control mode.
Fig. 2.
A: average (±SE) values of protocol H-reflex (HR) size for HR up-conditioned (HRup) and HR down-conditioned (HRdown) rats (▲ and ▼, respectively) for each 5-day period during the final 10 days of the control-mode period, the 50 days of the up- or down-conditioning period, and 100 days of the second control-mode period. The average (±SE) values of the locomotor reflexes of HRup and HRdown rats (△ and ▽, respectively) for the 3 locomotor sessions are also shown. After conditioning ends, the H-reflex continues to increase in HRup rats, while the H-reflex decrease in HRdown rats gradually disappears. The protocol and locomotor H-reflexes behave similarly. B: data from representative HRup and HRdown rats: average poststimulus EMG activity elicited in the conditioning protocol (top) or during locomotion (middle) and average soleus locomotor burst (bottom) on a day during the initial control-mode period (solid line), a day at the end of conditioning (dashed line), and a day at the end of the second control-mode period (dotted line).
Figure 2B, top, shows average daily peristimulus soleus EMG activity from representative HRup (left) and HRdown (right) rats for a day before conditioning, a day at the end of conditioning, and a day at the end of data collection 3 mo later. The persistence and further enhancement of the protocol H-reflex increase and the disappearance of the protocol H-reflex decrease are apparent. Background EMG activity (i.e., EMG activity at time 0) and M-response amplitude do not change.
Locomotor H-reflexes.
In Fig. 2A, the average (±SE) values of the locomotor reflexes for the three locomotor sessions are superimposed on the average courses of change in the protocol H-reflexes. The locomotor H-reflexes displayed changes with conditioning comparable to those of the protocol H-reflexes. In HRup rats, the locomotor H-reflex increased to 230(±53 SE, ±107 SD)% of initial size at the end of up-conditioning and 294(±68, ±118)% by the end of data collection (P = 0.048, ANOVA). The final HRup locomotor H-reflexes were significantly larger than their initial values (P = 0.048, Tukey test). In the HRdown rats, the locomotor H-reflex decreased to 38(±9, ±20)% of initial size at the end of down-conditioning and returned to 127(±49, ±109)% by the end of data collection (P = 0.12, ANOVA).
Figure 2B, middle, shows average peristimulus soleus EMG activity during locomotion from representative HRup (left) and HRdown (right) rats before conditioning, at the end of conditioning, and at the end of data collection 3 mo later. The persistence of the locomotor H-reflex increase and the disappearance of the locomotor H-reflex decrease after conditioning ends are apparent. Background EMG activity and M-response amplitude do not differ in the three traces from each rat.
Locomotion.
The persistent increases in the protocol and locomotor H-reflexes of HRup rats appeared to be accompanied by similarly persistent increases in the right soleus locomotor burst, which averaged 174(±50 SE, ±100 SD)% of its initial value at the end of up-conditioning and 187(±47, ±94)% at the end of data collection (P = 0.30, ANOVA). In HRdown rats, the right soleus locomotor burst showed little change, averaging 110(±9, ±20)% of initial at the end of conditioning and 119(±16, ±37)% at the end of data collection (P = 0.49, ANOVA).
Figure 2B, bottom, shows average right soleus locomotor bursts from an HRup rat (left) and an HRdown rat (right) before conditioning, at the end of conditioning, and at the end of data collection 3 mo later. The persistence of the burst increase with up-conditioning and the absence of burst change with down-conditioning are evident.
The persistent increase in the right soleus locomotor burst in HRup rats was associated with persistent increase in right ankle extension. Average right ankle angle during locomotion increased from 69(±2 SE, ±3 SD)° to 91(±2, ±3)° at the end of conditioning and remained high 85(±3, ±5)° at the end of data collection (P < 0.001, ANOVA; P < 0.001 for final vs. initial, Tukey test). Neither up-conditioning nor down-conditioning had any other significant effects on joint angles in the right or left leg.
Up-conditioning was also associated with a persistent increase in average right hip height during locomotion [to 106(±2 SE, ±4 SD)% of initial at the end of conditioning and 113(±3, ±7)% 3 mo later (P = 0.008, ANOVA; P = 0.006 for final vs. initial, Tukey test)]. Down-conditioning had no significant effect on hip height. Figure 3A summarizes these results, and Fig. 3B illustrates the impact of up-conditioning on locomotor kinematics in a representative rat.
Fig. 3.
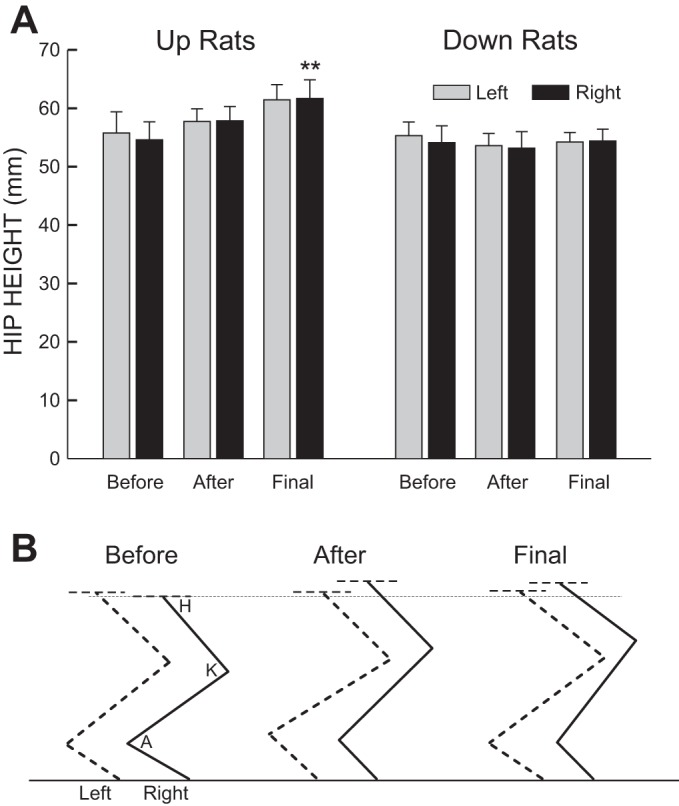
A: impact of up- or down-conditioning on right and left hip heights: average left and right hip heights (±SE) before and immediately after conditioning and at the end of data collection. Up-conditioning produced a persistent increase in right hip height (**P < 0.01 vs. before, Tukey test). Down-conditioning did not significantly decrease right hip height or increase the difference between left and right hip heights. B: average right and left leg positions during the stance phase of locomotion before and immediately after up-conditioning and 105 days later in a representative HRup rat. Up-conditioning increases right ankle angle and right (and left) hip height, and these changes persist. (The rat is walking toward the right; A, K, and H indicate ankle, knee, and hip angles, respectively.)
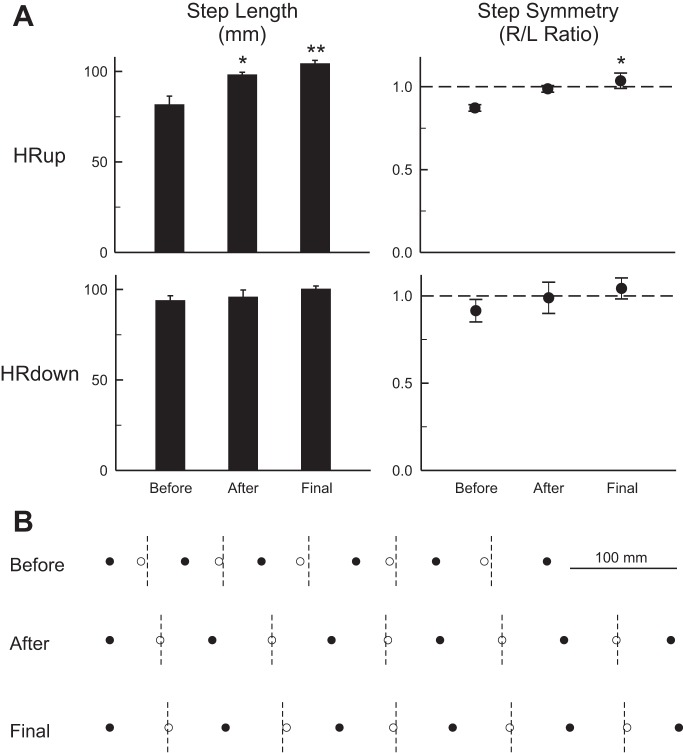
The persistent effects of up-conditioning on right ankle angle and right hip height were associated with a persistent effect on step cycle length, which increased from 82 (±5 SE, ±9 SD) mm initially to 98 (±1, ±3) mm at the end of conditioning and 104 (±2, ±4) mm at the end of data collection (P = 0.001, ANOVA; P = 0.001 for final vs. initial, Tukey test). This increase was accompanied by a similarly persistent significant improvement in step cycle right/left symmetry (P = 0.013, ANOVA; P = 0.012 for final vs. initial, Tukey test). In contrast, down-conditioning did not reduce step cycle length (P = 0.70, ANOVA) or further impair symmetry (P = 0.48, ANOVA). Figure 4A summarizes these results, and Fig. 4B illustrates them with data from an HRup rat. The longer steps and the improved right/left symmetry, and their persistence, are evident.
Fig. 4.
A: impact of H-reflex conditioning on step cycle length and step symmetry. A: average step-cycle length (left) and right/left step cycle symmetry (right) (±SE) before and immediately after up- or down-conditioning and at the end of data collection. Up-conditioning produced persistent improvements in step cycle length and right/left step cycle symmetry (*P < 0.05, **P < 0.01 vs. before; Tukey test). Down-conditioning had no significant effect on step cycle length or on right/left symmetry. B: data from a representative HRup rat illustrating the persistent impact of up-conditioning on step cycle length and symmetry. ● and ○, Onsets of right and left stance, respectively. Dashed vertical lines are halfway between right stance onsets, when left stance onset should occur. Prior to conditioning, right stance is not adequately maintained and left stance onset occurs too early (i.e., the rat limps). After up-conditioning, the steps are longer and left stance onset occurs on time. These improvements persist 100 days later.
DISCUSSION
Conditioned H-reflex change after conditioning ends.
This study is a sequel to a recent study (Chen et al. 2014) that examined the short-term locomotor impact of right soleus H-reflex up-conditioning or down-conditioning in rats in which a right LC contusion injury had weakened right stance and produced locomotor asymmetry (i.e., the rats limped). In these rats, up-conditioning increased the soleus H-reflex and improved locomotor symmetry, while down-conditioning decreased the H-reflex and had no effect on locomotion. Since conditioning began ≥56 days after the injury, when the deficit had stabilized (Basso et al. 1995; Chen et al. 2001, 2006), the improvement in up-conditioned rats could be confidently ascribed to the effects of H-reflex up-conditioning (i.e., the strengthened reflex pathway strengthened right stance). The absence of further impairment in down-conditioned rats is consistent with the occurrence of compensatory plasticity that prevented the weaker H-reflex pathway from further reducing the right soleus locomotor burst (see Chen et al. 2014 for discussion).
The present study examined the long-term impact of H-reflex conditioning in these spinal cord-injured rats. It asked two questions. First, do the H-reflex increases in up-conditioned rats and the H-reflex decreases in down-conditioned rats persist after the 50-day conditioning period ends? Second, what happens to locomotion; specifically, does the improvement produced by up-conditioning persist? The answer to this question is important for the potential therapeutic use of reflex conditioning. The results provide clear and encouraging answers to these questions.
In the spinal cord-injured rats of this study, the fates of the conditioned H-reflex increases and decreases after conditioning ended were different from those expected from the results in normal humans or monkeys. After cessation of conditioning of the biceps spinal stretch reflex (monkeys; Wolpaw et al. 1983) or the soleus H-reflex (humans; Thompson et al. 2009), conditioned reflex increase declined gradually, while conditioned reflex decrease persisted for at least 3 mo. In contrast, in the present study the H-reflex increase in up-conditioned rats continued to grow after conditioning ended, while the H-reflex decrease in down-conditioned rats gradually disappeared. These results are consistent with the difference in behavioral impact between up-conditioning and down-conditioning in these spinal cord-injured rats.
Persistence of the H-reflex increase.
In the HRup rats of the present study, the spinal cord plasticity responsible for the larger H-reflex also strengthened right stance and thereby improved step symmetry and step cycle length. Thus it was doubly adaptive: the stronger reflex pathway increased the probability that H-reflex elicitation would be followed by a food pellet reward, and it also improved locomotion. Furthermore, because it improved locomotion, it remained adaptive even after up-conditioning ended. Indeed, the H-reflex increased further after up-conditioning ended, and this was associated with further increase in hip height and step cycle length. This further increase was probably associated with and contributed to complex kinematic and EMG changes that together improved locomotion (see, e.g., Bennett et al. 2012).
These long-term results are consistent with those in humans with spasticity and foot drop due to incomplete spinal cord injuries (Manella et al. 2013; Thompson et al. 2013). In these individuals, soleus H-reflex down-conditioning (which reduced the abnormal hyperactivity of the H-reflex pathway) led to functional effects that went far beyond those directly attributable to the change in the reflex pathway: locomotor EMG activity improved in the muscles of both legs, and walking speed and symmetry improved (Thompson et al. 2013). In both rats and humans, H-reflex conditioning appropriate to the locomotor deficit (i.e., weak stance in rats, spasticity in humans) improved locomotion. This beneficial behavioral impact is likely to underlie the persistence of H-reflex increase after up-conditioning ended in the present study.
Disappearance of the H-reflex decrease.
In contrast, in the HRdown rats of this study the spinal cord plasticity responsible for the smaller H-reflex was expected to further weaken right stance and thus to further impair step symmetry. The fact that it did not do so implies that compensatory plasticity occurred [as it does in normal rats that undergo H-reflex conditioning (Chen et al. 2011)]. As illustrated in Fig. 2B, this additional plasticity apparently prevented the weakened H-reflex pathway from reducing the soleus locomotor burst (see Chen et al. 2014 for discussion). The conditioned H-reflex decrease was both adaptive and maladaptive: it increased reward probability, but it necessitated compensatory plasticity to prevent further impairment of locomotion. When down-conditioning ended, the H-reflex decrease became simply maladaptive. Thus its gradual disappearance is explicable as an adaptive process comparable to that triggered by the initial exposure to the reflex up- or down-conditioning protocol.
Therapeutic efficacy of appropriate H-reflex conditioning.
The spinal cord-injured rats of this study had been injured 56–93 days before conditioning began. Their spontaneous recovery had already occurred, and their deficits had stabilized (Basso et al. 1995; Chen et al. 2001, 2006). Thus the fact that up-conditioning produced lasting improvement in locomotion raises an important question: why did this improvement not occur in the course of spontaneous recovery? Since soleus H-reflex increase improved locomotion, why did it not occur spontaneously?
Activity-dependent plasticity can occur at many sites throughout the brain and spinal cord. The plasticity that does occur at each site depends on its activity, and thus on the animal's experience. While postinjury experience (e.g., moving about the cage) generally has beneficial effects, it does not necessarily produce the optimum pattern of plasticity in brain and spinal cord. H-reflex conditioning changes the rat's experience. Furthermore, it targets plasticity to the H-reflex pathway, and, in the case of up-conditioning, it produces plasticity in that pathway that improves locomotion, plasticity that did not occur during spontaneous recovery.
The beneficial locomotor effect of up-conditioning and the absence of locomotor effect for down-conditioning are both explicable in terms of the negotiated equilibrium hypothesis of the spinal cord's role in motor behavior (Chen et al. 2014; Thompson et al. 2013; Wolpaw 2010). According to this hypothesis, spinal neurons and synapses (including the pathway of the H-reflex) are continually maintained by the brain in an equilibrium that serves all the behaviors in the current repertoire. The interactive process that maintains this equilibrium is essentially a negotiation among the behaviors; each behavior repeatedly induces plasticity that maintains its key features despite the plasticity produced by other behaviors. For example, the key features of locomotion are likely to include step cycle symmetry (i.e., absence of limping).
When the acquisition of a new behavior, such as a larger or smaller H-reflex, changes the spinal cord, it affects older behaviors (e.g., locomotion) that use the same spinal circuitry, and it thereby triggers a new negotiation. In a normal CNS, this new negotiation is likely to produce compensatory plasticity that preserves the key features of older behaviors [e.g., step cycle and hip height symmetry (Chen et al. 2011)]. In contrast, when CNS function is impaired (e.g., by a spinal cord injury), the outcome of the new negotiation will depend on the plasticity produced by the new behavior and the nature of the deficit in old behaviors. If the new plasticity exacerbates the deficit, the new negotiation is likely to produce compensatory plasticity that prevents the exacerbation. This is apparently what occurred in the down-conditioned rats of the present study. And, when the cessation of down-conditioning removed the new behavior from the negotiation, the H-reflex decrease gradually disappeared. In contrast, if the new plasticity reduces the deficit, the new negotiation may lead to further plasticity that enhances the beneficial impact. This is apparently what occurred in the present up-conditioned rats, in which the H-reflex continued to increase and locomotion continued to improve even after up-conditioning ended. It also occurred in people with chronic spinal cord injury, in whom appropriate soleus H-reflex conditioning led to global improvements in locomotion (Thompson et al. 2013).
The present results extend previous animal and human data (Chen et al. 2006, 2014; Manella et al. 2013; Thompson et al. 2013) showing that a conditioning protocol that targets appropriate plasticity to a specific reflex pathway can help to improve function after partial spinal cord injuries. The targeted plasticity produced by the protocol improved locomotor function beyond the level reached by spontaneous recovery, and the improvement persisted. Thus spinal reflex conditioning protocols might complement current therapies and enhance neurorehabilitation. Protocols could be designed to target each person's particular deficits, and, with computer-based automatization, they would probably require only modest therapist involvement. They might be particularly useful when significant spinal cord regeneration becomes possible (e.g., Becker and McDonald 2012; Cregg et al. 2014; Tohda and Kuboyama 2011; Yoon and Tuszynski 2012) and precise methods for retraining the newly regenerated spinal cord become essential. Reflex conditioning protocols might also enhance rehabilitation in other situations such as after peripheral nerve injury and regeneration (e.g., Chen et al. 2010).
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HD-36020 (X. Y. Chen), NS-061823 (X. Y. Chen and J. R. Wolpaw), and NS-22189 (J. R. Wolpaw) and Program Project HD-32571 (A. W. English) and The NYS Spinal Cord Injury Trust Fund (X. Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.C., J.R.W., and X.Y.C. conception and design of research; Y.C., L.C., Y.W., and X.Y.C. performed experiments; Y.C., L.C., Y.W., J.R.W., and X.Y.C. analyzed data; Y.C., Y.W., J.R.W., and X.Y.C. interpreted results of experiments; Y.C., L.C., and X.Y.C. prepared figures; Y.C. and X.Y.C. drafted manuscript; Y.C., L.C., Y.W., J.R.W., and X.Y.C. approved final version of manuscript; J.R.W. and X.Y.C. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Rongliang Liu and Dr. Gerwin Schalk for technical assistance and Drs. Jonathan S. Carp, Dennis J. McFarland, and Elizabeth Winter Wolpaw for comments on the manuscript.
REFERENCES
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995. [DOI] [PubMed] [Google Scholar]
- Becker D, McDonald JW., 3rd Approaches to repairing the damaged spinal cord: overview. Handb Clin Neurol 109: 445–461, 2012. [DOI] [PubMed] [Google Scholar]
- Bennett SW, Lanovaz JL, Muir GD. The biomechanics of locomotor compensation after peripheral nerve lesion in the rat. Behav Brain Res 229: 391–400, 2012. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR, Jakeman LB. Corticospinal tract transection reduces H-reflex circadian rhythm in rats. Brain Res 942: 101–108, 2002. [DOI] [PubMed] [Google Scholar]
- Chen XY, Feng-Chen KC, Chen L, Stark DM, Wolpaw JR. Short-term and medium-term effects of spinal cord tract transections on soleus H-reflex in freely moving rats. J Neurotrauma 18: 313–327, 2001. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Dorsal column but not lateral column transection prevents down-conditioning of H reflex in rats. J Neurophysiol 78: 1730–1734, 1997. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol 87: 645–652, 2002. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR, Jakeman LB, Stokes BT. Operant conditioning of H-reflex in spinal-cord injured rats. J Neurotrauma 13: 755–766, 1996. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR, Jakeman LB, Stokes BT. Operant conditioning of H-reflex increase in spinal cord-injured rats. J Neurotrauma 16: 175–186, 1999. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci 25: 6898–6906, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu RL, Wang Y, Chen XY, Wolpaw JR. Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J Neurophysiol 111: 1249–1258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31: 11370–11375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Chen L, Sun C, English AW, Wolpaw JR, Chen XY. H-reflex up-conditioning encourages recovery of EMG activity and H-reflexes after sciatic nerve transection and repair in rats. J Neurosci 30: 16128–16136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol 253: 197–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella KJ, Roach KE, Field-Fote EC. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol 109: 2666–2679, 2013. [DOI] [PubMed] [Google Scholar]
- Metz GA, Dietz V, Schwab ME, van de Meent H. The effects of unilateral pyramidal tract section on hindlimb motor performance in the rat. Behav Brain Res 96: 37–46, 1998. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Operant conditioning of spinal reflexes: from basic science to clinical therapy. Front Integr Neurosci 8: 1–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohda C, Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol Ther 132: 57–71, 2011. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443–459, 1987. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in the primate spinal stretch reflex: initial development. J Neurophysiol 50: 1296–1311, 1983. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of reflexes. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford, UK: Academic, vol. 7, pp. 225–233, 2009. [Google Scholar]
- Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res 97: 31–39, 1993. [DOI] [PubMed] [Google Scholar]
- Yoon C, Tuszynski MH. Frontiers of spinal cord and spine repair: experimental approaches for repair of spinal cord injury. Adv Exp Med Biol 760: 1–15, 2012. [DOI] [PubMed] [Google Scholar]