Abstract
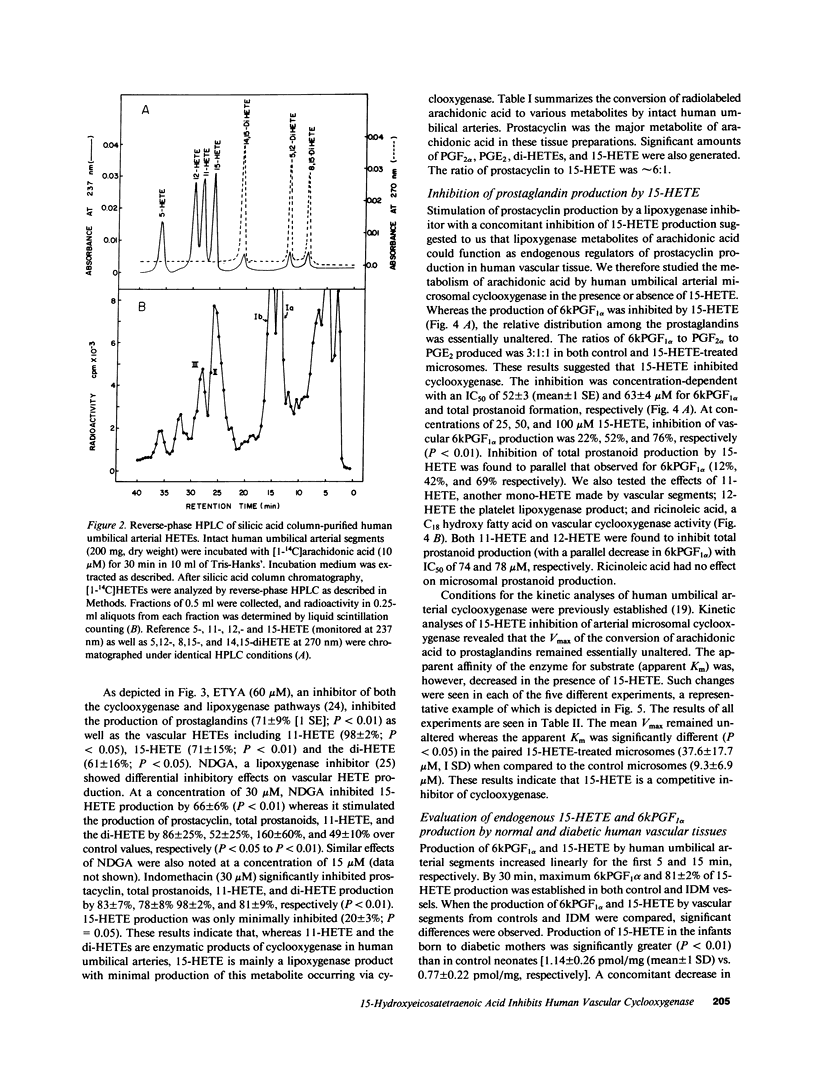
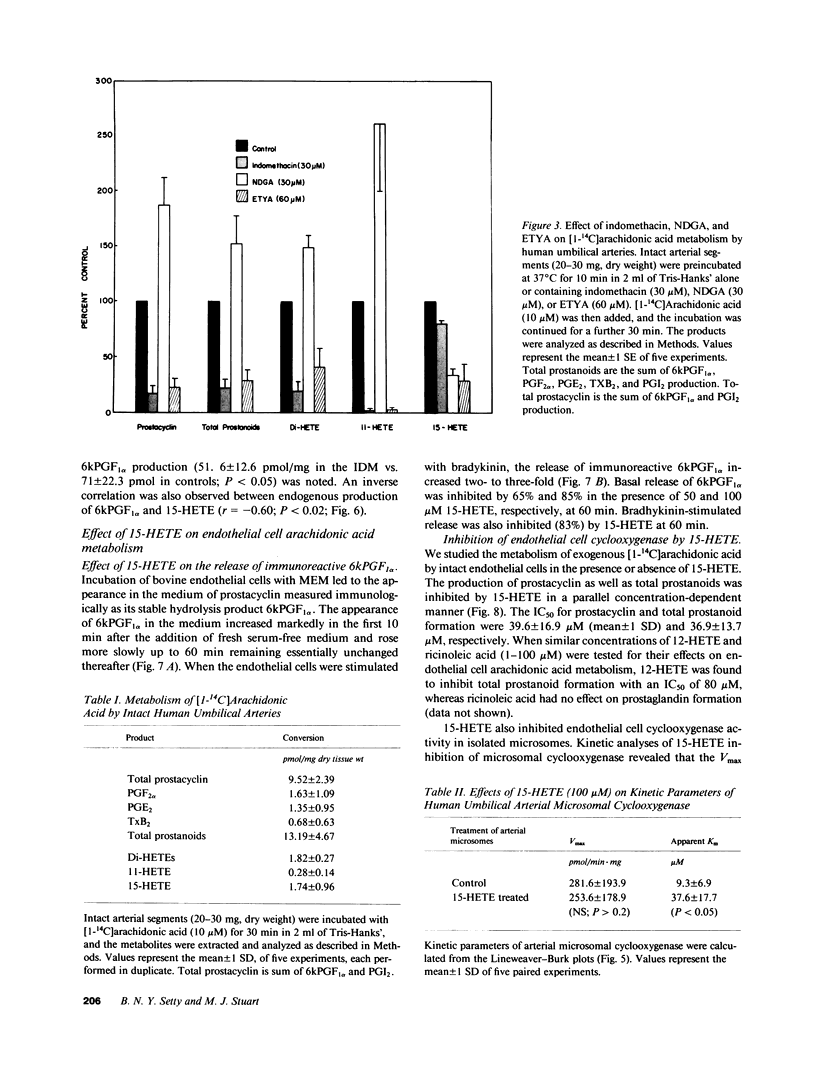
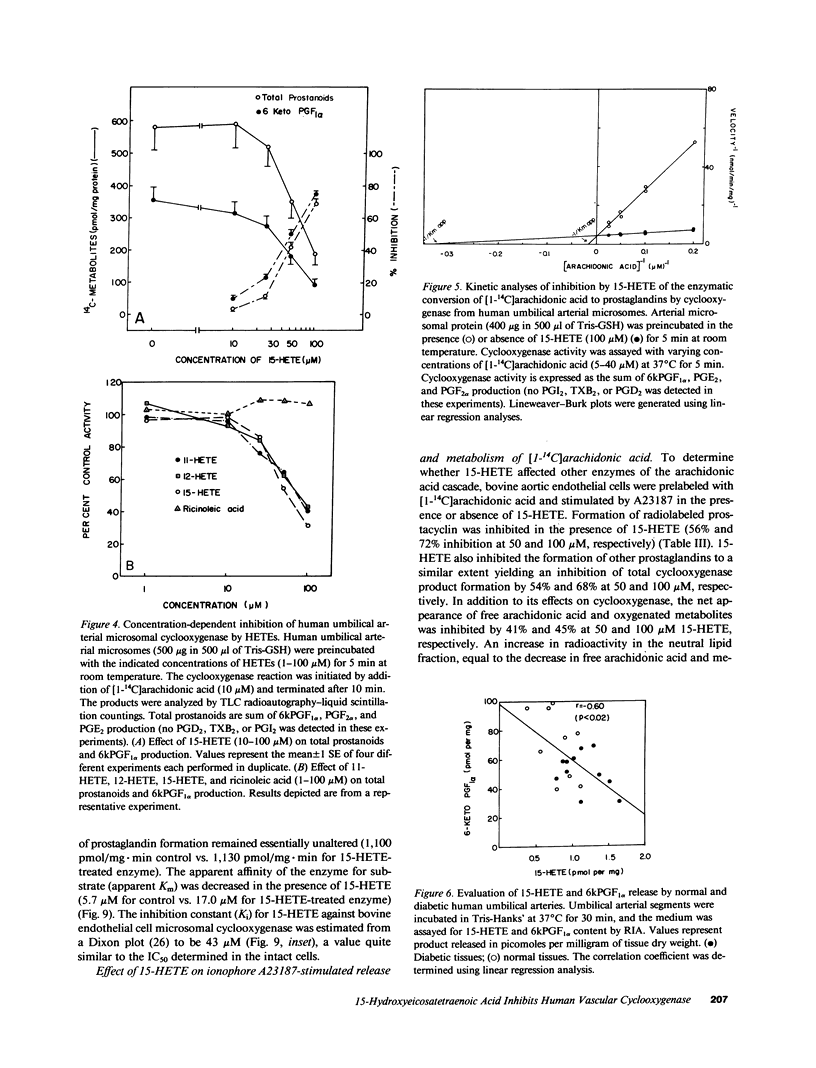
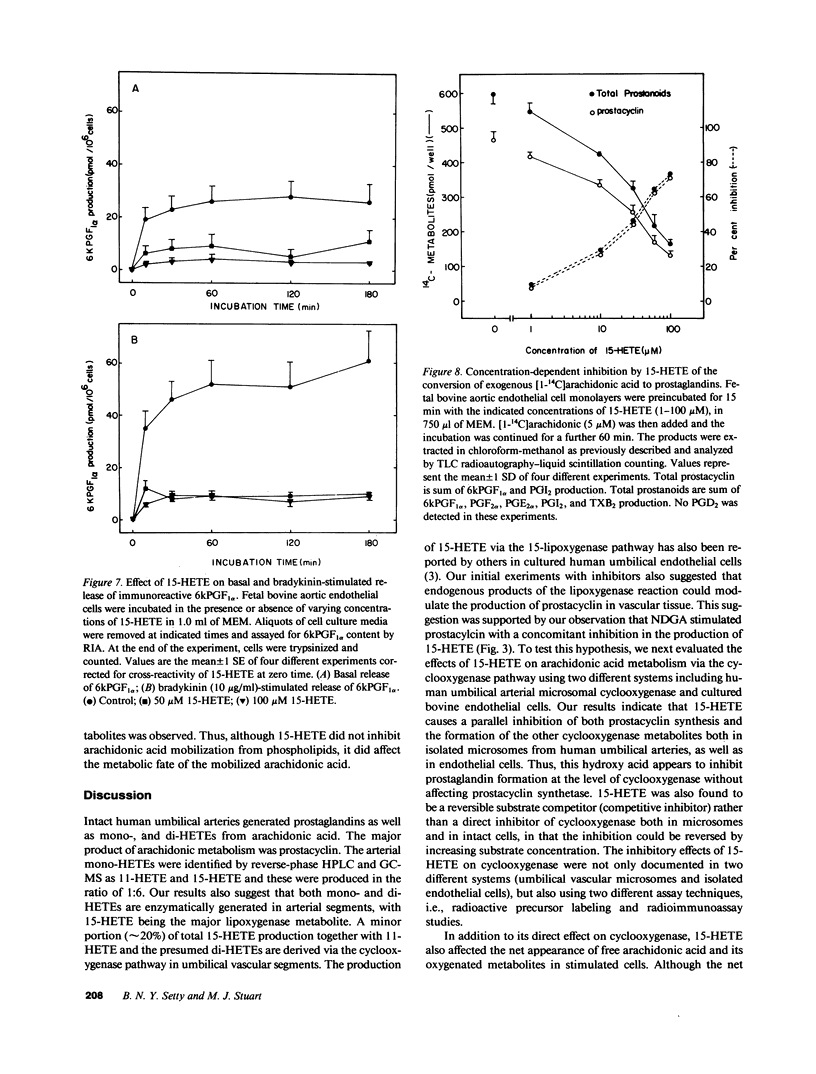
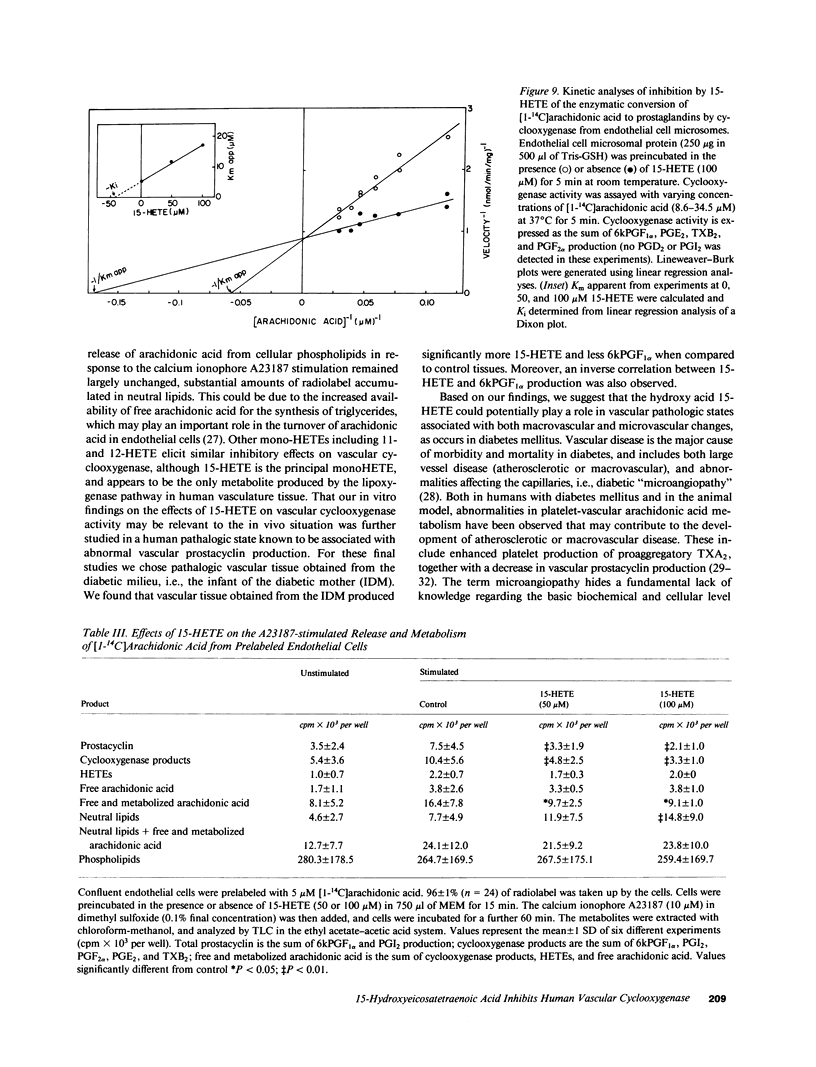
Human umbilical arteries converted arachidonic acid to three hydroxyeicosatetraenoic acids (HETEs) as well as prostaglandins. The mono-HETEs have been identified by reverse-phase high pressure liquid chromatography and gas chromatography-mass spectroscopy as 15-HETE and 11-HETE. 15-HETE in arterial segments appears to be derived mainly via the 15-lipoxygenase pathway, whereas 11-HETE, and the presumed di-HETE(s) were products of cyclooxygenase. Nordihydroguaiaretic acid, a lipoxygenase inhibitor, stimulated prostanoid production with a concomitant inhibition of 15-HETE formation. These results suggested that 15-HETE may function as an endogenous regulator of prostacyclin. In human umbilical arterial microsomes, 15-HETE was found to inhibit 6-keto-prostaglandin F1 alpha and total prostanoid production in a concentration-dependent manner (median inhibition constant [IC50] of 52 +/- 3 and 63 +/- 4 microM respectively). The relative distribution of prostaglandins, however, remained unaffected, indicating that the site of action was cyclooxygenase. Kinetic analysis revealed that 15-HETE was a competitive inhibitor of the enzyme. Although no changes in maximum velocity occurred, the apparent Km was significantly different (9.3 +/- 6.9 microM [1 SD] for control vs. 37.6 +/- 17.7 microM for the 15-HETE-treated enzyme). Furthermore, the inhibitory effect of 15-HETE on prostacyclin production was confirmed using cultured bovine endothelial cells. In this cell system, not only did 15-HETE inhibit endogenous prostacyclin production, but also the conversion of exogenous [1-14C]arachidonic acid to prostacyclin (IC50 of 40 +/- 17 microM). No effect on arachidonic acid release was noted. To investigate whether our in vitro finding that 15-HETE inhibited prostacyclin production could be relevant to the in vivo situation, our final studies were performed on vasculature obtained from the diabetic milieu. We found that the production of 15-HETE was significantly increased in vasculature obtained from the infant of the diabetic mother (1.14 +/- 0.26 pmol/mg) when compared to control neonates (0.77 +/- 0.22; P less than 0.01). A concomitant decrease in prostacyclin production was seen (51.6 +/- 12.6 pmol/mg in infants of diabetic mothers vs. 71 +/- 22.3 in controls). Moreover, an inverse correlation between these two eicosanoids was also noted. Our results suggest a potential in vivo regulatory role for 15-HETE on prostacyclin production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., OPPENHEIMER E. H., GORDON H. H. Renal-vein thrombosis in newborn infants of diabetic mothers; report of 2 cases. N Engl J Med. 1957 Jun 13;256(24):1134–1138. doi: 10.1056/NEJM195706132562403. [DOI] [PubMed] [Google Scholar]
- Adcock J. J., Garland L. G., Moncada S., Salmon J. A. Enhancement of anaphylactic mediator release from guinea-pig perfused lungs by fatty acid hydroperoxides. Prostaglandins. 1978 Aug;16(2):163–177. doi: 10.1016/0090-6980(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boeynaems J. M., Brash A. R., Oates J. A., Hubbard W. C. Preparation and assay of monohydroxy-eicosatetraenoic acids. Anal Biochem. 1980 May 15;104(2):259–267. doi: 10.1016/0003-2697(80)90073-1. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Colwell J. A., Halushka P. V. Platelet function in diabetes mellitus. Br J Haematol. 1980 Apr;44(4):521–526. doi: 10.1111/j.1365-2141.1980.tb08705.x. [DOI] [PubMed] [Google Scholar]
- Colwell J. A., Halushka P. V., Sarji K. E., Lopes-Virella M. F., Sagel J. Vascular disease in diabetes: pathophysiological mechanisms and therapy. Arch Intern Med. 1979 Feb;139(2):225–230. [PubMed] [Google Scholar]
- Crawford C. G., van Alphen G. W., Cook H. W., Lands W. E. The effect of precursors, products, and product analogs of prostaglandin cyclooxygenase upon iris sphincter muscle. Life Sci. 1978 Sep 25;23(12):1255–1262. doi: 10.1016/0024-3205(78)90503-9. [DOI] [PubMed] [Google Scholar]
- DITZEL J., WHITE P., DUCKERS J. Changes in the pattern of the smaller blood vessels in the bulbar conjunctiva in children of diabetic mothers: a preliminary report. Diabetes. 1954 Mar-Apr;3(2):99–106. doi: 10.2337/diab.3.2.99. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Figard P. H., Kaduce T. L., Spector A. A. Role of triglycerides in endothelial cell arachidonic acid metabolism. J Lipid Res. 1983 Aug;24(8):993–1001. [PubMed] [Google Scholar]
- Dollery C. T., Friedman L. A., Hensby C. N., Kohner E., Lewis P. J., Porta M., Webster J. Circulating prostacyclin may be reduced in diabetes. Lancet. 1979 Dec 22;2(8156-8157):1365–1365. doi: 10.1016/s0140-6736(79)92844-7. [DOI] [PubMed] [Google Scholar]
- Downing I., Shepherd G. L., Lewis P. J. Kinetics of prostacyclin synthetase in umbilical artery microsomes from normal and pre-eclamptic pregnancies. Br J Clin Pharmacol. 1982 Feb;13(2):195–198. doi: 10.1111/j.1365-2125.1982.tb01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Stuart M. J., Rao G. H., Steffes M. W., Mauer S. M., Brown D. M., White J. G. Alteration in the balance of prostaglandin and thromboxane synthesis in diabetic rats. J Lab Clin Med. 1980 Jun;95(6):950–958. [PubMed] [Google Scholar]
- Glaser B. M., D'Amore P. A., Michels R. G., Patz A., Fenselau A. Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol. 1980 Feb;84(2):298–304. doi: 10.1083/jcb.84.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granström E., Kindahl H. Radioimmunoassay of prostaglandins and thromboxanes. Adv Prostaglandin Thromboxane Res. 1978;5:119–210. [PubMed] [Google Scholar]
- Halushka P. V., Rogers R. C., Loadholt C. B., Colwell J. A. Increased platelet thromboxane synthesis in diabetes mellitus. J Lab Clin Med. 1981 Jan;97(1):87–96. [PubMed] [Google Scholar]
- Hamberg M., Hedqvist P., Rådegran K. Identification of 15-hydroxy-5,8,11,13-eicosatetraenoic acid (15-HETE) as a major metabolite of arachidonic acid in human lung. Acta Physiol Scand. 1980 Oct;110(2):219–221. doi: 10.1111/j.1748-1716.1980.tb06656.x. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967 Nov 25;242(22):5329–5335. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N. K., Oglesby T. D., Bundy G. L., Gorman R. R. Biosynthesis and metabolism of 15-hydroperoxy-5,8,11,13-eicosatetraenoic acid by human umbilical vein endothelial cells. J Biol Chem. 1984 Nov 25;259(22):14048–14053. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R. J., Bunting S., Vane J. R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976 Nov;12(5):715–737. doi: 10.1016/0090-6980(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Morse P. H., Duncan T. G. Ophthalmologic management of diabetic retinopathy. N Engl J Med. 1976 Jul 8;295(2):87–90. doi: 10.1056/NEJM197607082950206. [DOI] [PubMed] [Google Scholar]
- Oppenheimer E. H., Esterly J. R. Thrombosis in the newborn: comparison between infants of diabetic and nondiabetic mothers. J Pediatr. 1965 Oct;67(4):549–556. doi: 10.1016/s0022-3476(65)80424-3. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Formation of 6-oxoprostaglandin F1 alpha, 6,15-dioxoprostaglandin F1 alpha, and monohydroxyicosatetraenoic acids from arachidonic acid by fetal calf aorta and ductus arteriosus. J Biol Chem. 1982 Aug 25;257(16):9457–9463. [PubMed] [Google Scholar]
- Stuart M. J., Elrad H., Graeber J. E., Hakanson D. O., Sunderji S. G., Barvinchak M. K. Increased synthesis of prostaglandin endoperoxides and platelet hyperfunction in infants of mothers with diabetes mellitus. J Lab Clin Med. 1979 Jul;94(1):12–26. [PubMed] [Google Scholar]
- Sun F. F., McGuire J. C., Morton D. R., Pike J. E., Sprecher H., Kunau W. H. Inhibition of platelet arachidonic acid 12-lipoxygenase by acetylenic acid compounds. Prostaglandins. 1981 Feb;21(2):333–343. doi: 10.1016/0090-6980(81)90151-9. [DOI] [PubMed] [Google Scholar]
- Vanderhoek J. Y., Bryant R. W., Bailey J. M. Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J Biol Chem. 1980 Nov 10;255(21):10064–10066. [PubMed] [Google Scholar]
- Vanderhoek J. Y., Feinstein M. B. Local anesthetics, chlorpromazine and propranolol inhibit stimulus-activation of phospholipase A2 in human platelets. Mol Pharmacol. 1979 Jul;16(1):171–180. [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaja Setty B. N., Walenga R. W., Stuart M. J. Kinetic analyses of the effects of hyperoxia and hypoxia on vascular cyclooxygenase activity in vitro. Biochem Biophys Res Commun. 1984 Nov 30;125(1):170–176. doi: 10.1016/s0006-291x(84)80350-2. [DOI] [PubMed] [Google Scholar]