Abstract
Crowding, the inability to recognize an individual object in clutter (Bouma H. Nature 226: 177–178, 1970), is considered a major impediment to object recognition in peripheral vision. Despite its significance, the cortical loci of crowding are not well understood. In particular, the role of the primary visual cortex (V1) remains unclear. Here we utilize a diagnostic feature of crowding to identify the earliest cortical locus of crowding. Controlling for other factors, radially arranged flankers induce more crowding than tangentially arranged ones (Toet A, Levi DM. Vision Res 32: 1349–1357, 1992). We used functional magnetic resonance imaging (fMRI) to measure the change in mean blood oxygenation level-dependent (BOLD) response due to the addition of a middle letter between a pair of radially or tangentially arranged flankers. Consistent with the previous finding that crowding is associated with a reduced BOLD response [Millin R, Arman AC, Chung ST, Tjan BS. Cereb Cortex (July 5, 2013). doi:10.1093/cercor/bht159], we found that the BOLD signal evoked by the middle letter depended on the arrangement of the flankers: less BOLD response was associated with adding the middle letter between radially arranged flankers compared with adding it between tangentially arranged flankers. This anisotropy in BOLD response was present as early as V1 and remained significant in downstream areas. The effect was observed while subjects' attention was diverted away from the testing stimuli. Contrast detection threshold for the middle letter was unaffected by flanker arrangement, ruling out surround suppression of contrast response as a major factor in the observed BOLD anisotropy. Our findings support the view that V1 contributes to crowding.
Keywords: anisotropy, crowding, fMRI, suppression, V1
crowding, the inability to recognize an individual object in clutter (Bouma 1970), is ubiquitous in natural scenes and laboratory tasks (Farzin et al. 2009; Pelli et al. 2004). Crowding is considered a major impediment to object recognition in peripheral vision. It is often explained in terms of faulty integration of features, due to either an inappropriately large integration zone at a preattentive level (Levi et al. 2002; Pelli et al. 2004) or coarse spatial resolution of attention (He et al. 1996). Since feature integration and segmentation are core processes of form perception, understanding crowding is an important route toward a comprehensive understanding of object or form vision. Indeed, crowding has been linked to early-level feature integration (Kooi et al. 1994; Levi et al. 2002; Nandy and Tjan 2007; Pelli et al. 2004) as well as midlevel form vision phenomena such as grouping (Chicherov et al. 2014; Freeman et al. 2012), texture processing (Balas et al. 2009; Freeman and Simoncelli 2011; Parkes et al. 2001), and image statistics (Nandy and Tjan 2012). Various cortical areas have been proposed as likely candidates for the locus of crowding, including V2 (Bi et al. 2009; Freeman and Simoncelli 2011), V4 (Liu et al. 2009; Motter 2009), and areas involved in later stages of visual processing (Chicherov et al. 2014; Farzin et al. 2009).
Primary visual cortex (V1) has been implicated in some of the earliest behavioral studies on crowding (Levi et al. 1985; Levi and Klein 1985; Tripathy and Levi 1994), a view that was reiterated in some of the recent theories (Nandy and Tjan 2012; Pelli 2008). Specifically, Levi and colleagues found that vernier threshold was elevated by flankers placed far from the vernier target. Most importantly, they found that the target-flanker distance required for this crowding follows the cortical magnification of the striate cortex, and thus spans a constant distance in V1 regardless of eccentricity. These estimations by Levi and colleagues, along with a study using dichoptic interaction in the blind spot by Tripathy and Levi (1994) and the more recent calculation by Pelli (2008) based on letter crowding put the cortical distance of crowding somewhere between 3 and 8 mm in V1. This is broadly consistent with the estimated extent of horizontal connections in the striate cortex (∼6–8 mm; Gilbert 1992; Stettler et al. 2002). Nandy and Tjan (2012) extended this observation and proposed image encoding with faulty image statistics represented in V1 horizontal connections as the underlying cause of crowding. In particular, their theory provides an explanation of the radial-tangential anisotropy of crowding, the subject matter of the present study, and attributes it to V1. Hence, whether V1 is involved in crowding is important, as the answer provides crucial information about the mechanism of crowding and has implications for downstream visual processing.
Recent brain imaging studies have provided converging evidence that crowding attenuates neurophysiological signals (Anderson et al. 2012; Bi et al. 2009; Chen et al. 2014; Chicherov et al. 2014; Freeman et al. 2011a; Millin et al. 2013). However, these studies have painted a rather equivocal picture of the involvement of V1 in crowding. For example, despite both studies measuring functional magnetic resonance imaging (fMRI) adaptation effect on patterns with crowding-dependent percepts, Anderson et al. (2012) found an effect as early as V1 while Bi et al. (2009) did not. Similarly, when comparing mean fMRI response between crowded and noncrowded stimuli, Millin et al. (2013) observed an effect in V1 and beyond, while Freeman et al. (2011a) found an effect only in areas beyond V1. Using electroencephalography (EEG), Chicherov et al. (2014) found that crowding was associated with active suppression, with a source likely in a high-level visual area such as lateral occipital cortex, yet an fMRI and EEG study by Chen et al. (2014) found neural suppression in V1.
A robust hallmark of crowding is its anisotropy between the radial and tangential directions, i.e., the spatial extent of crowding is elongated along the radial axis connecting the target with the fovea (Chambers and Wolford 1983; Chung 2013; Petrov and Meleshkevich 2011; Toet and Levi 1992; van den Berg et al. 2010). To look for the neural correlates of crowding, we sought to detect this radial-tangential anisotropy physiologically in the visual cortex. Specifically, we measured the change in mean blood oxygenation level-dependent (BOLD) response due to the addition of a letter between a pair of flankers that were arranged either radially or tangentially. To focus on the feedforward component of crowding, we directed subjects' attention away from the crowding stimulus.
Study of the neural mechanisms of crowding in peripheral vision using fMRI has been challenged by the inability to isolate the signal evoked by a target from those evoked by the nearby flankers because of the small cortical magnification factor relative to imaging resolution. We overcame this difficulty in the present study by adopting a “target-addition” paradigm, which measured the signal evoked jointly by the target and flankers within a region of interest (ROI) that included both the target and flankers (Millin et al. 2013). We compared the mean signal level in the target-present condition against that in the target-absent condition to index the degree of cortical interaction between target letter and flankers. Using this paradigm, Millin et al. (2013) showed a systematic reduction in fMRI BOLD signal with increasing crowding (less spacing between the target and flankers) as early as V1, regardless of attention (attended toward the crowding stimulus and attended away). Note that adding a target (middle) letter is expected to bring about an increase in fMRI BOLD response simply because the addition increases the image contrast projected to ROIs. However, the observed effect of adding the target letter on BOLD response is the combination of an increase in BOLD response from the addition of a target signal and a decrease in BOLD response as a result of crowding. The relevant quantity is the relative effect on the BOLD response between the different crowding conditions when the target letter is added, rather than the absolute change in BOLD response.
In view of the previous finding that mean BOLD response is reduced in a more crowded condition, we expect a difference in the mean change in BOLD response due to addition of a target between radially and tangentially arranged flanker configurations. Specifically, since the radial configuration leads to more crowding, we expect a lower target-related BOLD response in the radial configuration relative to the tangential configuration. The aim of the present study was therefore to determine the cortical loci that exhibit a differential effect on the fMRI BOLD response of adding the middle letter between radially and tangentially arranged flankers.
We found as early as V1 a radial-tangential anisotropy in the mean BOLD response, even when attention was diverted away from the stimulus. This anisotropy cannot be explained in terms of the recently reported orientation bias (Freeman et al. 2011b), as it has the opposite sign. It is also unlikely to be caused by surround suppression, since we found no anisotropy in detection thresholds of the identical letter stimuli. While crowding is likely to occur at multiple stages of visual processing (Whitney and Levi 2011), our findings show that crowding is present at the earliest stage of cortical processing.
MATERIALS AND METHODS
Subjects
Seven subjects (2 women, 5 men) were recruited from the University of Southern California campus. They all had normal or corrected-to-normal vision without known cognitive or neurological impairments. Subjects received monetary compensation for their participation. They included one of the authors (R. Millin) and six naive subjects. The same subjects participated in each experiment, except for one subject who participated in all but the letter-detection experiment. The experimental protocols were approved by the Internal Review Board (IRB) of the University of Southern California, and written informed consent was obtained from all subjects prior to the experiment.
Behavioral Measurements
Stimuli.
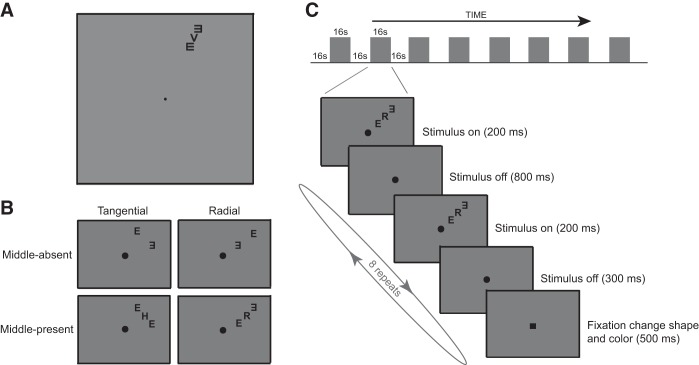
The stimuli consisted of a target letter flanked by two Es appearing on both sides of the target. Flankers were placed either radially or tangentially with respect to the fovea (Fig. 1B). The target letter was randomly drawn from the set of 10 Sloan letters (C, D, H, K, N, O, R, V, S, and Z). The flanker letter E was rotated 0°, 90°, 180°, or 270°, determined at random in each trial. The stimuli were black against a uniform gray background (23 cd/m2) with a contrast of 99%. Letter size was 0.75° in height at the viewing distance of 57 cm. The target letter was located at an eccentricity of 6.5° along the direction of either 25° clockwise from the upper vertical meridian (upper visual field) or 25° clockwise from the lower vertical meridian (lower visual field). At each location, the flanking Es were arranged either radially or tangentially.
Fig. 1.
Functional magnetic resonance imaging (fMRI) methods. A: example of the stimulus (upper visual field, radial, and middle-present condition). B: illustration of 4 stimulus conditions: flankers were arranged either radially or tangentially with respect to the fovea; the middle letter was either present or absent. C: schematic diagram of the block design. Note that the fixation change (to dark-colored square) also occurred with the same timing schedule during the fixation-only blocks where the letter stimulus was absent. Regardless of block type, subjects were asked to identify the color of the square at fixation.
The stimuli were generated and controlled with MATLAB (version 7.9) and Psychophysics Toolbox (Brainard 1997; Pelli 1997) running on a Dell PC computer with Windows 7. The display was a 19-in. CRT monitor (refresh rate: 85 Hz; resolution: 1,024 × 768). The CRT was controlled via a video attenuator (Li et al. 2003) using custom software to provide 10-bit fully linearized luminance control. The CRT was calibrated with a MINOLTA LS-100 Luminance Meter (Minolta Camera).
Measuring the spatial extent of crowding.
The spatial extent of crowding was defined as the center-to-center distance (spacing) between the high-contrast (99%) target and flankers that yielded a target identification accuracy of 79% correct. A subject's threshold spacing was estimated with a 3-down-1-up staircase procedure (Wetherill and Levitt 1965), with a step size of 1/20 log units. The mean of the log of spacing at the last 10 of 13 staircase reversals was taken as the threshold spacing for each staircase run. Log thresholds were averaged over four runs. We chose to use the nonparametric 3-down-1-up staircase to estimate the spacing threshold because the slope of the underlying psychometric function of accuracy vs. spacing was unknown. Assuming its slope, which would be necessary for parametric adaptive procedures such as QUEST (Watson and Pelli 1983), might bias our estimates.
In each trial, a subject was presented with three letters, a target at the center flanked by two tumbling Es. The stimuli appeared for 150 ms at a given location on the CRT monitor, followed by 500 ms of blank. A response screen with the 10 target letters (1.2° × 1.2° in size) then appeared. A subject identified the target by clicking on one of the letters. Auditory feedback was given whenever a correct answer was made. Throughout the experiment, a small dot in the center of the screen served as a fixation mark to minimize eye movements. A subject's stable fixation on the dot was confirmed with a high-speed eye tracker (EyeLink 1000/Tower Mount, SR Research, Mississauga, ON, Canada). A chin rest was used to minimize head movements. Subjects were given a series of practice trials prior to the experiment.
The experiment consisted of four stimulus conditions: two flanker configurations (radial and tangential) × two test locations (upper and lower visual fields). The conditions were blocked, and each condition block was tested four times per subject. All subjects participated in all the conditions. The order of blocks was randomized across subjects.
Measuring contrast threshold for detecting a flanked letter.
The test stimuli and locations were the same as those described above, except that the center-to-center distance (spacing) between the target and the flanking Es was fixed at 0.94°. The contrast thresholds for detecting the middle (target) letter were measured with a temporal two-interval-alternative forced-choice (2IAFC) paradigm. The subject's threshold contrast for each condition was estimated with the QUEST procedure (Watson and Pelli 1983), with an accuracy criterion of 79% correct and a β of 1.2 for a 55-trial block. Log thresholds were averaged over two blocks.
In each trial there were two 150-ms intervals each marked by an auditory beep, separated by 500 ms. One interval, selected at random, contained the target and flankers, while the other interval contained only the flankers. The target was a randomly selected Sloan letter. The subjects' task was to indicate which interval contained a target by pressing one of two keys. (They did not have to identify the letter.) Auditory feedback was given whenever a correct answer was made. Subjects were given a series of practice trials before the experiment.
The detection experiment consisted of four stimulus conditions: two flanker configurations (radial and tangential) × two test locations (upper and lower visual fields).
fMRI Measurements
Stimuli.
The test stimuli were the same as those in the psychophysical experiments (with a letter size of 0.75° at an eccentricity of 6.5°), except that the stimuli were counter-flickered (alternating between positive and negative 99% contrast) at 10 Hz for 200 ms (4 flickers) every second during a 16-s stimulus presentation block (Fig. 1C). Unlike the psychophysical stimuli, the center-to-center-distance (spacing) between the middle and flanking letters was fixed at 0.94°. The display was controlled and generated by a SONY VAIO Laptop computer running MATLAB (version 7.11) on Windows 7 with Psychtoolbox (Brainard 1997; Pelli 1997). Stimuli were projected by a three-chip DLP projector (Christie DLV 1280-DX) with a frame rate of 60 Hz and a native linear gamma. The projection surface was a translucent screen placed inside the scanner bore. Subjects viewed the stimuli through a mirror located above their eyes. The viewing distance was 91 cm. The stimulus was displayed on a uniform gray field (156 cd/m2).
Procedure.
Throughout each scan, subjects maintained fixation and performed a highly demanding central fixation task every 2 s. During the last 500 ms of each 2-s period, the black fixation dot changed to a dark-colored square (either green or red). Subjects were to press one of two buttons to indicate the color of the square (red or green). A 3-down-1-up staircase procedure was used to adjust the brightness of the color of the test square to maintain accuracy at 79% correct. All subjects were able to maintain a mean brightness threshold around 26 cd/m2, indicating that subjects devoted their attention to the color identification task at fixation. Since subjects were strongly encouraged to minimize the threshold brightness, they understood that there was no advantage in deviating from the intended fixation or paying attention to the peripheral stimuli. Subjects were given a series of practice trials outside the scanner.
The (unattended) crowding stimuli were presented in the periphery. They were counter-flickering high-contrast letter stimuli in either the upper right (Fig. 1A) or lower left visual field. At each stimulus location, there were four stimulus conditions: radially or tangentially arranged flankers with the middle letter either present or absent (Fig. 1B). The mean BOLD responses for the flickering letter stimuli were measured within ROIs that included both the flankers and the middle letter with a block-design paradigm (Fig. 1C). Each scan consisted of nine fixation-only blocks interleaved with eight stimulus blocks, each lasting 16 s. The initial fixation-only block was excluded from the analysis. Each scan lasted 272 s (∼4.5 min). Each block consisted of eight 2-s subintervals (Fig. 1C, bottom). For each subinterval, a counter-flickering letter stimulus was switched on for 200 ms at the beginning of every second. A different stimulus of the same type was used for each subinterval.
The eight stimulus conditions (2 stimulus conditions: middle-absence and middle-presence × 2 flanker configurations: radial and tangential × 2 test locations: upper and lower visual fields) were measured in the same scan, interleaved with fixation-only blocks. The set of eight stimulus conditions was repeated eight times in a counterbalanced order across scans. Eight scans were performed in one session, which on average lasted an hour. Each subject participated in two separate sessions—one to define retinotopic areas and ROIs and one for the main experiment.
An independent scan was used to define ROIs. The stimulus consisted of a middle letter along with four flankers arranged along the radial and tangential directions (Fig. 2). The position of the middle letter, presentation timing, and the subject's fixation task (color identification) were identical to the main experiment. The two testing locations (upper and lower visual fields) were measured in the same scan, and the set of two stimulus conditions was repeated twice in a counterbalanced order. We defined an ROI as the contiguous set of voxels within a retinotopic region that were significantly activated by this stimulus. It should be noted that such an ROI included the middle letter and the flankers of both configurations. We measured the signal evoked jointly by the target and flankers within the ROI and relied on subtraction between the middle-present and middle-absent conditions to index the degree of mutual interaction between middle letter and flankers (Millin et al. 2013).
Fig. 2.
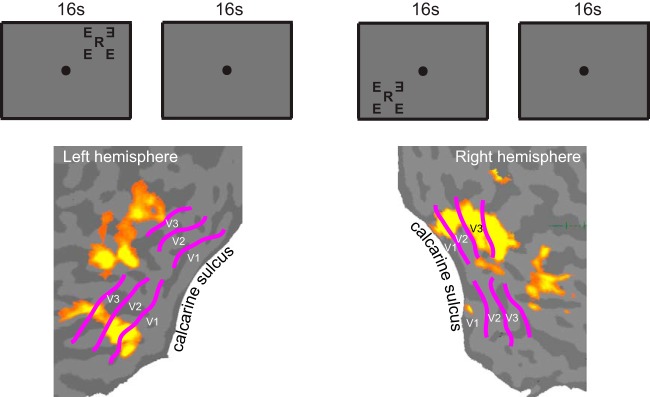
Defining the regions of interest (ROIs). An ROI was defined independently in a separate scan as the contiguous set of voxels within a retinotopically defined visual area (V1–V3) that were significantly activated by a counter-flickering stimulus. The stimulus consisted of a middle letter and 4 flankers simultaneously presented along the radial and tangential directions. The position of the middle letter, presentation timing, and the fixation task were identical to the main experiment. Two testing locations (upper and lower visual fields) were measured in the same scan, and the set of 2 stimulus conditions was repeated twice in a counterbalanced order. The activation maps show the V1, V2, and V3 ROIs determined for 1 subject. Retinotopic boundaries of V1, V2, and V3 were obtained with a standard retinotopic mapping method (Engel et al. 1997; Sereno et al. 1995).
We followed a standard retinotopic mapping method (Engel et al. 1997; Sereno et al. 1995) to identify the various visual areas. A rotating wedge was used to map the cortical representation of polar angle and to define the boundaries between visual areas. A 45° wedge with an 8.5° radius was abruptly rotated counterclockwise by 11.25° every second, sweeping the entire visual field in 32 s. An expanding ring was used to map the representation of eccentricity. Rings expanded in equal logarithmic steps from the center of the display, where the subjects fixated. The width of the ring and its eccentricity increased every second and reached a maximum radius of 8.5° in 20 s. The fixation marker of the display changed from “+” to “×” or vice versa randomly at an interval between 5 and 10 s (uniform distribution); the subject pressed a button as soon as a change of the fixation mark was detected. Each ring and wedge scan lasted 376 s and 424 s, respectively.
fMRI data acquisition.
The fMRI data were collected with a 3-T Siemens Tim Trio scanner with a 32-channel head coil using a T2*-weighted echo planar imaging sequence (TE/TR/flip angle = 25 ms/1,000 ms/60°). Nineteen slices with 3 × 3 × 3-mm3 isotropic voxels were prescribed to be perpendicular to the calcarine sulcus and covering the occipital pole. A high-resolution T1-weighted anatomical data set (3D MPRAGE; 1-mm3 isotropic voxels, TE/TR/flip angle/TI = 2.98 ms/2,300 ms/9°/900 ms) was collected in the same session before the functional runs and used to assist the prescription of the functional slices.
fMRI data analysis.
fMRI data analyses were done separately for each subject. Data analysis was performed with BrainVoyager QX (Brain Innovation, Maastricht, The Netherlands) and custom MATLAB code. The anatomical volume for each subject obtained in the retinotopic mapping session was inflated to generate a model of the cortical surface. Functional volumes were preprocessed, which included 3D motion correction, linear trend removal, and high-pass filtering (>0.0118 Hz). The functional images were aligned to the anatomical volume and transformed into Talairach space. The first 16 s of BOLD measurements (a blank interval with the ongoing color identification task at fixation) were discarded to ensure equilibrium in longitudinal magnetization and subject state.
A general linear model was used to identify the ROIs based on data from the ROI-mapping scan. The ROIs in V1, V2, and V3 were defined as areas that responded more strongly to the ROI-defining stimulus (Fig. 2) than the blank interval (false discovery rate < 0.05) and bounded by the retinotopic boundaries. For each experimental scan, the preprocessed fMRI voxel data were z-transformed and averaged within each ROI. ROI averaged data were concatenated across scans, and the results were deconvolved against an indicator function formed by placing a Dirac δ function at the onset of the stimulus interval of the same stimulus condition while allowing for a constant per-scan offset of no interest, resulting in one time course per stimulus condition.
RESULTS
Radial-Tangential Anisotropy in fMRI BOLD Response
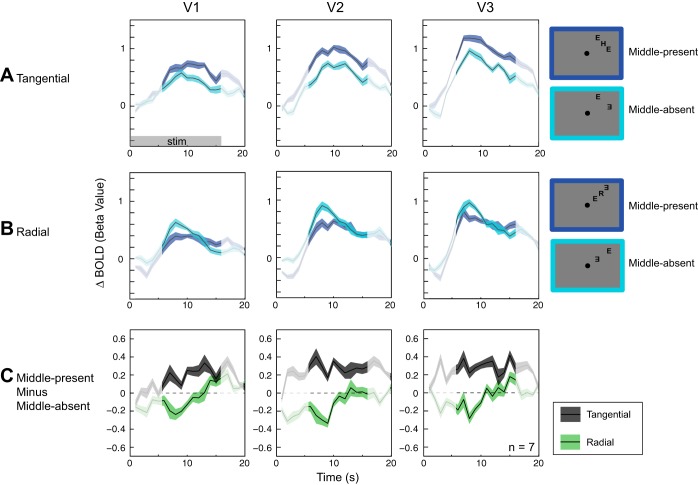
Previous studies have demonstrated that a reduced fMRI BOLD response is associated with crowding (Bi et al. 2009; Millin et al. 2013). If crowding indeed occurs at one of the early visual cortical areas, we would expect to see the behavioral radial-tangential anisotropy effect reflected in the fMRI BOLD response in these cortical areas. Figure 3, A and B, show fMRI BOLD responses to middle-present and middle-absent conditions for the tangential and radial flanker configurations, respectively. In each ROI of V1–V3, which encompassed the middle letter and the flankers in both flanker configurations, adding a middle letter in the tangential configuration led to a slight increase in BOLD response (Fig. 3A). In contrast, adding a middle letter in the radial configuration brought about a considerable decrease in BOLD response (Fig. 3B). Note that an increase in BOLD response due to the addition of the middle letter should not be confused with lack of signal suppression or reduction. This is because the magnitude of the mutual suppression between the middle letter and flankers relative to any signal gain caused by the middle letter is not known. What is relevant for the present study is whether mutual suppression between the middle and flankers is stronger in the radial condition than in the tangential condition. This relative difference in BOLD response between the tangential and radial conditions indicates differential cortical interactions between target and flankers, mirroring the radial-tangential anisotropy of crowding.
Fig. 3.
Anisotropy in blood oxygenation level-dependent (BOLD) response. A: tangentially arranged flankers. Cyan lines indicate fMRI BOLD responses to the middle-absent condition, while blue lines represent BOLD responses to the middle-present condition. Adding a middle letter led to a slight increase in mean BOLD response in the ROIs that encompassed the flankers and the middle letter. B: radially arranged flankers. Adding a middle letter resulted in a noticeable decrease in mean BOLD response in the ROIs. C: difference in BOLD response between middle-present and middle-absent for the tangential configuration (gray lines) and the radial configuration (green lines). Significant signal reduction was observed in the radial condition compared with the tangential condition. Highlighted time points (6–16 s) were used in the statistical analysis (see text). Shaded areas represent ±1 within-subject SE. Because the present study adopted a within-subject design for its statistical analysis, the relevant SE is the within-subject SE (Loftus and Masson 1994). Unless otherwise stated, all SEs are within-subject SEs.
The distinction in the response to the middle letter between radial and tangential flanker configurations can be visualized by plotting the difference in BOLD response between middle-present and middle-absent conditions for both radial and tangential flanker configurations (Fig. 3C). The difference values from the radial configuration were generally negative, while those from the tangential configuration were positive.
The radial-tangential effect on fMRI BOLD response was consistent across all three cortical areas. Importantly, the radial-tangential effect on BOLD response was present as early as V1, indicating that the effect of crowding likely impacts visual processing in V1.
To test the statistical significance of the radial-tangential effect on BOLD response, we performed a repeated-measures ANOVA on the average difference in BOLD signal changes (middle-present − middle-absent) between 6 and 16 s from the onset of a stimulus block. This period of time points was chosen because it reflects the hemodynamic response to the stimulus while minimizing any effect due to baseline variation and stimulus offset transient. The within-subject factors were flanker configuration (tangential and radial) and ROI (V1, V2, and V3).
There was a significant main effect of flanker configuration on the difference in BOLD [F(1,6) = 24.85, P = 0.002], reflecting that the difference values from the radial configuration were significantly lower than those from the tangential configuration [difference in parameter estimates (β) = 0.26 ± 0.04 for tangential vs. −0.06 ± 0.06 for radial]. We did not find any significant main effect of ROI [F(2,12) = 1.35, P = 0.30] or any significant interaction between flanker configuration and ROI [F(2,12) = 2.47, P = 0.13]. Pairwise comparisons further confirmed that the radial-tangential anisotropy was significant as early as V1 [t(6) = 2.89, P = 0.028] and remained significant in V2 [t(6) = 5.70, P = 0.001] and V3 [t(6) = 5.38, P = 0.002].
Behavioral Radial-Tangential Anisotropy of Crowding (Letter Identification)
To confirm the subjects' behavioral anisotropy, we measured the spatial extent of crowding behaviorally outside of the scanner. Figure 4A shows the average spatial extent of crowding measured at two testing locations (upper and lower visual fields). Consistent with previous findings (e.g., Pelli et al. 2004; Toet and Levi 1992), we observed a significantly larger spatial extent of crowding for the radially arranged flankers than for the tangentially arranged flankers [2-tailed pairwise t-test: t(6) = 3.61, P = 0.01]. Mean spatial extent averaged across two testing locations was 2.18 ± 0.26° for the radial configuration and 1.51 ± 0.10° for the tangential configuration (Fig. 4B).
Fig. 4.
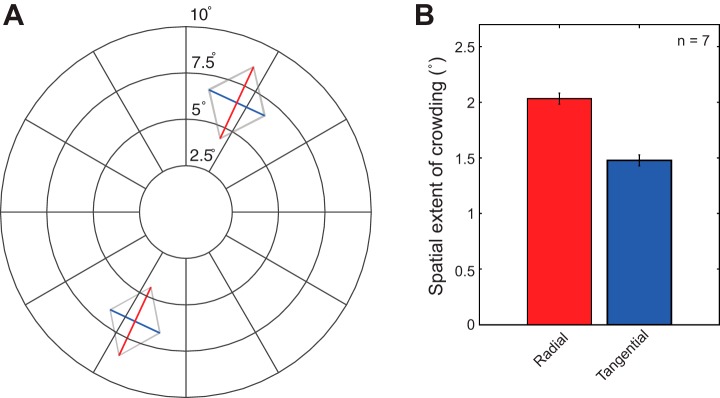
Anisotropy in the spatial extent of crowding. A: spatial extent of crowding measured at the 2 testing locations used in the fMRI experiment, averaged across subjects. Red lines indicate the spatial extent of crowding for the radial configuration, while blue lines represent that for the tangential configuration. It is evident that the spatial extent of crowding is elongated along the radial axis connecting the target (middle letter) with the fovea for both testing locations. Thin shaded contours indicate ±1 within-subject SE. B: mean spatial extent of crowding averaged across testing locations and subjects. Our results showed a significantly larger spatial extent of crowding for the radial configuration than the tangential configuration [2-tailed pairwise t-test: t(6) = 3.61, P = 0.01]. Error bars represent ±1 within-subject SE.
Relationship Between Behavioral and BOLD Anisotropies
To see whether behavioral anisotropy of crowding is related to fMRI BOLD anisotropy, we quantified both behavioral and BOLD anisotropy in terms of an anisotropy index. The behavioral anisotropy index was defined as the difference between radial and tangential extents of crowding divided by the sum of radial and tangential extents of crowding, i.e., (radial − tangential)/(radial + tangential). Similarly, the BOLD anisotropy index was computed as the negative difference between radial and tangential β values (parameter estimates) from middle-present minus middle-absent divided by the sum of radial and tangential β values from the middle-absent conditions, i.e., −(radial difference − tangential difference)/(radial middle-absent + tangential middle-absent). Note that the BOLD anisotropy index includes a factor of −1 in order to reverse the sign of the index values, since high BOLD response is associated with less crowding. Figure 5 shows the behavioral anisotropy index plotted against the BOLD anisotropy index from the lower and upper visual fields. The lower visual field exhibits a larger behavioral anisotropy index than the upper visual field [t(6) = −5.17, P = 0.002], which is in good agreement with the previous studies of crowding (Nandy and Tjan 2012; Petrov and Meleshkevich 2011). We found that, averaged across subjects, the difference in the behavioral anisotropy between the upper and lower visual fields was consistent with the difference in the BOLD anisotropy between the upper and lower visual fields [r(4) = 0.96, P = 0.002]. This pattern of results held true for all three visual areas (V1, V2, and V3), supporting a qualitative linkage between the behavioral and fMRI anisotropies.
Fig. 5.
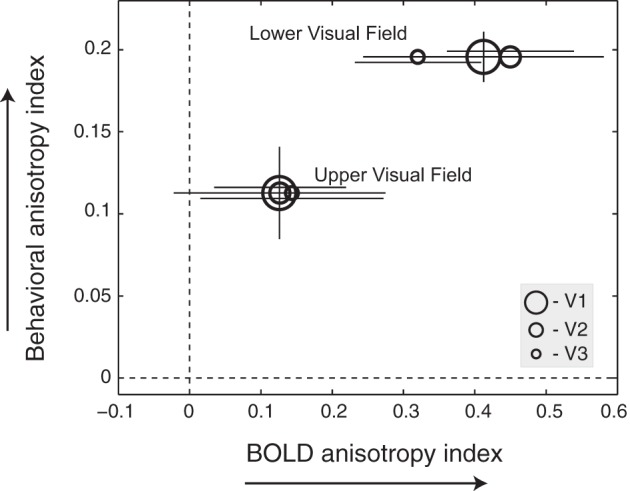
Relationship between behavioral and BOLD anisotropies across visual fields. Mean behavioral anisotropy index is plotted against BOLD anisotropy index from the lower and upper visual fields. Each data point represents a group average from each cortical area (V1, V2, and V3). Dashed lines indicate the absence of radial-tangential anisotropy. Error bars indicate ±1 SE across 7 subjects. Horizontal error bars are offset vertically from each other to add visibility. There is only 1 behavioral index for each visual field. The corresponding (vertical) error bars are placed on the V1 symbols.
However, we did not find any statistically significant correlation between behavioral and fMRI measurements of anisotropy at an individual level. Several differences in the way we measured and defined behavioral and fMRI anisotropies might have obscured this finer-scale relationship. For instance, behavioral anisotropy was measured with subjects attending to the crowded target (middle) letter and defined as the difference in the spatial extent of crowding (estimated threshold spacing yielding 79% recognition accuracy) between the tangential and radial conditions. fMRI anisotropy was measured with subjects attending away from the stimulus and defined as the difference in BOLD responses between the tangential and radial conditions to a fixed target-flanker spacing (at 0.94°). These methodological differences together with a relatively small sample size make it difficult to detect a statistically significant correlation between behavioral and fMRI measurements of anisotropy at an individual level. Averaging across subjects by another meaningful grouping variable, such as visual field, reduces the effect of intersubject variability in the neural response functions (Tjan et al. 2006) and unmasks this correlation.
Lack of Behavioral Radial-Tangential Anisotropy in Letter Detection
Crowding is an impairment of target identification by its surround, when target contrast is above detection threshold (Pelli et al. 2004). Surround is also known to elevate contrast thresholds for target detection (Chubb et al. 1989; Xing and Heeger 2000; Zenger-Landolt and Heeger 2003), a phenomenon known as surround suppression. Physiologically, surround suppression occurs when a visual stimulus outside of a neuron's classical receptive field causes a reduction in neural response (Hubel and Wiesel 1968; Maffei and Fiorentini 1976). This suppressive effect by surrounding stimuli has also been observed in fMRI BOLD response (Kastner et al. 1998; Zenger-Landolt and Heeger 2003).
To our knowledge, there is no electrophysiological or fMRI evidence showing that surround suppression is anisotropic. However, one behavioral study (Petrov and McKee 2006) found evidence for radial-tangential anisotropy in surround suppression. To test for a possible role of anisotropy in contrast detection in our experiment, we measured contrast threshold for detecting the presence of the middle letter in the stimuli we used for the crowding task. Subjects did not need to identify the middle letter, only to detect its presence.
We did not find any significant effect of flanker configuration on the contrast threshold for detecting the middle letter (Fig. 6). The mean ratio of radial to tangential detection threshold was 1.01 ± 0.03 and was not statistically different from unity [2-tailed 1-sample t-test: t(5) = 0.54, P = 0.61]. Visual field dependence was also absent in our detection experiment. The mean ratio of radial detection threshold to tangential threshold was 1.00 ± 0.05 for the upper visual field and 1.03 ± 0.05 for the lower visual field. These results help to rule out anisotropy in contrast response or surround suppression as an explanation for the observed radial-tangential anisotropy in fMRI BOLD response.
Fig. 6.
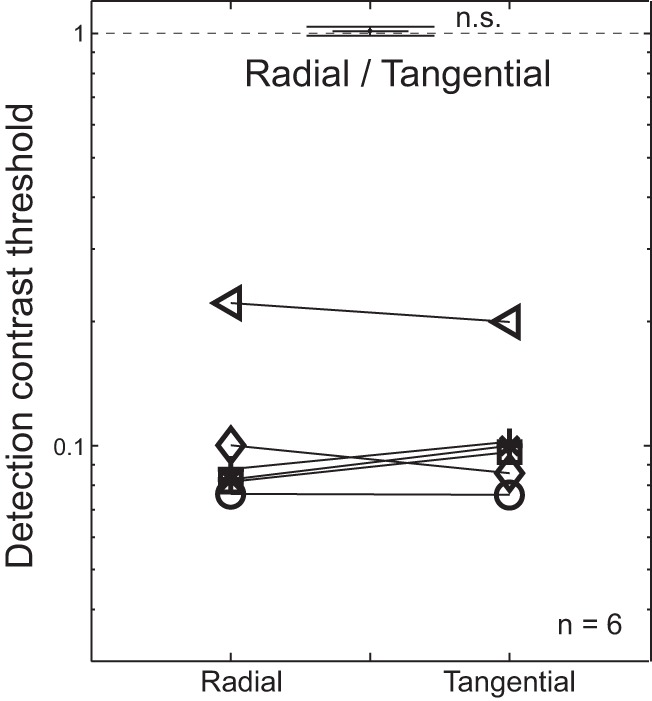
No anisotropy in flanked letter detection threshold. Contrast thresholds for middle letter detection are plotted as a function of flanker configuration. Each symbol represents the detection threshold of a single subject. The mean ratio of radial to tangential detection threshold across subjects is shown at top. Error bars indicate ±1 within-subject SE. n.s., Not significant.
DISCUSSION
We observed significant reduction of the BOLD signal evoked by a middle letter with flankers in the radial compared with tangential arrangement in V1, V2, and V3. This anisotropy in the BOLD response mirrors the behavioral anisotropy of crowding, with radially arranged flankers causing stronger crowding (Chung 2013; Petrov and Meleshkevich 2011; Toet and Levi 1992; van den Berg et al. 2010).
With ROIs that included the flankers and target (middle letter), Millin et al. (2013) showed that the otherwise expected increase in BOLD response due to the addition of the middle letter between a pair of flankers was attenuated or even eliminated in the crowded condition in V1–V4. This was the case regardless of whether attention was directed to the stimuli or not. In light of these findings, we used the unattended design of Millin et al. in the present study, with subjects performing an attention-demanding task at fixation that was unrelated to the peripheral stimuli. For each subject, we confirmed crowding and anisotropy of crowding off-line. We reasoned that since the magnitude of crowding parametrically affected the BOLD response in the Millin et al. task, BOLD response should show the same anisotropy as behavior. This is indeed our main finding.
We observed in V1 a greater suppression in the BOLD response evoked by the middle letter when it was added to the radially configured flankers compared with when it was added to the tangential configuration. In fact, there was even a net reduction in the total BOLD response when the middle letter was added to the radial configuration. However, this net reduction in an ROI that included the middle and flanking letters is not a necessary consequence of crowding. We expect that in both radial and tangential configurations crowding leads to a suppression of BOLD response to the letter triplet below the sum of responses to the individual middle and flanking letters. However, only in the radial condition was this suppression strong enough to completely override any added response due to the addition of the middle letter.
It is also noteworthy that there was a larger BOLD response to letters in the radial arrangement (0.83 ± 0.1) than in the tangential arrangement (0.57 ± 0.1) when the middle letter was absent (the middle-absent condition in Fig. 3, A and B). This pattern of results is consistent with Sasaki et al. (2006)'s radial orientation bias found in human and nonhuman primates (see also Freeman et al. 2011b) and may also be explained in terms of the difference in cortical magnification associated with different flanker configurations. However, adding the middle letter reverses the radial bias: we found a lower BOLD response to the radial triplet arrangement (0.62 ± 0.12) and a higher BOLD response to the tangential arrangement (0.83 ± 0.11). This differential effect of adding the middle letter means that the anisotropy we report here, which we attribute to crowding, is a separate phenomenon from the radial orientation bias. This finding cannot be explained by a floor effect (i.e., no room for reduction in the tangential condition). The change in the response associated with the middle letter was relatively small, considerably less than one-half of the flanker-evoked response (see Fig. 3). Furthermore, the anisotropy showed up in different signs of similar amplitude: an increase in BOLD response in the tangential condition versus a decrease in BOLD response in the radial condition.
We also found evidence of a qualitative linkage between the behavioral and fMRI anisotropies. Although no significant correlation between behavioral and fMRI measurements of anisotropy was found at an individual level, our results demonstrated that the difference in the behavioral anisotropy between the upper and lower visual fields was consistent with the difference in BOLD anisotropy between the upper and lower visual fields. We speculate that differences in how we measured and defined behavioral and fMRI anisotropies might have obscured the finer-scale relationship at the individual level. Future study will be needed to establish the quantitative relationship between the two.
Surround Suppression and Crowding
For our stimuli, the observed anisotropy in the BOLD response is not attributable to surround suppression in contrast response. Surround suppression is similar to crowding in many ways, and the two phenomena may not be fully dissociable. Surround suppression is often described as a decrease in neuronal responses due to lateral inhibition (Hubel and Wiesel 1968; Maffei and Fiorentini 1976; Zenger-Landolt and Heeger 2003). Crowding, while resembling surround suppression, appears to be distinct from it in several aspects (Levi 2008; Petrov et al. 2007). Crowding is emphasized over surround suppression when the target and flankers are of the same contrast (Pelli et al. 2007; Petrov and McKee 2006). Crowding exhibits inward-outward asymmetry (i.e., an outer flanker yields more crowding than an inner flanker) (Bouma 1973) and radial-tangential anisotropy (Toet and Levi 1992). To our knowledge, no anisotropy has yet been found with physiological measurements in stimulus conditions favoring surround suppression. With a behavioral experiment, Petrov and McKee (2006) did observe a higher detection threshold for the radial flanker configuration than the tangential configuration. However, this behavioral anisotropy, obtained with a large surround (4.5° in radius), was relatively weak (0.12 log units in contrast threshold) and with high intersubject variability. It is unclear whether this reported anisotropy in detection is of significance to a typical crowding experiment with small flankers (0.75° in height) such as ours.
We measured contrast threshold for detecting the middle letter of our stimuli and found no evidence of radial-tangential anisotropy in detection threshold (Fig. 6), nor did we find any effect of visual field (upper vs. lower field). These results argue against surround suppression being a determining factor for the observed BOLD anisotropy in our experiment. The covarying nature of BOLD anisotropy and behavioral anisotropy of crowding, not detection threshold, implies a close relation between BOLD anisotropy and crowding.
Spatial Attention and Crowding
In our experiment, significant BOLD anisotropy was observed when subjects' attention was directed away from the stimulus, suggesting that the neural correlates of crowding are dependent on neither attention nor the behavioral relevance of the stimuli. Subjects performed a highly demanding near-threshold color identification task at fixation throughout the scan. Although we cannot completely rule out the deployment of any attentional resources to the stimulus, it is unlikely that the observed anisotropy was the result of subjects systematically paying more attention to a particular stimulus configuration. In fact, subjective reports obtained during debriefing indicated that subjects were unware of the properties of the stimuli such as letter identity or flanker configuration, further suggesting that endogenous attention was not a factor.
The pattern of our results is also inconsistent with an exogenous attentional account. The color task at fixation might not have completely eliminated any exogenous attention evoked by the stimulus in the periphery. However, there is also no obvious reason to expect that the middle-present condition would have elicited more attention than the middle-absent condition when the flankers were arranged tangentially but less attention than the middle-absent condition when the flankers were arranged radially. The presence of the middle letter adds contrast to the stimulus, resulting in a higher probability of eliciting more attention in the middle-present condition than in the middle-absent condition, regardless of flanker configuration. It is therefore unlikely that exogenous attention alone played a significant role in the fMRI anisotropy observed in the present study. Moreover, Millin et al. (2013) found crowding-induced suppression of fMRI BOLD response with a fixation task that completely overlapped in time with the peripheral stimulus presentation, further corroborating our finding that crowding-induced suppression was present when attention was not directed toward the stimuli.
A recent study by Chen et al. (2014) investigated crowding using both event-related potential (ERP) and fMRI and produced results that compliment ours. They found that crowding is associated with suppressed fMRI BOLD response in V1 and with suppressed ERP C1 component, which is the earliest ERP component typically attributed to V1 sources. Similar to the present study, Chen et al. also found that crowding-induced cortical suppression was more pronounced in the radial condition relative to the tangential condition. However, unlike the present study, they observed suppression and anisotropy only when subjects attended to the crowding stimuli. They concluded that spatial attention is necessary for the manifestation of the suppression. This discrepancy between the study of Chen et al. and our study might have been due to methodological differences between the two studies. Specifically, Chen et al. used event-related paradigms, which are often less sensitive in detecting an effect than the block design used in the present study. Indeed, using a block design, Freeman et al. (2011a) and Millin et al. (2013) also found crowding-induced suppression in the attended-away condition. The question of whether spatial attention is required for crowding-induced cortical suppression to occur is important for understanding the underlying mechanisms of crowding. A coherent picture suggested by the present study and previous studies is that attention modulates (increases) crowding-induced suppression and anisotropy. Nevertheless, a study designed specifically to test the role of spatial attention in fMRI anisotropy is needed to fully resolve the apparent discrepancy between the two studies.
Early Origin(s) of Crowding
While crowding appears to occur at multiple stages of visual processing (Whitney and Levi 2011), locating the earliest cortical area affected by crowding is a critical step toward identifying the underlying mechanism of crowding and understanding its impact on downstream visual processing. Although a number of behavioral studies attributed crowding to cortical constraints from V1 (Levi et al. 1985; Levi and Klein 1985; Pelli 2008; Tripathy and Levi 1994), the recent prevailing view has been that crowding occurs in areas beyond V1 (Bi et al. 2009; Farzin et al. 2009; Freeman and Simoncelli 2011; He et al. 1996; Motter 2002).
Probing the effect of crowding on BOLD response has been challenged by the difficulty of isolating target-evoked activity from flanker-evoked activity. Previous studies used a large peripheral target or indirectly measured crowding via an adaptation paradigm (Bi et al. 2009; Fang and He 2008). These approaches, while conservative, are often less sensitive because of a weak crowding effect. For instance, Fang and He (2008) reported no significant effect of crowding in V1. They used a relatively large target stimulus (i.e., a checkered patch with a size of 2.6° at 5° eccentricity), which produced only a marginal difference in behavioral performance between crowded and noncrowded conditions.
Anderson et al. (2012) found evidence of V1 involvement in crowding. In a change-detection paradigm, they showed that fMRI BOLD response correlated with the percept that was affected by crowding in as early as V1; they also found that the modulation grew stronger in V2 through V4. However, their result may have conflated crowding with changes in attention state induced by the percept.
It was not until recently that a more robust crowding effect was observed in V1 with the more traditional crowding stimuli commonly used in behavioral testing. Millin et al. (2013) showed that crowding is associated with a suppressed BOLD response in V1 and beyond. In contrast with earlier studies, Millin et al. measured the average signal evoked jointly by the target and flankers within an ROI that retinotopically included both the target and flankers and relied on subtraction to index the degree of mutual suppression between target and flankers. This approach allowed them to use letter stimuli of a much smaller size, typical of the crowding stimuli used in behavioral testing, and induce a large crowding effect. They found a suppressed BOLD response for crowded stimuli when subjects attended toward or away from the stimuli, and thereby removed any attention confound in their interpretation.
Using inclusive ROIs similar to those of Millin et al., the present study as well as the study of Chen et al. (2014) replicated and extended these V1 findings by showing radial-tangential anisotropy in BOLD response in V1. Chen et al. also found that the C1 component of ERP signals was associated with crowding and target-flanker configuration, further implicating V1. The present study compliments Chen et al. (2014) by showing that the BOLD anisotropy persists in V1 even in an unattended condition.
Radial-Tangential Anisotropy and Crowding in V1
Until recently, there was no proposed explanation for the elongated shape of crowding zones. All previous models of crowding either ignored the prominent radial-tangential anisotropy or explicitly modeled it with shape parameters fit to data (van den Berg et al. 2010; Wilkinson et al. 1997). Nandy and Tjan (2012) provided a parameter-free explanation of this telltale sign of crowding. They argued that an attention-gated mechanism for acquiring image statistics, which works well for foveal vision, leads to the acquisition of erroneous image statistics, confounded by eye movements, in peripheral vision. By using the known saccade statistics and anatomical data of V1 (eccentricity scaling of receptive fields, cortical extents of horizontal connections and feedback projections from V2, etc.), Nandy and Tjan were able to quantitatively account for the three most salient properties of crowding zones: scaling with eccentricity (often referred to as Bouma's law), inward-outward asymmetry (larger extent of crowding zone on the side of the target further away from the fovea), and radial-tangential anisotropy. Their theory relies on anatomical properties of V1 and the finding that the activity in V1 is least affected by saccadic suppression. These requirements pin both their theory of crowding and their explanation of radial-tangential anisotropy to V1 (or feedback from V2 to V1). Their theory thus anticipates neural activity in V1 to be modulated with crowding, which is what we found.
Nandy and Tjan, however, did not predict whether crowding would lead to an increase or a decrease of neural activity. The sign of the effect depends on how an input image is represented by neurons in V1. The findings in the present study inform on this topic of V1 coding. A reduction in the evoked activity with increased crowding is consistent with a neural code that becomes sparser, perhaps inappropriately so, for the more crowded stimulus. A caricature example of this view would be if V1 (mis-)codes the entire crowded letter triplet as a single entity with missing features, while devoting a separate code for each component in a noncrowded stimulus. Such a coding scheme will impede processing in the later stages since the components in a crowded stimulus are not individually represented. Further studies are required to test this hypothesis on image coding.
In summary, we observed a pronounced suppression in BOLD response when flankers were arranged radially compared with tangentially. Our results suggest that the radial-tangential anisotropy of crowding is present as early as V1, implicating a role of V1 in crowding. Our findings further support the view that crowding occurs at an early stage of visual processing.
GRANTS
This work was supported by National Eye Institute Grant R01 EY-017707.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.K., P.B., R.M., and B.S.T. conception and design of research; M.K. and P.B. performed experiments; M.K. and P.B. analyzed data; M.K., P.B., R.M., and B.S.T. interpreted results of experiments; M.K. prepared figures; M.K. drafted manuscript; M.K., P.B., R.M., and B.S.T. edited and revised manuscript; M.K., P.B., R.M., and B.S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Benjamin T. Files for his helpful suggestions and comments on this manuscript.
REFERENCES
- Anderson EJ, Dakin SC, Schwarzkopf SS, Rees G, Greenwood JA. The neural correlates of crowding-induced changes in appearance. Curr Biol 22: 1199–1206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas B, Nakano L, Rosenholtz R. A summary-statistic representation in peripheral vision explains visual crowding. J Vis 9: 13.1–13.18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi T, Cai P, Zhou T, Fang F. The effect of crowding on orientation-selective adaptation in human early visual cortex. J Vis 9: 13.1–13.10, 2009. [DOI] [PubMed] [Google Scholar]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature 226: 177–178, 1970. [DOI] [PubMed] [Google Scholar]
- Bouma H. Visual interference in the parafoveal recognition of initial and final letters of words. Vision Res 13: 767–782, 1973. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Chambers L, Wolford G. Lateral masking vertically and horizontally. Bull Psychonom Soc 21: 459–461, 1983. [Google Scholar]
- Chen J, He Y, Zhu Z, Zhou T, Peng Y, Zhang X, Fang F. Attention-dependent early cortical suppression contributes crowding. J Neurosci 34: 10465–10474, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicherov V, Plomp G, Herzog MH. Neural correlates of visual crowding. Neuroimage 93: 23–31, 2014. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proc Natl Acad Sci USA 86: 9631–9635, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST. Cortical reorganization after long-term adaptation to retinal lesions in humans. J Neurosci 33: 18080–18086, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex 7: 181–192, 1997. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Crowding alters the spatial distribution of attention modulation in human primary visual cortex. J Vis 8: 6.1–6.9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Whitney D. Holistic crowding of Mooney faces. J Vis 9: 1811–1815, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Brouwer GJ, Heeger DJ, Merriam EP. Orientation decoding depends on maps, not columns. J Neurosci 31: 4792–4804, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Chakravarthi R, Pelli DG. Substitution and pooling in crowding. Atten Percept Psychophys 74: 379–396, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Donner TH, Heeger DJ. Inter-area correlations in the ventral visual pathway reflect feature integration. J Vis 11: 1–23, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Simoncelli EP. Metamers of the ventral stream. Nat Neurosci 14: 1195–1201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron 9: 1–13, 1992. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature 383: 334–337, 1996. [DOI] [PubMed] [Google Scholar]
- Hess RF, Dakin SC, Kapoor N, Tewfik M. Contour interaction in fovea and periphery. J Opt Soc Am A 17: 1516–1524, 2000. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol 195: 215–243, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 282: 108–111, 1998. [DOI] [PubMed] [Google Scholar]
- Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spat Vis 8: 255–279, 1994. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding—an essential bottleneck for object recognition: a mini review. Vision Res 48: 635–654, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Hariharan S, Klein SA. Suppressive and facilitatory spatial interactions in peripheral vision: peripheral crowding is neither size invariant nor simple contrast masking. J Vis 2: 167–177, 2002. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res 25: 979–991, 1985. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Res 25: 963–977, 1985. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, Xu P, Jin J, Zhou Y. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. J Neurosci Methods 130: 9–18, 2003. [DOI] [PubMed] [Google Scholar]
- Liu T, Jiang Y, Sun X, He S. Reduction of the crowding effect in spatially adjacent but cortically remote visual stimuli. Curr Biol 19: 127–132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychon Bull Rev 1: 476–490, 1994. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res 16: 1131–1139, 1976. [DOI] [PubMed] [Google Scholar]
- Millin R, Arman AC, Chung ST, Tjan BS. Visual crowding in V1. Cereb Cortex (July 5, 2013). 10.1093/cercor/bht159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Crowding and object integration within the receptive field of V4 neurons. J Vis 2: 274a, 2002. [Google Scholar]
- Motter BC. Modulation of transient and sustained response components of V4 neurons by temporal crowding in flashed stimulus sequences. J Neurosci 26: 9683–9694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Central V4 receptive fields are scaled by the V1 cortical magnification and correspond to a constant-sized sampling of the V1 surface. J Neurosci 29: 5749–5757, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC, Simoni DA. The roles of cortical image separation and size in active visual search performance. J Vis 7: 6.1–6.15, 2007. [DOI] [PubMed] [Google Scholar]
- Nandy AS, Tjan BS. The nature of letter crowding as revealed by first- and second-order classification images. J Vis 7: 5.1–5.26, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy AS, Tjan BS. Saccade-confounded image statistics explain visual crowding. Nat Neurosci 15: 463–469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nat Neurosci 4: 739–744, 2001. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Pelli DG. Crowding: a cortical constraint on object recognition. Curr Opin Neurobiol 18: 445–451, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis 4: 1136–1169, 2004. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Tillman KA, Freeman J, Su M, Berger TD, Majaj NJ. Crowding and eccentricity determine reading rate. J Vis 7: 20.1–20.36, 2007. [DOI] [PubMed] [Google Scholar]
- Petrov Y, McKee SP. The effect of spatial configuration on surround suppression of contrast sensitivity. J Vis 6: 224–238, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, Meleshkevich O. Asymmetries and idiosyncratic hot spots in crowding. Vision Res 51: 1117–1123, 2011. [DOI] [PubMed] [Google Scholar]
- Petrov Y, Popple AV, McKee SP. Crowding and surround suppression: not to be confused. J Vis 7: 121–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Rajimehr R, Kim BW, Ekstrom LB, Vanduffel W, Tootell RB. The radial bias: a different slant on visual orientation sensitivity in human and nonhuman primates. Neuron 51: 661–670, 2006. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893, 1995. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron 36: 739–750, 2002. [DOI] [PubMed] [Google Scholar]
- Tjan BS, Lestou V, Kourtzi Z. Uncertainty and invariance in the human visual cortex. J Neurophysiol 96: 1556–1568, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Res 32: 1349–1357, 1992. [DOI] [PubMed] [Google Scholar]
- Tripathy SP, Levi DM. Long-range dichoptic interactions in the human visual cortex in the region corresponding to the blind spot. Vision Res 34: 1127–1138, 1994. [DOI] [PubMed] [Google Scholar]
- van den Berg R, Roerdink JB, Cornelissen FW. A neurophysiologically plausible population code model for feature integration explains visual crowding. PLoS Comput Biol 6: e1000646, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys 33: 113–120, 1983. [DOI] [PubMed] [Google Scholar]
- Wetherill GB, Levitt H. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol 18: 1–10, 1965. [DOI] [PubMed] [Google Scholar]
- Whitney D, Levi DM. Visual crowding: a fundamental limit on conscious perception and object recognition. Trends Cogn Sci 15: 160–168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson F, Wilson HR, Ellemberg D. Lateral interactions in peripherally viewed texture arrays. J Opt Soc Am A Opt Image Sci Vis 14: 2057–2068, 1997. [DOI] [PubMed] [Google Scholar]
- Xing J, Heeger DJ. Center-surround interactions in foveal and peripheral vision. Vision Res 40: 3065–3072, 2000. [DOI] [PubMed] [Google Scholar]
- Zenger-Landolt B, Heeger DJ. Response suppression in V1 agrees with psychophysics of surround masking. J Neurosci 23: 6884–6893, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]