Abstract
Endocannabinoids (eCBs) released from postsynaptic neurons mediate retrograde suppression of neurotransmitter release at central synapses. eCBs are crucial for establishing proper synaptic connectivity in the developing nervous system. Mobilization of eCBs is driven either by a rise in intracellular Ca2+ (depolarization-induced suppression of inhibition, DSI) or postsynaptic G protein-coupled receptors (GPCRs) that activate phospholipase C beta (PLCβ). To determine whether eCB mobilization changes between neonatal and juvenile ages, we used whole cell voltage-clamp recordings of CA1 neurons from rat hippocampal slices at postnatal days 1–18 (neonatal) and 19–43 (juvenile), because many neurophysiological parameters change dramatically between approximately postnatal days 18–20. We found that DSI was slightly greater in juveniles than in neonates, while eCB mobilization stimulated by GPCRs was unchanged. However, when DSI was elicited during GPCR activation, its increase was much greater in juveniles, suggesting that eCB mobilization caused by the synergy between the Ca2+ and GPCR pathways is developmentally upregulated. Western blotting revealed significant increases in both metabotropic type glutamate receptor 5 (mGluR5) and PLCβ1 proteins in juveniles compared with neonates. Responses to pharmacological activation or inhibition of PLC implied that eCB upregulation is associated with a functional increase in PLC activity. We conclude that synergistic eCB mobilization in hippocampal CA1 neurons is greater in juveniles than in neonates, and that this may result from increases in the mGluR5-PLCβ1 eCB pathway. The data enhance our understanding of the developmental regulation of the eCB system and may provide insight into diseases caused by improper cortical wiring, or the impact of cannabis exposure during development.
Keywords: DSI, CA1 region, G protein-coupled receptors, mAChRs, whole cell voltage-clamp, Western blot
unlike conventional neurotransmitters that are released from presynaptic nerve terminals and mediate anterograde neurotransmission in central synapses, endocannabinoids (eCBs) are produced and released from postsynaptic neurons and act via retrograde transmission on presynaptic cannabinoid type I receptors (CB1R) to suppress release of neurotransmitters, e.g., GABA and glutamate (Kano et al. 2009). Mobilization of eCBs can be achieved in three ways. One is via a strong depolarization of postsynaptic neurons which opens voltage-gated calcium (Ca2+) channels and increases intracellular Ca2+ concentration, [Ca2+]i, to micromolar levels (Lenz and Alger 1999) which mobilizes eCBs via a phospholipase C (PLC)-independent pathway (Hashimotodani et al. 2005). Since Ca2+-dependent eCB mobilization can produce either depolarization-induced suppression of inhibition (DSI) (Pitler and Alger 1992) or of excitation (Kreitzer and Regehr 2001), depending on whether excitatory or inhibitory synapses are involved, this purely Ca2+-dependent eCB process can be designated generally as “eCBCa”. The second mechanism, requiring activation of postsynaptic G protein-coupled receptors (GPCRs) (Maejima et al. 2005; Varma et al. 2001), is largely independent of Ca2+ and involves a diacylglycerol-lipase-α-dependent (Gao et al. 2010; Tanimura et al. 2010) pathway that may include PLC. This process can be designated generally as “eCBGPCR”. The third mechanism is a synergistic interaction between Ca2+ and the GPCR- activated pathways (Kim et al. 2002; Varma et al. 2001). The increase in [Ca2+]i interacts with the GPCR activation and produces an elevated level of eCB release, a process designated “eCBCa+GPCR”.
In the hippocampus, eCB-mediated short-term depression of synaptic GABA release, i.e., DSI (Pitler and Alger 1992), is produced when eCBs are released from pyramidal cells (Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001) and suppress inhibitory postsynaptic currents (IPSCs) by activating CB1Rs located mainly on cholecystokinin (CCK)-containing GABAergic inhibitory interneurons (Katona et al. 1999; Marsicano and Lutz 1999; Tsou et al. 1999). Activation of either the muscarinic acetylcholine receptors (mAChRs; Kim et al. 2002; Ohno-Shosaku et al. 2003) or group I metabotropic type glutamate receptors (mGluR1 and mGluR5) (Ohno-Shosaku et al. 2002; Varma et al. 2001) enhance DSI, which are examples of eCBCa+GPCR in adult hippocampus. [Note: Instead of “eCBCa”, Kano et al. (2009) use “CaER”, where “ER” is “endocannabinoid release,” instead of “eCBGPCR”, they use “basal RER,” “receptor-activated ER,” and instead of “eCBCa+GPCR”, they use “Ca2+-assisted RER.” There is no difference in the phenomena themselves.]
Although modulation of synaptic transmission by eCB signaling is increasingly well understood in adult brain, less is known about eCB system regulation during postnatal development. CB1Rs (Harkany et al. 2007; Wang et al. 2003), eCBs (Berrendero et al. 1999; Fernandez-Ruiz et al. 2000), together with their synthesizing (Berghuis et al. 2007; Watson et al. 2008) and degrading enzymes (Harkany et al. 2007), can all be detected from the earliest stages of embryonic development and throughout the pre- and postnatal periods (Basavarajappa et al. 2009; Deshmukh et al. 2007) in various brain areas, including hippocampus. Moreover, CB1R mRNA expression levels increase gradually in the hippocampus from the fetal period to adulthood (Harkany et al. 2007). During embryonic and prenatal development, eCB signaling regulates neuronal migration (Berghuis et al. 2007; Morozov and Freund 2003b), axonal elongation (Gomez et al. 2008; Mulder et al. 2008) and synaptogenesis (Kim and Thayer 2001). Moreover, disruption of eCB signaling during early postnatal development alters cortical activity patterns (Bernard et al. 2005), suggesting a role for eCBs in activity-dependent synaptic refinement (Deshmukh et al. 2007). How the mobilization of eCBs is regulated during postnatal development in hippocampus remains to be elucidated, however.
To investigate the developmental issues related to eCB signaling, we have used whole cell voltage-clamp recordings of electrically (field stimulation) evoked inhibitory postsynaptic currents (eIPSCs) in hippocampal CA1 neurons and Western blot analysis of proteins that are involved in eCB signaling. We focused mainly on DSI and the enhancement of DSI that is induced by the group I mGluR agonist, (S)-3,5-dihydroxyphenylglycine (DHPG), but also tested mAChRs in some experiments. We now show that a developmentally dependent, functional increase in eCB mobilization occurs between the neonatal and juvenile stages. The difference correlates with increases in the levels of protein expression of mGluR5 and PLCβ1, suggesting that alterations in these proteins may account for the increased eCB mobilization. In tests of this hypothesis, we used pharmacological tools to inhibit or activate PLCβ and observed results consistent with it. We conclude that molecular components of the eCB system change during development and may thereby contribute to the normal maturation of neuronal circuits. Finally, our results may help the understanding of behavioral dysfunctions related to abnormal hippocampal maturation or cannabis use during development.
MATERIALS AND METHODS
Animals.
Sprague-Dawley rats were bred in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facilities of the University of Maryland School of Medicine, USA, or the Chang Gung University, Taiwan, and maintained on a standard light cycle with ad libitum food and water. All procedures were approved by the Institutional Animal Care and Use Committees for both institutions. Pregnant dams were checked each morning, and the day when pups were first found was deemed postnatal day 0 (PN0). Animals were divided into neonatal (PN1–18) and juvenile (PN19–43) groups because a large number of physiological parameters in the hippocampus change dramatically in the range of PN18–20, which is also essentially the time of weaning (PN19–21) in the animal colonies. Parameters changing include the relative abundance of α1- vs. α2-subunits of the GABA-A receptor (Davis et al. 2000), endogenous levels of GABA and glutamate (Davis et al. 1999) and the changes in depolarizing vs. hyperpolarizing GABA action (Ben-Ari et al. 2012). Juvenile animals are independent but not yet reproductively mature.
Brain slice preparation.
Brains of neonatal and juvenile rats (114 males and 63 females) were rapidly removed following sedation and decapitation, in accordance with standard approved protocols. Horizontal, 400-μm-thick brain slices that included the hippocampus were prepared using a vibratome (Vibratome series 1000, St. Louis, MO) in ice-cold sucrose solution, where NaCl in the artificial cerebral spinal fluid (ACSF) was replaced by an isosmotic concentration of sucrose. The ACSF composition was as follows (in mM): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgCl2, 2 CaCl2 (saturated with 95% O2/5% CO2). The slices were incubated at 34°C for 20 min and then allowed to recover for at least 1 h at room temperature in an interface holding chamber filled with humid 95% O2/5% CO2 before the experiment. In most studies, the slices were incubated with ω-agatoxin GVIA (agatoxin, 300 nM), an irreversible P/Q-type Ca2+ channel blocker, for 30–60 min prior to experiments (“pretreatment”) to depress occurrence of eCB-insensitive IPSCs (e.g., Lenz et al. 1998; Wilson et al. 2001) during recordings. Agatoxin reduces the total GABAA receptor-mediated IPSC elicited with extracellular stimulation in CA1 by ∼60% within ∼25 min of treatment in our hands (Lafourcade and Alger 2008). However, it is too expensive to use in routine perfusion and since agatoxin is essentially irreversible (within the time frame of the experiments, e.g., Wheeler et al. 1994), we pretreated slices with it. Stimulus intensities required to evoke IPSCs in agatoxin-pretreated slices appear to be higher than in untreated slices, as expected, since a large fraction of the interneurons, especially parvalbumin-expressing interneurons, depend exclusively on activation of agatoxin-sensitive (P/Q-type) Ca2+ channels to release GABA.
Whole cell voltage-clamp recordings.
Visualized whole cell voltage-clamp recordings from CA1 pyramidal neurons were made in hippocampal slices at room temperature (20–22°C) on a fixed stage upright microscope (Nikon Eclipse E600FN, Nikon, Tokyo, Japan) equipped with a charge-coupled device camera (DAGE-MTI, Michigan City, IN) using a ×60 water immersion objective. Carrying out the experiments at room temperature improves the viability of slices in the visualized preparation and is quite common in experiments like these. Slices were superfused with oxygenated ACSF containing 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and d-(-)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM) to block ionotropic glutamatergic responses. The ACSF flowed through the recording chamber at a rate of ∼1.5 ml/min. Patch electrodes (borosilicate glass, WPI, Sarasota, FL) had resistances of 4–6 MΩ in the bath when filled with the internal solution, which contained the following (in mM): 90 CsCH3SO3, 50 CsCl, 0.2 Cs4-BAPTA, 10 HEPES, 1 MgCl2, 2.5 phosphocreatine-2Na, 2 Mg-ATP, and 0.3 GTP-Tris, 5 lidocaine N-ethylbromide (QX-314) titrated to pH 7.2–7.3 with 3 M CsOH, osmolarity titrated to 295–300 mosM with sucrose.
At the holding potential of −70 mV, monosynaptic eIPSCs were elicited by 100-μs-long extracellular stimulation pulses delivered via concentric bipolar stimulating electrodes (stainless steel, FHC; A360 Stimulus Isolator, World Precision Instruments, Sarasota, FL) placed in stratum radiatum. Synaptic currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2 kHz, sampled at 10 kHz, digitized (Digidata 1200; Molecular Devices) and stored for offline analysis [IGOR Pro, Wavemetrics, Portland, OR (Hwang and Copenhagen 1999)]. Access resistance stability (≤20 MΩ; 80% compensation) was monitored using a −2-mV voltage step, and data collection was stopped when a change >20% occurred. To measure DSI, the eIPSCs were elicited at 4-s intervals, and the postsynaptic cell was usually depolarized to 0 mV for 1 s, although, in some experiments, the depolarizing step lasted for 5 s, as noted, to elicit DSI at 120-s intervals.
Western blot analysis.
Following electrophysiological recordings, in a number of experiments the hippocampi containing the CA1 area were dissected from the brain slices, rapidly frozen in dry ice and stored at −80°C freezer before being homogenized in 400 μl of lysis buffer (pH 7.4; 0.8% trizma base, 0.9% NaCl, 1% igepal, 1 mM deoxycholic acid, 1% EDTA, 0.1% phosphatase inhibitor and 0.1% protease inhibitor). The sections were centrifuged for 30 min at 3,000 rpm, and the supernatant was collected. The homogenate was then subjected to a Bradford protein assay to determine standardize protein levels in subsequent Western blotting analyses. Ten micrograms of protein from each animal were electrophoresed in separate lanes on an 8% Tris-glycine precast SDS-polyacrylamide gel (Invitrogen, San Diego, CA) and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). Membranes were blocked in 5% skim milk powder in Tween 20-Tris-buffered saline with 0.1% Tween 20 for 1 h at room temperature and then incubated either with rabbit anti-mGluR5 polyclonal IgG antibody (1:1,000; Upstate Biotechnology, New York, NY) or with rabbit anti-PLCβ1 polyclonal IgG antibody (1:2,000; Santa Cruz Biotechnology, Dallas, TX) in 2.5% skim milk overnight. A 30-min incubation with goat anti-rabbit horseradish peroxidase-conjugated IgG (New England Biolabs, Beverly, MA) followed. Membranes were then stained with Ponceau S solution (0.5% Ponceau S in 1% glacial acetic acid made in dH2O), which appears at ∼45 kDa for standardization to correct for any errors in sample loading (Olesen and Auger 2005). Metabotropic GluR5 blots were exposed on hyperfilm-enhanced chemiluminescent (Amersham, Arlington Heights, IL), and bands were visualized with Lumiglo system (Cell Signaling Technologies). The integrative gray-scale pixel area density of mGluR5 bands was captured with a charge-coupled device camera and quantified using National Institutes of Health Image software (developed at the US National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/). The membranes containing PLCβ1 bands were developed with enhanced chemiluminescent horseradish peroxidase substrate (Bionovas Biotechnology, Ann Arbor, MI) and then exposed to autoradiography film, and band intensity was measured with Image J software. The mGluR5 and PLCβ1 proteins were detected as bands of relative molecular mass of 130 and 150 kDa, respectively. Data were expressed as the ratios of either mGluR5 or PLCβ1 to respective Ponceau S signals.
Data analysis.
DSI was calculated as follows: DSI (%) = (1 − mean amplitude of three eIPSCs after depolarization pulse/mean amplitude of five eIPSCs before depolarization pulse) × 100%. Values of two or three DSI trials were averaged to obtain a mean DSI in a given condition. Normalized DSI was calculated as {[DSI tested in drug(s)/control DSI] × 100%}. Data are expressed as means ± SE, and P values are calculated from Student's unpaired or paired t-tests or one-way ANOVA followed by Newman-Keuls test for multiple comparisons, with significance levels assessed at P < 0.05, P < 0.01 or P < 0.005, with two-tailed tests.
Chemicals sources.
DHPG, DNQX, and d-AP5 were purchased from Tocris Cookson (Bristol, UK). ω-Agatoxin IVA was obtained from Peptides International (Louisville, KY). U-73122, U-73343 and 2,4,6,-trimethyl-N-(meta-3-trifluoromethyl-phenyl)-benzenesulfonamide (m-3M3FBS) were purchased from Calbiochem (Gibbstown, NJ). 2,4,6,-Trimethyl-N-(ortho-3-trifluoromethyl-phenyl)-benzenesulfonamide (o-3M3FBS) was ordered from Tocris (Minneapolis, MN). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
RESULTS
Developmental change in eCBCa in response to a strong depolarization of CA1 neurons.
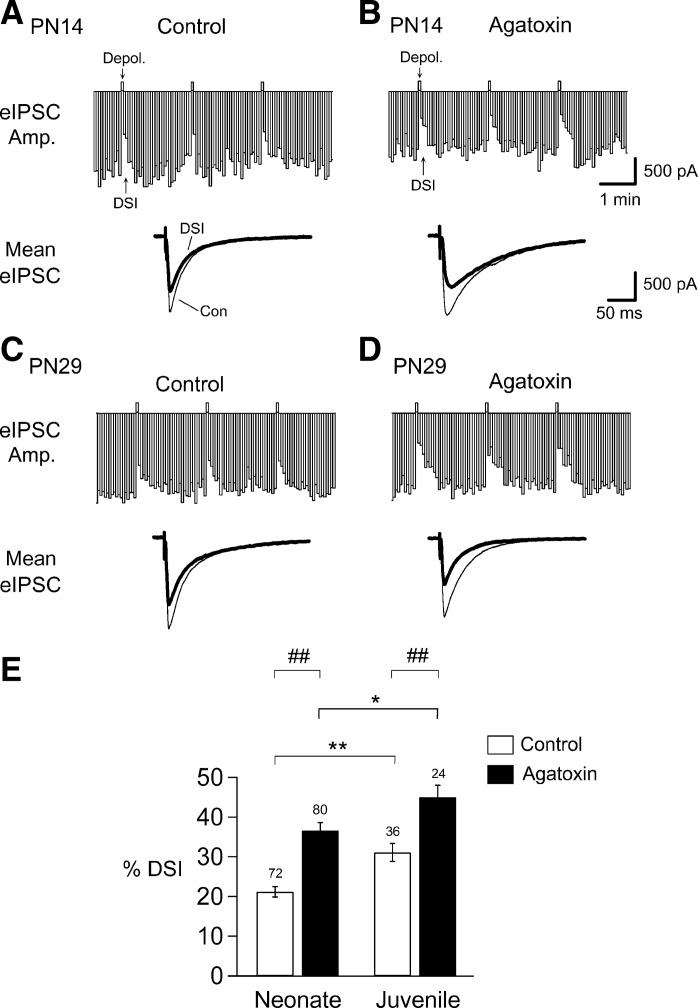
A transient depolarization of hippocampal CA1 neurons causes DSI (Pitler and Alger 1992), which is mediated by eCBs (Wilson and Nicoll 2001), in juvenile and PN1–7 neonatal rats (Bernard et al. 2005). Since synthesis and release of eCBs cannot be distinguished electrophysiologically, we refer to the combination of both processes as “mobilization”. To examine whether eCB mobilization exhibits a developmental change, we first tested DSI by giving cells 1-s-long depolarizing voltage steps every 2 min through the whole cell pipette. However, in the youngest cohort of animals (PN1–9), essentially no DSI could be observed in normal ACSF, and accordingly we used 5-s-long depolarizing voltage steps (as numerous others have done, e.g., Wilson and Nicoll 2001) and recorded eIPSCs that were evoked at 4-s intervals during neonatal and juvenile stages (see materials and methods). DSI in neonates amounted to an average depression of 21 ± 1% of the eIPSC amplitude (Fig. 1, A and E), which is consistent with published results (Bernard et al. 2005). Since there were no sex differences in the DSI values recorded from neonatal (n = 43 males and n = 29 females) or juvenile (n = 18 for each sex) groups, and there was no sex and age interaction (P value = 0.78, nonsignificant, two-way ANOVA), the male and female results were lumped together within the two age cohorts. DSI in juveniles was significantly greater than in neonates (a depression of 31 ± 2%, an increase to 147% of the neonate value, n = 72 in neonates, n = 36 in juveniles, P < 0.01; Fig. 1, A, C, and E).
Fig. 1.
Endocannabinoid (eCB) mobilization induced by depolarization-induced suppression of inhibition (DSI) (eCBCa) is significantly larger in juveniles than in neonates in hippocampal CA1 neurons. A and B: DSI, the reduction in evoked inhibitory postsynaptic current (eIPSC) amplitude (Amp.) indicated in the top bar histograms, was induced by a strong (5-s) depolarization to 0 mV given at the upward deflections (Depol.). Representative traces are of eIPSCs recorded from neurons in slices with (B) or without (A) agatoxin (300 nM) pretreatment from postnatal day 14 (PN14) rats. Traces below are the means of five eIPSCs before [thin trace, control (Con)] and three eIPSCs after the voltage step (thick trace; DSI) averaged from the top panels of recorded Con and agatoxin-pretreated neurons. C and D: representative eIPSCs recorded from neurons in slices without and with agatoxin pretreatment from PN29 rats. Traces below are means of eIPSCs before and after a DSI step averaged from the top panels. E: histograms show a significantly larger magnitude of DSI in juveniles (PN19–43) than in neonates (PN1–18) (t-test, **P < 0.01). Removal of eCB-insensitive IPSCs by agatoxin (see text) significantly increases DSI with respective neonatal and juvenile groups (t-test, ##P < 0.01). A statistically significantly greater DSI in juveniles compared with neonates is also evident in agatoxin-treated neurons (t-test, *P < 0.05). The no. on the top of each bar denotes the no. of recorded neurons in all figures, except Fig. 4.
The main receptor for eCBs in the brain, CB1R, is heavily concentrated on CCK-containing GABAergic inhibitory interneurons in hippocampus (Katona et al. 1999; Marsicano and Lutz 1999; Tsou et al. 1999). Although CCK-expressing cells constitute one of the two major basket cell classes, non-CCK interneurons are present, and our extracellular stimulation will activate them as well. Many of the non-CCK/non-CB1R expressing interneurons release GABA via P/Q-type Ca2+ channels (e.g., Freund and Katona 2007; c.f., Lenz et al. 1998; Wilson et al. 2001), which are blocked by the selective P/Q-type Ca2+ channel blocker, agatoxin, whereas CCK interneurons release GABA exclusively via N-type Ca2+ channels, which are insensitive to agatoxin. Therefore, to reduce co-occurrence of eCB-insensitive IPSCs that could confound the measurement of DSI, slices were pretreated with agatoxin (300 nM) for 30–60 min prior to the experiment (see materials and methods). Agatoxin is irreversible within the time frame of the experiments (c.f., Wheeler et al. 1994). Following pretreatment with agatoxin, DSI recorded from CA1 neurons of both neonates and juveniles showed significant increases above their respective non-agatoxin treated groups (DSI increased from 21 ± 1% to 37 ± 2% in neonates, n = 80, P < 0.01; and from 31 ± 2% to 45 ± 3% in juveniles, n = 24, P < 0.01; Fig. 1, B, D, and E). This indicates that the difference between neonatal and juvenile responses can mainly be attributed to a relatively smaller contribution of the eCB-sensitive IPSCs to the total neonatal eIPSCs. For these and all further experiments in agatoxin-pretreated slices, we used 1-s depolarizing voltage steps to induce DSI, to avoid any possible “ceiling effect” that might occur with 5-s steps and maximal DSI.
After agatoxin pretreatment, the difference between neonatal and juvenile DSI was smaller but still significant (37% vs. 45%; an increase of 22%; P = 0.04; Fig. 1E). The reduction in the difference probably reflects developmental changes in the relative populations of agatoxin-sensitive and -insensitive interneurons (Tricoire et al. 2011). The remaining difference reflects the true developmental change in eCBCa. Again, there were no sex differences in the DSI values recorded in neonatal (n = 45 for males and n = 35 for females) or juvenile (n = 12 for each sex) groups, and there was no sex and age interaction (P value = 0.23, nonsignificant, two-way ANOVA, data not shown). The male and female results were lumped together within the two age cohorts in this and all subsequent experiments.
Developmental increase in eCBCa+GPCR initiated by a mAChR agonist.
Activation of mAChRs induces the mobilization of eCBs in young adult hippocampal CA1 neurons (Kim et al. 2002). Muscarinic AChRs are present in the hippocampus of neonatal rats (Guo-Ross et al. 2007). We found that, in the absence of a DSI step, application of carbachol (CCh, 5 μM) alone had almost no effect on the eIPSCs in either neonates or juveniles (10 ± 7%, n = 7 in neonates; 9 ± 2%, n = 5, in juveniles; P = 0.86). This did not reflect an absence of CB1Rs, because the recorded cells were capable of producing robust DSI before the CCh application (data not shown). Moreover, the results did not differ between neonates and juveniles. Hence, there was no detectable developmental change in eCBGPCR in hippocampal CA1 neurons, although we cannot rule out the occurrence of subtle changes below our level of resolution.
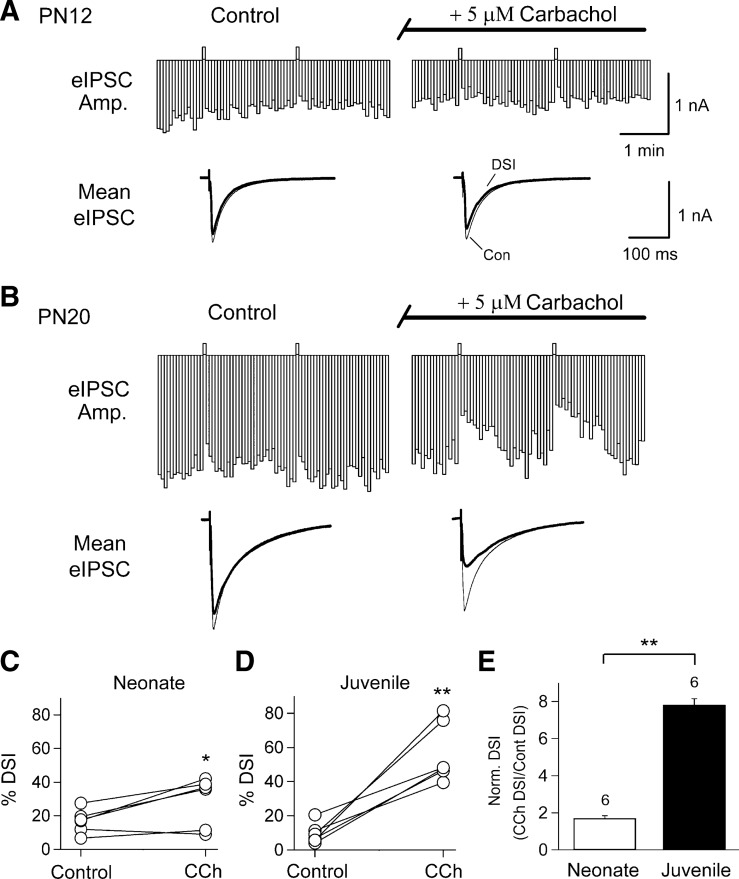
In agatoxin-pretreated slices, DSI produced by a 1-s depolarization caused an average eIPSC depression of 17 ± 3% in neonates (Fig. 2, A and C; n = 6), and application of CCh for 5 min significantly increased DSI (mean DSI was 29 ± 6%; P < 0.03, paired t-test, Fig. 2, A and C). On the other hand, DSI recorded from juveniles in this group was on average 10 ± 2% in control ACSF, but, following application of CCh, was increased to 57 ± 7%, (Fig. 2, B and D; n = 6, P < 0.001, paired t-test). The fact that CCh increased DSI in juveniles to a greater extent than in neonates (Fig. 2E; n = 6, P < 0.01, unpaired t-test) suggests there is a developmental increase in the combined GPCR plus Ca2+ mechanism, i.e., in eCBCa+GPCR, (e.g., Varma et al. 2001; see Kano et al. 2009 for review). Note that, in addition to increasing the peak magnitude of DSI, the eCBCa+GPCR process also prolongs its duration, undoubtedly reflecting the more complex kinetics associated the biochemical pathway stimulated by the GPCR compared with that of the Ca2+ transient alone, which largely determines the duration of DSI.
Fig. 2.
Carbachol (CCh), a muscarinic acetylcholine receptor (mAChR) agonist, significantly increases DSI in cells from juvenile and neonatal slices; the effects are greater in juveniles. For this and all remaining figures, the physiological experiments were done in agatoxin-pretreated slices. A: representative time course of eIPSCs (top) and traces of averaged eIPSC before and after DSI steps (below) recorded from a CA1 neuron before (left) and during (right) 5-min bath application of CCh in a PN12 rat. B: same as in A for a CA1 neuron in a PN20 rat. C and D: plot of changes in %DSI of individual cells in control and in the presence of CCh for experiments in A and B, respectively (paired t-tests, *P < 0.05; **P < 0.01). E: histogram shows that CCh caused a significantly greater increase of DSI in juveniles than that in neonates (unpaired t-test, **P < 0.01).
Developmental increase in eCBCa+GPCR initiated by group I mGluR agonist.
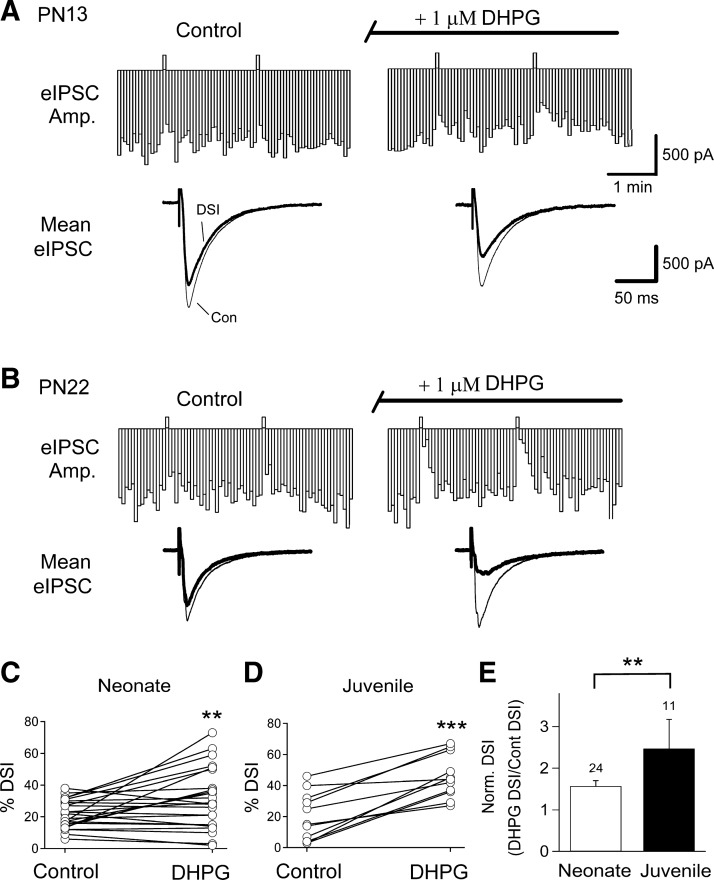
DSI is also increased in adult animals by the selective group I mGluR agonist, DHPG (Ohno-Shosaku et al. 2002; Varma et al. 2001). To determine whether the developmental difference in eCBCa+GPCR occurs only when mAChRs are stimulated, or is also found when other GPCRs, in particular group I mGluRs (mGluR1 and mGluR5) are stimulated, we recorded DSI before and after application of the selective group I mGluR agonist, DHPG (1 μM), in agatoxin-pretreated slices. DSI in neonates was increased by a 5-min application of DHPG (from 22 ± 2% to 31% ± 4% in DHPG; Fig. 3, A and C; n = 24, P < 0.01, paired t-test). In comparison, juvenile DSI increased on average from 20 ± 5% to 46 ± 4% by DHPG (Fig. 3, B and D; n = 11, P < 0.001, paired t-test). The increase in DSI caused by DHPG was much greater in juveniles than in neonates (Fig. 3E; P < 0.01, unpaired t-test). The results with DHPG are consistent with those with CCh and suggest that a common mechanism is involved in the developmental increase in eCBCa+GPCR.
Fig. 3.
Activation of group I metabotropic type glutamate receptors (mGluRs) by (S)-3,5-dihydroxyphenylglycine (DHPG), a mGluR1/5 agonist, increases DSI to a greater degree in juveniles than in neonates. A: representative time course of eIPSCs (top) and traces of averaged eIPSCs (bottom) recorded before (left) and during (right) 5-min bath application of DHPG in a PN13 rat. B: same as in A for a PN22 rat. C and D: plot of changes in %DSI of individual cells in Con and in the presence of DHPG for experiments in A and B, respectively (paired t-tests, **P < 0.01; ***P < 0.005). E: histogram shows that DHPG caused a significantly greater increase of DSI magnitude in juveniles than that in neonates (unpaired t-test, **P < 0.01).
Developmental increases in both mGluR5 and PLCβ1 protein expression in hippocampus.
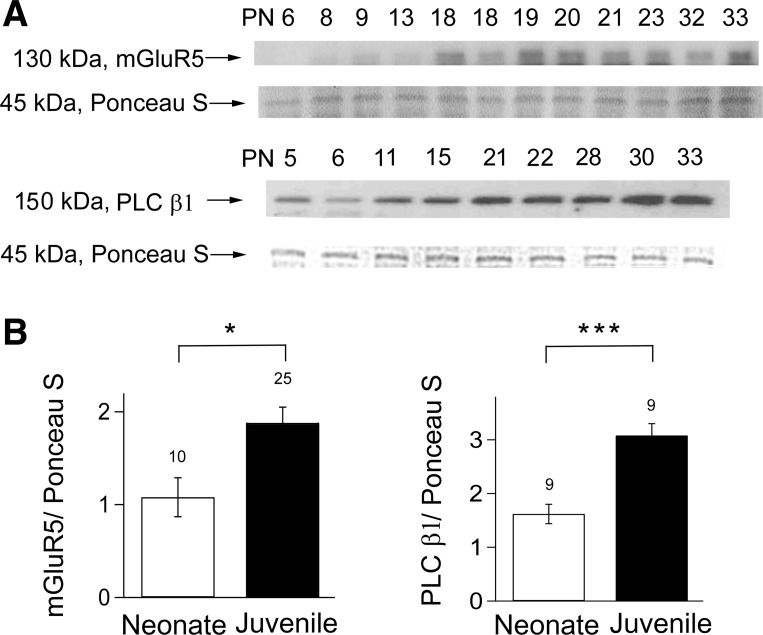
Because the results obtained with both mAChR and mGluR agonists were essentially the same, we chose to focus on the group I mGluRs. mGluR5 is abundantly expressed in hippocampal CA1 neurons (Shigemoto et al. 1993) and may be involved in low-frequency, stimulation-enhanced DSI in neonates (Zhu and Lovinger 2010). PLCβ1 is proposed to mediate eCBGPCR and to be the coincidence detector for eCBCa+GPCR in hippocampal neurons (Hashimotodani et al. 2005). If the developmental increase in DSI caused by DHPG is related to changes in the eCBCa+GPCR pathway, we predicted that there might be developmental increases in the protein expression of mGluR5, PLCβ1, or both. To test this prediction, we saved the recorded hippocampal slices and carried out Western blot analyses on them (see materials and methods). We observed increases in protein expression levels in juveniles over the neonatal values for both mGluR5 (an increase of 58%; n = 10 for neonates, n = 25 for juveniles, P < 0.05) and PLCβ1 (an increase of 89%; n = 9 for both neonates and juveniles, P < 0.001; see Fig. 4, A and B). These results suggest that the developmental increase in eCBCa+GPCR may be accounted for by increases in these components of the GPCR pathway.
Fig. 4.
Juveniles have significantly greater amounts of mGluR5 and phospholipase C (PLC) β1 protein than neonates. A: representative photomicrographs of Western blots of mGluR5 and PLCβ1 proteins from hippocampal slices after electrophysiological DSI recordings. Note two lanes labeled “18” for mGluR5 protein are from two different PN18 animals and illustrate the variability possible at this critical age. B: significant increase in the amounts of mGluR5 and PLCβ1 proteins in juveniles compared with neonates (t-test, *P < 0.05; ***P < 0.005). The no. on the top of each bar denotes the no. of animals analyzed.
Inhibitor of PLC activity reduces eCBCa+GPCR in both juveniles and neonates.
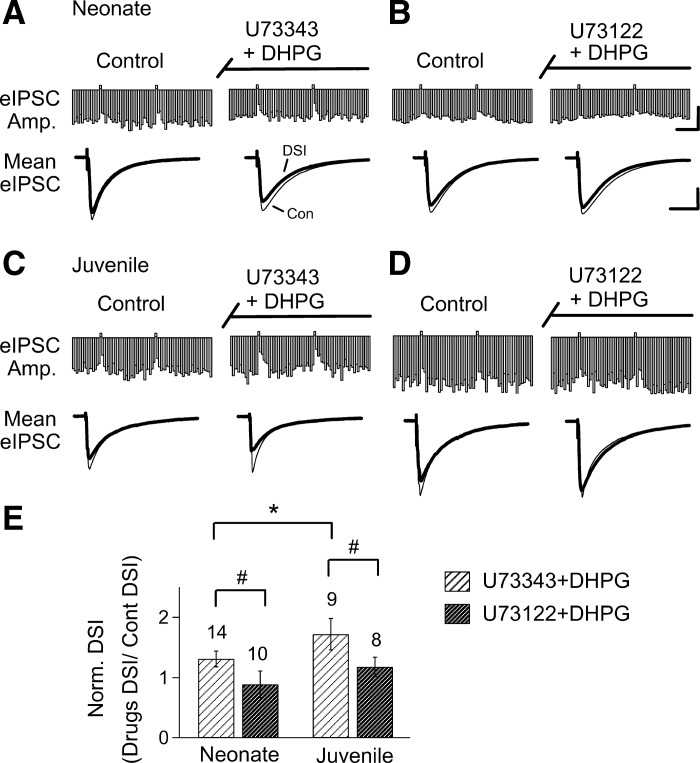
If the eCBCa+GPCR process is less effective in neonates, as our data suggest, perhaps because of the differences in mGluR5 or PLC, then altering the efficacy of the pathway should have differential effects in neonates and juveniles. To test this prediction, we used pharmacological agents to affect the activity of PLC in agatoxin-pretreated slices. We began by testing the PLC inhibitor U-73122 (5 μM), and its inactive analog, U-73343 (5 μM). Neither of these agents significantly affected DSI in control conditions in neonates or juveniles (18 ± 3% to 22 ± 3%, n = 7, paired t-test, P = 0.07 in neonates and 19 ± 2% to 20 ± 3%, n = 12, paired t-test, P = 0.65, in juveniles in U-73343; 15 ± 2% to 11 ± 6%, n = 8, paired t-test, P = 0.46 in neonates and 15 ± 2% to 19 ± 4%, n = 8, paired t-test, P = 0.13 in juveniles in U-73122). The inactive analog, U-73343, had no effect on the increase in DSI caused by DHPG either in neonates (n = 15, P < 0.02 paired t-test; Fig. 5A), or in juveniles (n = 10, P < 0.001; Fig. 5C), and, as before, DHPG caused a greater increase in DSI in the juveniles (n = 10) than in the neonatal (n = 15) animals (P < 0.05, unpaired t-test). However, the active PLC inhibitor, U-73122 prevented the enhancement of DSI by DHPG in both neonates (n = 10, P = 0.9; Fig. 5B), and in juveniles (n = 8; P = 0.06, paired t-test, Fig. 5D). Summary data are shown in Fig. 5E.
Fig. 5.
DHPG-mediated enhancement of DSI is prevented by coapplication of a PLC inhibitor, U-73122, in both neonates and juveniles. Representative time course is shown of eIPSCs recorded from CA1 neurons in the presence of DHPG plus either U-73122 (B and D), or its inactive analog, U-73343 (A and C), in slices obtained from neonatal and juvenile rats, as indicated. Traces below are averages of eIPSCs recorded before and during DSI and before and during bath application of the respective chemicals. E: histograms show that U-73122 prevented the DHPG-mediated potentiated response of DSI in both neonates and juveniles (unpaired t-tests, #P < 0.05). Note DSI magnitude is greater in juveniles than in neonates in the presence of DHPG plus U-73343 (unpaired t-test, *P < 0.05). All the slices were preincubated with agatoxin before the DSI recordings. Calibration: continuous traces = 1 nA, 1 min; mean traces = 1 nA, 100 ms.
Activator of PLC activity induces eCBCa+GPCR in neonates but not juveniles.
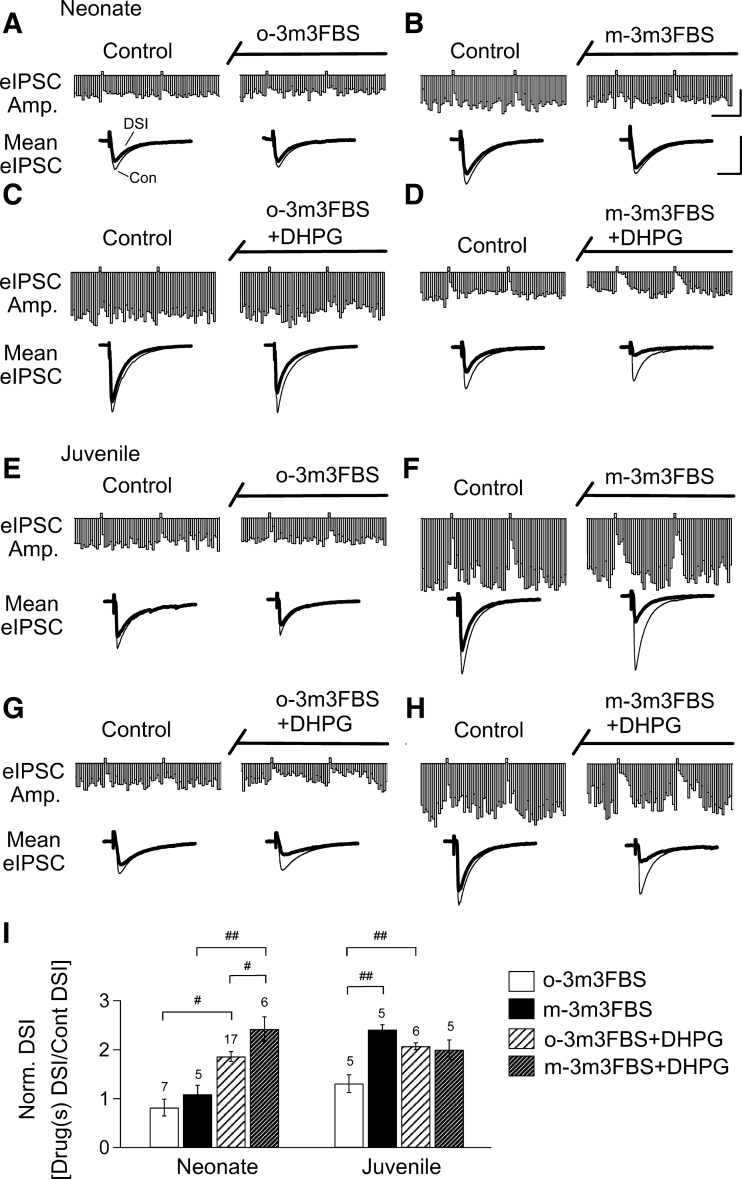
Although the PLC antagonist blocked the increase in DSI caused by DHPG in both groups, the difference in the initial effects of DHPG is compatible with a developmental increase in eCBCa+GPCR. This suggests that increasing PLC activity might have a proportionately greater effect in neonates. We tested this prediction by applying a PLC activator (m-3M3FBS, 30 μM), with or without DHPG (1 μM) for 20 min in slices pretreated with agatoxin. For comparison, we also tested o-3M3FBS (30 μM), an inactive analog of m-3M3FBS, and observed that it had no effect on DSI in neurons recorded from either neonates (Fig. 6, A and I, n = 7) or juveniles (Fig. 6, E and I, n = 5). The active compound, m-3M3FBS applied for 20 min, had no effect on neonatal DSI at either 30 μM (Fig. 6, B and I, n = 5) or 100 μM (n = 3, data not shown). In contrast, in juveniles, DSI was increased from 26 ± 2% to 59 ± 5% by 30 μM m-3M3FBS (Fig. 6, E, F, and I, n = 5, P < 0.01 compared with the results from the o-3M3FBS-treated group). The higher concentration of m-3M3FBS (100 μM) had no further effect in juveniles (data not shown).
Fig. 6.
A PLC activator potentiates the DHPG-enhanced DSI in neonates to the level of juveniles. Representative time course is shown of eIPSCs recorded from CA1 neurons in the presence of a PLC activator, m-3M3FBS (right, B, D, F, H) or its inactive analog, o-3M3FBS (left, A, C, E, G) in slices obtained from neonatal and juvenile rats, with or without DHPG coapplication as indicated. Traces below are representative averaged eIPSCs before and during bath application of the chemicals. I: histograms show that o-3M3FBS had no effect on basal DSI or the action of DHPG on DSI in either group (ANOVA, #P < 0.05; ##P < 0.01). m-3M3FBS significantly increased basal DSI in juveniles but not neonates (ANOVA, ##P < 0.01). When coapplied with DHPG, m-3M3FBS increased neonatal DSI to the juvenile level (ANOVA, #P < 0.05, ##P < 0.01); the juvenile level was not further enhanced by m-3M3FBS. All of the slices were preincubated with agatoxin. Calibration: continuous traces = 1 nA, 1 min; mean traces = 1 nA, 100 ms.
We then tested the ability of PLC enhancement to affect eCBCa+GPCR. DHPG continued to increase DSI in the presence of o-3M3FBS in neonates (16 ± 3% to 27 ± 3%; n = 17, P < 0.001, paired t-test, Fig. 6, A, C, and I) and juveniles (24 ± 4% to 53 ± 7%, n = 6, P < 0.002, paired t-test; Fig. 6, E, G, and I; P < 0.01). When m-3M3FBS was coapplied with DHPG in neonates, DSI was enhanced from 23 ± 3% to 55 ± 7% (n = 6; P < 0.002); i.e., to the level of juvenile DSI in DHPG (Fig. 6, B, D, and I). This enhanced DSI effect was mediated via increasing PLC activity because it was blocked when U-73122 (5 μM) was coapplied with m-3M3FBS and DHPG for 20 min (17 ± 6% to 22 ± 9%, n = 7, P = 0.51, paired t-test; data not shown). On the other hand, m-3M3FBS did not affect the DHPG-induced DSI increase in juveniles, which went from 26 ± 4% to 54 ± 7% (n = 5, P < 0.01, paired t-test, Fig. 6, H and I). These results are consistent with the hypothesis that DHPG and m-3M3FBS act via a common pathway to increase eCBCa+GPCR. The differential effects of drugs acting on PLC support the conclusion that the developmental increase in eCBCa+GPCR is related to upregulation of this combined pathway.
DISCUSSION
It has been proposed that the eCB system undergoes developmental upregulation from PN7 to PN21, and that this effect is attributable to a postsynaptic increase in eCB production (Zhu and Lovinger 2010). However, the mechanisms involved in this change have remained almost entirely unknown. Using a combination of whole cell voltage-clamp recording and Western blot analysis, we have investigated these issues in rats while expanding the age range being tested from PN1 to PN43. Our major findings are that, over the age range tested, 1) of the three known mechanisms of eCB mobilization, there were substantial changes in only the combined mechanism, eCBCa+GPCR, manifested as an enhancement of DSI caused by simultaneous stimulation of GPCRs, showed a large developmental increase. 2) The purely GPCR-dependent mechanism, eCBGPCR, as assessed by application of mAChR or group I mGluR agonists alone, was largely ineffective in inducing eCB release. 3) Although we cannot rule out a minor effect, our data suggest that there is no significant change in functional CB1R levels, in agreement with the report of Zhu and Lovinger (2010).
While the full biochemical details of the eCBCa+GPCR pathway have not been worked out, probably PLCβ (Hashimotodani et al. 2005) and diacylglycerol-α (Gao et al. 2010; Tanimura et al. 2010) are central components. We observed developmental increases in protein levels of both mGluR5 and PLCβ1, which are consistent with the proposed mechanism and, furthermore, that manipulations of PLCβ had age-dependent effects on eCB mobilization. The findings suggest that both of these factors contribute to the developmental differences in eCBCa+GPCR. This is the first report of developmental regulation of a molecular mechanism of eCBCa+GPCR and could have important implications for understanding the role of eCBs during this growth period. The fact that increases in the synergistic mechanism occur suggests that eCBs could play a role when pre- and postsynaptic activity are simultaneously active in sculpting neuronal circuitry in the growing brain. This possibility is discussed below.
Molecular mechanism of developmentally enhanced eCBCa+GPCR: the involvement of PLC.
Inhibition of PLC alone had no effect on baseline DSI (in ACSF) in either neonates or juveniles, consistent with the report that elimination of PLC activity does not affect DSI (Hashimotodani et al. 2005). On the other hand, PLC is involved in the modulation of DSI by GPCR-coupled transmitters. DSI is enhanced by DHPG in both neonates and juveniles, but this effect is greater in the juveniles. Nevertheless, inhibition of PLC did prevent the DSI enhancement in both cases, indicating PLC is an integral part of the mechanism throughout the developmental period.
An activator of PLC showed a more complex pattern: it was more effective in increasing baseline DSI (in ACSF) in juveniles than in neonates, yet, conversely, more effective in increasing DHPG-enhanced DSI in neonates than in juveniles. These results are consistent with the hypothesis that the PLC activity associated with eCB mobilization continually rises from the neonatal to the juvenile age ranges. In the older animals, the basal PLC activity would be sufficient that its enhancement by the activator makes a noticeable contribution to the net mobilization of eCBs, and thereby increases DSI. In the neonates, the basal PLC activity may be so low that even increasing it does not make a measurable difference. On the other hand, the low level of basal PLC activity in neonates will be moderately increased by an mGluR agonist, and hence allow the PLC activator to boost eCB mobilization significantly. In contrast, in the juveniles after mGluR stimulation, the PLC activity is apparently already maximal and, therefore, incapable of being further elevated by the PLC activator. Importantly, there is no quantitative difference between maximally activated DHPG-enhanced DSI in neonates (with the PLC activator) and juveniles (with or without the PLC activator), which is consistent with a ceiling effect of PLC stimulation.
Our results suggest that m-3M3FBS potentiates the DHPG enhancement of DSI by increasing PLC activity, which is in agreement with studies showing that m-3M3FBS strongly enhances PLC-dependent superoxide-generating activity and stimulates production of inositol phosphates (Bae et al. 2003). Moreover, inhibition of PLC prevents m-3M3FBS-induced apoptosis in a human renal cell line (Jung et al. 2008). On the other hand, because m-3M3FBS can stimulate a transient increase in [Ca2+]i in neurons (Bae et al. 2003), we cannot totally exclude the possibility that part of the enhancement of eCBCa+GPCR by m-3M3FBS in neonates reflects an increase in [Ca2+]i. Nevertheless, any increase in [Ca2+]i caused by m-3M3FBS would have had to be relatively minor, because DSI in m-3M3FBS was not enhanced in neonates, as it would have been if [Ca2+]i had been greatly elevated.
Molecular mechanism of developmentally enhanced eCBCa+GPCR: eCB and CB1R.
The eCB responsible for most signaling processes, including eCBCa, eCBGPCR, and eCBCa+GPCR, is 2-arachidonoylglycerol (2-AG) rather than anandamide (see Kano et al. 2009 for review). PLC is directly involved in the synthesis of 2-AG, whereas anandamide, which mediates eCB signaling in certain circumstances (e.g., Ade and Lovinger 2007; Azad 2004; Chavez et al. 2010; Grueter et al. 2010; Kim and Alger 2010), does not require PLC. Our data are, therefore, consistent with the conclusion that developmental upregulation of 2-AG mobilization mediates the increase in eCBCa+GPCR.
CB1R and CCK interneurons are present in the hippocampus at birth (Morozov and Freund 2003a, 2003b), and DSI is present at an early neonatal stage (Bernard et al. 2005). Although CB1R mRNA expression levels gradually increase in the hippocampus between the fetal period and adulthood (Harkany et al. 2007), our functional assays did not detect strong evidence for changes in CB1R levels. Compared with the eCBCa+GPCR, there were no substantial developmental changes in eCBCa or eCBGPCR, and, had functional CB1R levels been rising significantly, there would have been. Similarly, Zhu and Lovinger (2010) reported that, in hippocampal slices, eIPSCs response to bath application of the CB1R agonist, WIN55212-2, did not change over the similar developmental period between neonates and early juveniles, which is inconsistent with an increase in CB1Rs. This conclusion is further supported by our observation of a large increase in DSI when preceded by DHPG or CCh application; neither of these GPCRs affects CB1R levels. Finally, the PLC activator is unlikely to affect CB1R, but had significant effects on eCBs mobilization. Thus the increase in GPCR-enhanced DSI is most likely to be explained principally by increased eCB mobilization and not increased CB1R.
Lack of substantial changes in eCBCa or eCBGPCR.
We found that, in the presence of agatoxin, DSI was greater in juveniles than in neonates, but that the difference between the two groups was much reduced compared with those in the absence of agatoxin. Agatoxin does not enhance eCB mobilization. The extracellular electrical stimulation excites both DSI-sensitive (CB1R-expressing) and DSI-insensitive (non-CB1R-expressing) interneurons. Thus agatoxin enhances the relative degree of DSI of eIPSCs by eliminating DSI-insensitive eIPSCs (Lenz et al. 1998).
The fact that agatoxin pretreatment reduced the magnitude of the developmental increase in DSI suggests that a shift in the balance of non-CB1R-expressing and CB1R-expressing interneurons probably takes place during this time period. If the percentage of CB1R-expressing neurons increases with development, the measured DSI would increase, even if the actual eCBCa process was not much more effective in these animals. Detailed morphological analysis of the development of different types of interneurons in the hippocampus (Tricoire et al. 2011), showed that, from PN5 to PN30, interneurons lacking CB1R (including parvalbumin-expressing cells) always outnumber CB1R-expressing interneurons, but the ratio of non-CB1R-expressing to CB1R-expressing cells drops from PN5 to PN30. This implies that the ability of agatoxin to enhance DSI should be less in juveniles than neonates, which is what we found.
While we confirmed the developmental increase in DSI reported by Zhu and Lovinger (2010), our data suggest that this may not be entirely attributed to a true increase in eCBCa. The developmental decrease in non-DSI-sensitive eIPSCs could have affected their results as well, although they observed significant effects of 1-amino-1,3-dicarboxycyclopentane and CCh on eIPSCs in neonates, indicating the existence of other differences between the two studies. Their data do show an increase in eCBCa+GPCR with 1-amino-1,3-dicarboxycyclopentane or CCh (Zhu and Lovinger 2010; Fig. 4), however, they do not comment on whether the relative effects of the GPCR agonists on DSI increased with age.
Functional significance of increases in eCBCa+GPCR.
The synergistic interactions between DSI and mGluR or mAChR activation are probably not mediated by an increase in [Ca2+]i (Kim et al. 2002). Hashimotodani et al. (2005) showed that eCBGPCR is absent in PLCβ1−/− mice, whereas eCBCa is unaffected by the knockout. This suggested that PLCβ, which is both Ca2+ and G protein dependent, serves as a “coincidence detector” for the two pathways of eCB mobilization. This simple hypothesis is somewhat controversial (e.g., Edwards et al. 2006), and Edwards et al. (2008) proposed instead that Ca2+ actually primes the eCBGPCR pathway and the priming effect outlasts the actual period of increased [Ca2+]i, i.e., that true temporal coincidence of the two signals is not required. This opens up the possibility that PLCβ, although a key upstream regulator, might not actually be the molecular integrator. Regardless of the details, a synergistic interaction between Ca2+ and the products of GPCR activation is an especially potent stimulus for eCB mobilization. We now report that developmental increases in PLCβ activity accompany the increased eCBCa+GPCR, yet, because protein levels of both mGluR5 and in PLCβ1 occurred, upregulation of PLCβ1 per se cannot be identified as the only mechanism.
However, the molecular details of this issue are ultimately resolved, our demonstration that development affects primarily the synergistic eCBCa+GPCR pathway is itself quite significant. Unlike the purely postsynaptic Ca2+-dependent eCB mechanism, or the purely GPCR-dependent eCB mechanism, the combined mechanism is equally dependent on both pre- and postsynaptic factors. This means that it is ideally suited to take part in the formation and shaping of critical neuronal circuitry, which requires coordinated presynaptic and postsynaptic activity (Shatz 1990). The near simultaneous occurrence of presynaptic release of glutamate and significant postsynaptic depolarization to activate voltage-gated Ca2+ channels will result in a heightened mobilization of eCBs. If released near inhibitory CB1R-expressing synapses, the eCBs will suppress the release of GABA, thereby increasing net excitation in the neuronal circuits. Moreover, even weak excitatory synaptic activity, too weak to induce long-term potentiation on its own, if it occurs during DSI-induced IPSC depression, can undergo long-term potentiation (Carlson et al. 2002). Increased DSI caused by release of glutamate should be even more effective in this function. Thus the combination of strong postsynaptic and presynaptic activity could lead to the strengthening of synapses that are crucial for appropriate circuit development.
GRANTS
This work was supported by grants CMRPD1C0181 and CMRPD1C0182 (to S.-L. Liang) from Chang Gung Medical Research Foundation of Taiwan, ROC.; and National Institutes of Health Grants R01-NS-050525-01 (to M. M. McCarthy) and R01-DA-014625 (to B. E. Alger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.-L.L., B.E.A., and M.M.M. conception and design of research; S.-L.L. performed experiments; S.-L.L. analyzed data; S.-L.L., B.E.A., and M.M.M. interpreted results of experiments; S.-L.L. prepared figures; S.-L.L. and B.E.A. drafted manuscript; S.-L.L., B.E.A., and M.M.M. edited and revised manuscript; S.-L.L., B.E.A., and M.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the Alger and McCarthy laboratories for valuable discussion.
REFERENCES
- Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci 27: 2403–2409, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 24: 9953–991, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol 63: 1043–1050, 2003. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Nixon RA, Arancio O. Endocannabinoid system: emerging role from neurodevelopment to neurodegeneration. Mini Rev Med Chem 9: 448–462, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18: 467–486, 2012. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316: 1212–1216, 2007. [DOI] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci U S A 102: 9388–9393, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33: 181–191, 1999. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5: 723–724, 2002. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–1518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Penschuck S, Fritschy JM, McCarthy MM. Developmental switch in the expression of GABA(A) receptor subunits alpha(1) and alpha(2) in the hypothalamus and limbic system of the rat. Brain Res Dev Brain Res 119: 127–138, 2000. [DOI] [PubMed] [Google Scholar]
- Davis AM, Ward SC, Selmanoff M, Herbison AE, McCarthy MM. Developmental sex differences in amino acid neurotransmitter levels in hypothalamic and limbic areas of rat brain. Neuroscience 90: 1471–1482, 1999. [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Onozuka K, Bender KJ, Bender VA, Lutz B, Mackie K, Feldman DE. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience 145: 279–287, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol 95: 67–75, 2006. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Zhang L, Alger BE. Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc Natl Acad Sci U S A 105: 8142–8147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci 23: 14–20, 2000. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron 56: 33–42, 2007. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30: 2017–2024, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M, Hernandez ML, Pazos MR, Tolon RM, Romero J, Fernandez-Ruiz J. Colocalization of CB1 receptors with L1 and GAP-43 in forebrain white matter regions during fetal rat brain development: evidence for a role of these receptors in axonal growth and guidance. Neuroscience 153: 687–699, 2008. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13: 1519–1525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Chambers JE, Meek EC, Carr RL. Altered muscarinic acetylcholine receptor subtype binding in neonatal rat brain following exposure to chlorpyrifos or methyl parathion. Toxicol Sci 100: 118–127, 2007. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci 28: 83–92, 2007. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase C beta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45: 257–268, 2005. [DOI] [PubMed] [Google Scholar]
- Hwang TN, Copenhagen DR. Automatic detection, characterization, and discrimination of kinetically distinct spontaneous synaptic events. J Neurosci Methods 92: 65–73, 1999. [DOI] [PubMed] [Google Scholar]
- Jung EM, Lee TJ, Park JW, Bae YS, Kim SH, Choi YH, Kwon TK. The novel phospholipase C activator, m-3M3FBS, induces apoptosis in tumor cells through caspase activation, down-regulation of XIAP and intracellular calcium signaling. Apoptosis 13: 133–145, 2008. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380, 2009. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci 21: RC146, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci 13: 592–600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10182–10191, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001. [DOI] [PubMed] [Google Scholar]
- Lafourcade CA, Alger BE. Distinctions among GABAA and GABAB responses revealed by calcium channel antagonists, cannabinoids, opioids, and synaptic plasticity in rat hippocampus. Psychopharmacology (Berl) 198: 539–549, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz RA, Alger BE. Calcium dependence of depolarization-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiol 521: 147–157, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz RA, Wagner JJ, Alger BE. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol 512: 61–73, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci 25: 6826–6835, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11: 4213–4225, 1999. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci 18: 1213–1222, 2003a. [DOI] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Postnatal development and migration of cholecystokinin-immunoreactive interneurons in rat hippocampus. Neuroscience 120: 923–939, 2003b. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A 105: 8760–8765, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729–738, 2001. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci 18: 109–116, 2003. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15: 953–961, 2002. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol 17: 255–261, 2005. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci 12: 4122–4132, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Competitive interactions between retinal ganglion cells during prenatal development. J Neurobiol 21: 197–211, 1990. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163: 53–57, 1993. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65: 320–327, 2010. [DOI] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci 31: 10948–10970, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93: 969–975, 1999. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118: 681–694, 2003. [DOI] [PubMed] [Google Scholar]
- Watson S, Chambers D, Hobbs C, Doherty P, Graham A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol Cell Neurosci 38: 89–97, 2008. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science 264: 107–11, 1994. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31: 453–62, 2001. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J Neurophysiol 103: 1123–1129, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]