Abstract
Oral all-trans retinoic acid (atRA) has been shown to reduce the formation of neointimal hyperplasia; however, the dose required was 30 times the chemotherapeutic dose, which already has reported side effects. As neointimal formation is a localized process, new approaches to localized delivery are required. This study assessed whether atRA within a citrate-based polyester, poly(1,8 octanediolcitrate) (POC), perivascular membrane would prevent neointimal hyperplasia following arterial injury. atRA-POC membranes were prepared and characterized for atRA release via high-performance liquid chromatography with mass spectrometry detection. Rat adventitial fibroblasts (AF) and vascular smooth muscle cells (VSMC) were exposed to various concentrations of atRA; proliferation, apoptosis, and necrosis were assessed in vitro. The rat carotid artery balloon injury model was used to evaluate the impact of the atRA-POC membranes on neointimal formation, cell proliferation, apoptosis, macrophage infiltration, and vascular cell adhesion molecule 1 (VCAM-1) expression in vivo. atRA-POC membranes released 12 μg of atRA over 2 wk, with 92% of the release occurring in the first week. At 24 h, atRA (200 μmol/l) inhibited [3H]-thymidine incorporation into AF and VSMC by 78% and 72%, respectively (*P = 0.001), with negligible apoptosis or necrosis. Histomorphometry analysis showed that atRA-POC membranes inhibited neointimal formation after balloon injury, with a 56%, 57%, and 50% decrease in the intimal area, intima-to-media area ratio, and percent stenosis, respectively (P = 0.001). atRA-POC membranes had no appreciable effect on apoptosis or proliferation at 2 wk. Regarding biocompatibility, we found a 76% decrease in macrophage infiltration in the intima layer (P < 0.003) in animals treated with atRA-POC membranes, with a coinciding 53% reduction in VCAM-1 staining (P < 0.001). In conclusion, perivascular delivery of atRA inhibited neointimal formation and restenosis. These data suggest that atRA-POC membranes may be suitable as localized therapy to inhibit neointimal hyperplasia following open cardiovascular procedures.
Keywords: endothelium/vascular, nitric oxide, peripheral vascular disease, restenosis, smooth muscle cell proliferation and differentiation
atherosclerosis is the leading cause of death and disability in the United States (27). Current surgical treatment options include angioplasty with stent placement, endarterectomy, and surgical revascularization. Unfortunately, the long-term durability of these interventions is limited because of the formation of neointimal hyperplasia and vascular remodeling that result in subsequent restenosis (12, 14, 17, 20–22, 25). Although not fully elucidated, both of these processes involve a complex pathophysiological response of the vasculature to injury. This response affects multiple cell types and results in the proliferation, dedifferentiation, and migration of vascular smooth muscle cells (VSMC) and adventitial fibroblasts (AF) (4, 5). Thus the ideal therapeutic agent would prevent the dedifferentiation and migration of multiple cell types and have selective effects on the proliferation of endothelial cells vs. AF and VSMC.
All-trans retinoic acid (atRA), a vitamin A derivative, acts on retinoic acid nuclear receptors regulating cellular proliferation and differentiation (9). It has been used clinically in patients to treat proliferative disorders, such as acute promyelocytic leukemia and other cancers (10, 29). Furthermore, it has been investigated as a potential agent to inhibit neointimal hyperplasia in animal studies (7, 13, 23, 31). Specifically, investigators have demonstrated that oral delivery of atRA (10–24 mg/kg) using the rat carotid artery injury model or the atherosclerotic rabbit femoral artery injury model resulted in a reduction of neointimal hyperplasia or positive remodeling following arterial injury (7, 23, 31). Although atRA has been proven to be relatively safe clinically, it is not without systemic effects. The oral doses utilized in these animal studies to achieve efficacy were ∼30-fold higher than the doses utilized to treat cancer in humans (8). Furthermore, the systemic doses used in both the animal and human studies have resulted in elevation of both triglycerides and alkaline phosphatase levels (10, 23). Thus a safer approach to atRA delivery would be beneficial.
As the formation of neointimal hyperplasia is a localized process, localized delivery of atRA would greatly decrease the dose required to achieve efficacy and the incidence of systemic side effects. Whereas localized endoluminal delivery of atRA after injury did not significantly reduce intimal formation or stenosis at later time points, it did significantly reduce early neointimal proliferation rates (13). These data suggest that locally delivered atRA might be effective at inhibiting neointimal hyperplasia; however, the lack of improved outcomes highlights the importance of finding the most effective biomedical delivery method.
atRA is a relatively unstable drug that can rapidly decompose into nonactive isomers when exposed to heat or light (9). When systemically delivered, atRA is bound by albumin and retinol-binding proteins in the serum and rapidly transferred to tissue stores found in the liver (3). Therefore, the ideal delivery system should preserve the stability and activity, allow slow release, avoid systemic clearance, and be biocompatible and degradable. Periadventitial delivery via a biodegradable polymer membrane provides the benefit of local delivery over time that initially bypasses clearance by the systemic circulation.
Our prior work has investigated the use of the biodegradable elastomer, poly (1,8-octanediolcitrate) (POC), which we showed to be cell and tissue compatible in several applications (18, 28, 33). POC can be fabricated into soft porous elastomer membranes suitable for periadventitial delivery of drugs. In this work, we set out to develop a new method to locally deliver atRA to the site of vascular injury using perivascular membranes fabricated from POC. Thus the objective of this research was to assess whether periadventitial delivery of atRA via a POC membrane would inhibit the development of neointimal hyperplasia. We hypothesize that atRA-POC membranes will result in sustained release of atRA, inhibit both VSMC and AF proliferation in vitro, and inhibit the development of neointimal hyperplasia following arterial injury in vivo.
MATERIALS AND METHODS
Cell culture.
VSMC and AF were harvested and cultured from the abdominal aortas of 10–12-wk-old male Sprague Dawley rats (Harlan, Indianapolis, IN). The animals were induced with 5% isoflurane via an anesthetic chamber and then maintained on 1.5–2.5% isoflurane via nose cone delivered through a nonbreathable circuit. Once anesthetized, as evident by absence of response to toe pinch, the animals were euthanized via a bilateral thoracotomy. The aortas were removed, and the cells were isolated and maintained as previously described (30). Briefly, after the aorta is removed, the endothelial layer is gently removed by scraping. The remaining aorta is briefly exposed to collagenase, which allows for separation of the adventitia from the media. Each component separately undergoes further digestion in a collagenase/elastase solution to isolate and grow these cells in culture. VSMC phenotype is confirmed by staining for α-smooth muscle cell actin. Although no specific cell surface marker exists for AF, we confirmed that the AF are negative for smooth muscle myosin heavy chain. All cells were used for in vitro experimentation between passages 3 and 9.
[3H]-thymidine incorporation.
VSMC and AF were plated at a density of 3,000 cells/cm2. Twenty-four hours after being plated, these cells were growth arrested for 24 h with media lacking fetal bovine serum. Cells were then treated with complete medium containing [3H]-thymidine with or without varying concentrations of atRA (1–200 μmol/l) for 18–24 h. DNA was precipitated by treatment with 5% trichloroacetic acid at 4°C for at least 1 h. The plates were rinsed with cold water and allowed to dry overnight. After dissolution in sodium hydroxide (0.1 mol/l), the [3H]-thymidine incorporated into trichloroacetic-acid-precipitated DNA was measured using a WinSpectral 1414 liquid scintillation counter (Wallac, Turku, Finland).
Assessment of cell number, apoptosis, and necrosis.
Cells treated with atRA (50 or 100 μmol/l) were also analyzed for early apoptosis, late apoptosis, and necrosis via flow cytometry using the Muse with the Annexin V and dead cell assay kit (EMD Millipore, Boston, MA). Briefly, after treatment with atRA, the media was collected, and the cells were collected by trypsinization. After centrifuge for 10 min at 1,200 revolution/min, the effluent was decanted off, and the cell pellet was resuspended in 100–200 μl of media. With the use of the Annexin V Live Dead Kit, the specimens were then processed per the manufacturer's instructions.
Fabrication of atRA-eluting periadventitial POC membranes (atRA-POC).
The membrane consisted of a porous thin film of POC with atRA immobilized via entrapment throughout the membrane. Briefly, POC prepolymer was synthesized from equimolar amounts of citric acid and 1,8-octanediol as previously described (28). This mixture was heated to 140°C for 1 h to create the prepolymer and dissolved in ethanol to a concentration of 30% (wt/vol). The membrane was fabricated from POC prepolymer using a salt-casting and particulate-leaching technique as previously described (33). The membrane was then soaked in the dark for 12 h in a 20 mg/ml solution of atRA dissolved in dimethyl sulfoxide (DMSO). After being soaked, the membranes were removed, rinsed with water, and lyophilized in the dark overnight. Once prepared, the membranes were gas sterilized, protected from light, and stored at −20°C.
Release profile of atRA eluted from the membranes.
To measure atRA release, atRA-POC discs 6 mm in diameter and 1 mm in height with a volume of ∼28 mm3 were soaked in sterile liquid chromatography with mass spectrometry (LC-MS) grade water at 37°C and protected from light. The water was removed and replaced daily. Collected water was lyophilized to recover the released atRA, which was dissolved in acetonitrile and quantified using high-performance LC-MS (HPLC-MS). HPLC-MS analysis was carried out using an LCMS-2020 single quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a Shimadzu HPLC system with UV-Vis spectrophotometer similar to published methods (16). Samples of the atRA released were injected into an Ascentis RP-Amide column (150 mm × 2.1 mm, 3-mm particle size) (Sigma-Aldrich, St. Louis, MO). The solvent system consisted of HPLC-MS-grade water (solvent A) and acetonitrile (solvent B) [each containing 0.1% (vol/vol) formic acid] with the following linear gradient at 400 μl/min: 0–3 min, hold at 70% B; 3–15 min, 70% B to 95% B; 15–20 min, hold at 95% B; 20–21 min, 5% B to 70% B; 21–25 min, reequilibrate at 70% B. atRA was monitored using both the UV-Vis spectrophotometer at 350 nm and mass spectrometer (16). Quantification was based on the UV-Vis and HPLC-MS peak area of retinoic acid and comparison to a standard curve of known concentration. Because atRA is prone to isomerization, the standard curve also included 9-cis atRA and 13-cis atRA as controls. Samples from the supernatant were collected daily for 2 wk for atRA concentration measurement (n = 6).
Animal surgery.
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85-23, 1996) and approved by the Northwestern University Animal Care and Use Committee. Thirty 11-wk-old male Sprague Dawley rats weighing between 350 and 400 g underwent the carotid artery balloon injury model, as previously described (17, 28, 30). Animals were anesthetized with isoflurane (1.5–5%) and treated preoperatively with carprofen subcutaneously (0.15 mg/kg) for pain control. After injury and reestablishment of flow, a 10 mm × 10 mm × 2 mm portion of atRA-POC or POC membrane was measured and rehydrated in PBS for 15 min while being protected from light. The final volume after rehydration was 200 mm3. The membrane was then placed under the common carotid artery (CCA) and wrapped circumferentially 1.5 times around the CCA just inferior to the CCA bifurcation and extending proximally to the inferior border of the sternocleidomastoid muscle. The neck incision was closed in two layers, and carotid arteries were harvested 14 days after injury. Groups included injury alone, injury + POC membrane, injury + atRA-POC membrane, and no injury (n = 6 per group). At 2 wk, the carotid arteries were harvested. The animals were induced with 5% isoflurane via an anesthetic chamber and then maintained on 1.5–2.5% isoflurane via nose cone delivered through a nonrebreathable circuit. Once anesthetized, as evident by absence of response to toe pinch, the animals were euthanized via a bilateral thoracotomy. After in situ perfusion-fixation with PBS (250 ml) and 2% paraformaldehyde (500 ml) via a cannula inserted into the left ventricle, the carotid was exposed as previously described and harvested from the inferior border of the sternocleidomastoid muscle to just distal of the carotid bifurcation. The tissue was processed as previously described (17, 28, 30).
Morphometric analysis.
Specimens were examined histologically for evidence of neointimal hyperplasia using 5-μm hematoxylin and eosin (H and E)-stained cross-sections. Six equally spaced sections from within the area of injury were stained from each animal. Digital images of stained sections were collected with light microscopy using a Zeiss Imager-A2 microscope (Hallbergmoos, Germany). The luminal area, intimal area, medial area, arterial circumference, intima-to-media area ratio (I/M), percent of the arterial wall composed of the intima (intimal area/intimal area + medial area or I/I+M), and percent stenosis (intima area/intimal area + lumen area * 100) were determined using ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry.
The harvested carotid arteries were examined for evidence of inflammation using immunohistochemical staining against ED1, a marker for monocytes and macrophages, or vascular cell adhesion molecule 1 (VCAM-1), a cell adhesion protein that mediates the adhesion of various inflammatory cells to the vascular endothelium. Arteries were also assessed for proliferation via Ki67, a marker of proliferation. Briefly for ED1 staining, sections fixed with 2% paraformaldehyde were permeabilized with Triton X-100 in PBS for 20 min. Sections were then exposed to a primary antibody against ED1 (CD68, cat no. sc-59103; Santa Cruz Biotechnology, Santa Cruz, CA) diluted (1:50) in immunohistochemistry (IHC)-Tek for 1 h at room temperature (IHC-Tek diluent, pH 7.4, cat. no. 1W-1000; IHC World, Woodstock, MD) followed by PBS rinses and secondary antibody application (Alexa Fluor 555, goat anti-mouse IgG, 1:500 dilution, cat. no. A-21424; Invitrogen, Carlsbad, CA) in PBS. Nuclei were stained with DAPI in PBS (1:500, cat. no. D3571, Invitrogen), and the slides were rinsed in sterile water and coverslipped with ProLongGold (cat. no. P36930, Invitrogen). To assess proliferation, sections were exposed to a primary antibody against Ki67 (cat. no. ab15580; Abcam, Cambridge, MA) diluted 1:100 in IHC-Tek diluent overnight at 4°C, rinsed, and then exposed to the secondary antibody (Alexa Fluor 555, goat anti-rabbit IgG, 1:1,000 dilution, Invitrogen) in PBS for 1 h at room temperature. Nuclei were stained with DAPI in PBS (1:500, cat. no. D3571, Invitrogen), and the slides were rinsed in sterile water and coverslipped with ProLongGold (cat. no. P36930, Invitrogen). To assess VCAM-1 staining, endogenous peroxidases were blocked with Bloxall (cat. no. SP-6000; Vector Laboratories, Burlingame, CA). Sections were then exposed to primary antibody against VCAM-1 (cat. no. ab134047, Abcam) diluted 1:2,000 in IHC-Tek diluent for 1 h at room temperature followed by a 1-min PBS rinse and application of secondary biotinylated antibody (Rabbit ABC staining kit, 1:1,000 dilution, cat. no. PK-4002, Vector Laboratories) diluted in PBS for 1 h at room temperature. Diaminobenzidine (DAB) solution was then applied (cat. no. 3 SK4100, Vector Laboratories) for 5 min and counterstained with aqueous hematoxylin for 5 s. For all stained sections, digital fluorescent (ED1, KI67) or bright-field (VCAM-1) images were acquired using Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) on a Nikon Eclipse 50i Microscope (Nikon Instruments, Melville, NY). To quantify the staining, four equally spaced out sections per each artery and six arteries per treatment group were analyzed. Fluorescent images for Ki67 slides were graded qualitatively on a scale of 0 to 4, with 0 indicating no staining, 1 indicating minimal staining, 2 indicating mild staining, 3 indicating moderate staining, and 4 indicating intense staining. Detailed instructions and representative images of each grade were provided to three independent, blinded observers to guide their evaluation. The grades within and between reviewers were assessed for intra- and intergrader consistency. Once grading was completed, the slides were unblinded, and the difference in the mean grade for the intima, media, adventitia, and overall was analyzed with ANOVA on ranks. Images for VCAM-1 staining were converted to CMYK format using Adobe Photoshop CS, and the integrated density cumulatively and for the for each layer (intima, media, and adventitia) was measured using ImageJ software (NIH), using the method described by Pham et al. (26). Briefly, with the use of Adobe Photoshop CS, all images were changed to CMYK format with black generation set to maximum, and all but the yellow color channel was deleted. The inverse of this image was saved to create a black and white representation of each image. The integrated density for each image was then measured with Image J software. Similarly, ED1 fluorescent images were analyzed with Image J software measuring the integrated density of the red channel for each layer (intima, media, and adventia). These values were then summed to calculate the cumulative integrated density for each image.
TUNEL staining.
To assess atRA-POC effect cell apoptosis in vivo, the harvested rat carotid artery cross-sections were stained with the Dead End Colorimetric Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) system (Promega, Madison, WI), per the manufacturer's protocol. This process has previously been defined (30). Briefly, sections were washed in 0.85% NaCl, washed in 1× PBS, and fixed in 4% paraformaldehyde. The specimens were rinsed with 1× PBS, treated with 20 μg/ml proteinase K, washed in 1× PBS, and fixed in 4% paraformaldehyde again. The specimens were then washed in 1× PBS and covered with equilibration buffer, then covered with biotinylated nucleotide mix and a plastic coverslip, and incubated for 60 min at 37°C. The plastic coverslips were removed, and the end-labeling reaction was terminated by incubation in 2× sodium chloride-sodium citrate for 15 min at room temperature. The specimens were then washed three times in 1× PBS and blocked for endogenous peroxidases and developed with DAB chromogen, as described above. The sections were coverslipped with gevatol, and sections were digitally imaged as above. Eight cross-sections from each group were stained, and counts of the TUNEL-positive stained nuclei were collected for the intima, media, and adventitial layers; an overall cumulative score was also calculated.
Statistical analysis.
To determine the number of animals required in each treatment group to reach statistical significance, a sample size analysis was conducted using an α = 0.05, power of 0.8, difference in means of 0.2, and a standard deviation of 0.1. Analysis revealed that a sample size of six for each treatment group was required. All results are expressed as means ± SE. The difference between two groups was analyzed using Student's t-test, whereas the differences between multiple groups were analyzed using one-way ANOVA with the Student-Newman-Keuls post hoc test to assess all pairwise comparisons. Nonparametric data were analyzed using ANOVA on ranks (SigmaStat; SPSS, Chicago, IL). Statistical significance was assumed when P ≤ 0.05.
RESULTS
Effect of atRA on incorporation of [3H]-thymidine is dependent on concentration and cell type.
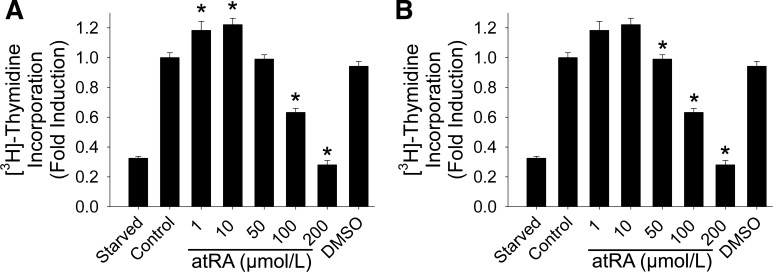
For rat VSMC, we observed a biphasic effect with a slight increase in [3H]-thymidine incorporation at lower concentrations of atRA but significant inhibition of uptake at higher concentrations. As seen in Fig. 1A, incorporation of [3H]-thymidine in VSMC increased by 18% and 22% in cells treated with 1 and 10 μmol/l atRA, respectively (P < 0.001), and decreased by 37% and 72% in cells treated with 100 μmol/l and 200 μmol/l atRA, respectively (P < 0.001, n = 4). For rat AF, we observed a trend toward an increase in [3H]-thymidine incorporation in cells treated with 1 μmol/l atRA, but this did not reach statistical significance. We did observe a dose-dependent decrease in [3H]-thymidine incorporation of 22%, 60%, and 78% in cells treated with 50 μmol/l, 100 μmol/l, and 200 μmol/l, respectively (P < 0.001, n = 5; Fig. 1B).
Fig. 1.
Effect of all-trans retinoic acid (atRA) on [3H]-thymidine incorporation is dependent on atRA concentration. [3H]-thymidine incorporation in vascular smooth muscle cells (VSMC) (A) and adventitial fibroblasts (AF) (B) was assessed following exposure to atRA (1–200 μmol/l) over 24 h (*P < 0.001 vs. control). We observed a maximum decrease in proliferation of 72% and 78% in VSMC and AF, respectively. Data are representative of 4–5 separate experiments (n = 8/treatment group). DMSO, dimethyl sulfoxide.
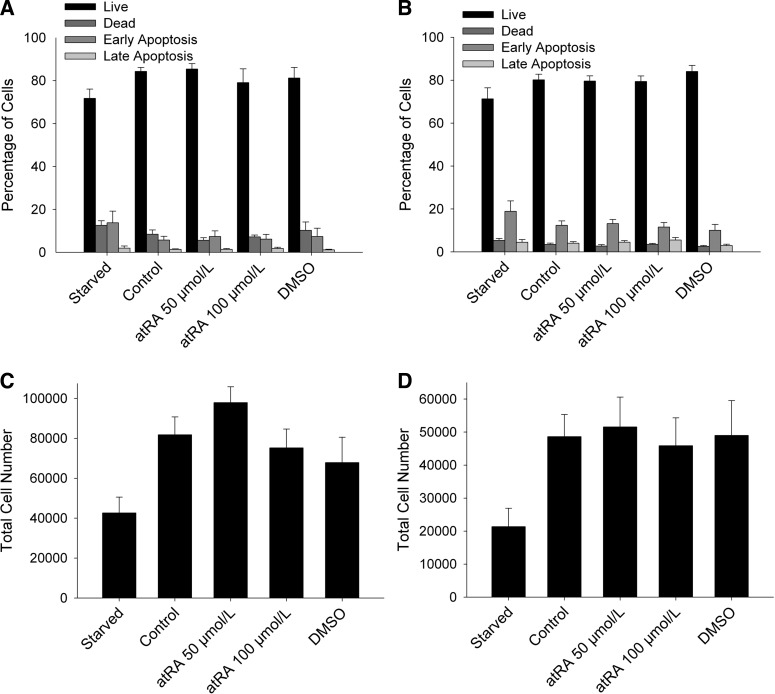
To determine whether the above findings were due to a decrease in cell number, an increase in cellular apoptosis or necrosis, or a combination thereof, we conducted similar experiments and assessed for early apoptosis, late apoptosis, necrosis, and overall cell number. The decrease in [3H]-thymidine incorporation was not associated with an increased rate of cell death or apoptosis (Fig. 2, A and B). VSMC and AF exposed to 50 and 100 μmol/l atRA exhibited minimal early apoptosis, late apoptosis, and necrosis. In fact, levels were similar across all treatment groups. Similarly, the percent of live cells was similar among all treatment groups. Lastly, we analyzed the effect of atRA on cell number. Surprisingly, although there was a trend toward fewer VSMC upon exposure to 100 μmol/l atRA, this did not reach statistical significance. No differences in cell numbers were observed in AF up to 100 μmol/l atRA. These data indicate that, although atRA readily reduces [3H]-thymidine incorporation in both VSMC and AF, there is no appreciable effect on cell number, apoptosis, or death; however, observations over longer periods of time (e.g., 48, 72, and 96 h) would likely demonstrate differences in cell number.
Fig. 2.
atRA induced negligible apoptosis and necrosis in VSMC and AF. VSMC (A) and AF (B) exposed to atRA (50–100 μmol/l) exhibited negligible early apoptosis, late apoptosis, and necrosis between the treatment groups. All treatment groups displayed a similar percentage of live cells. VSMC (C) and AF (D) exposed to atRA (50–100 μmol/l) showed no significant decrease in cell count over 24 h. Data are representative of 4 separate experiments (n = 3–5/treatment group).
Periadventitial POC membranes slowly release bioactive atRA for 2 wk in vitro.
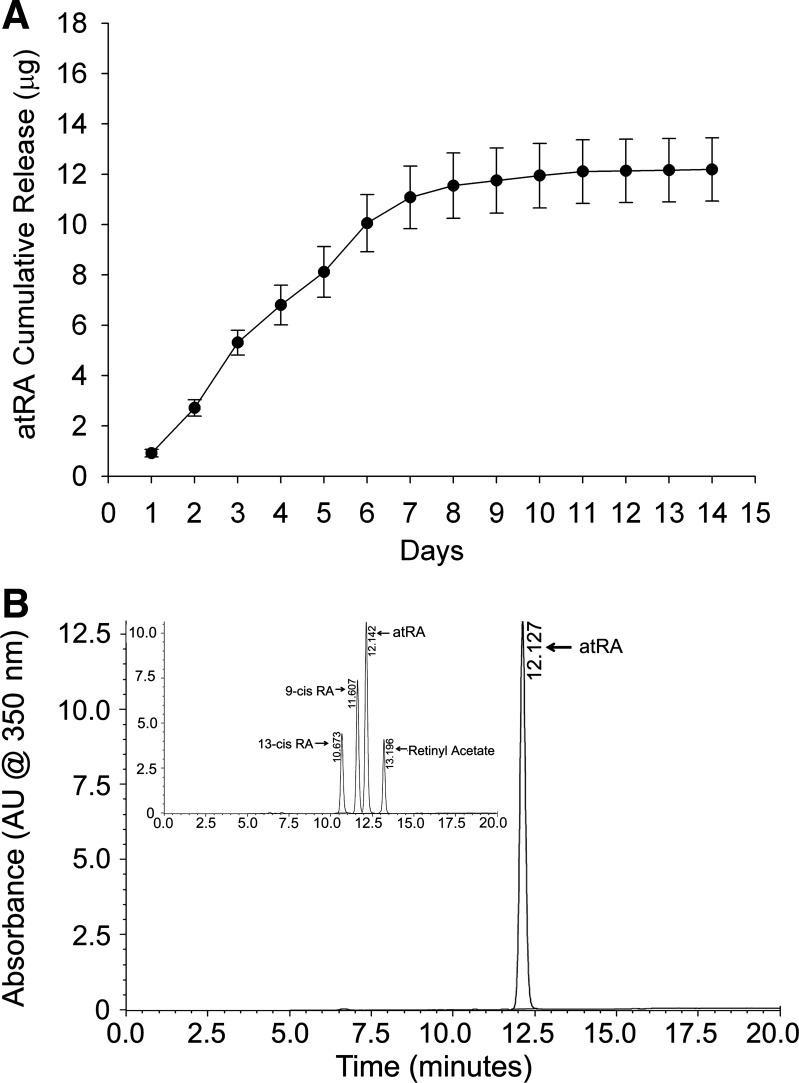
atRA (20 mg) was loaded onto POC membranes, which released ∼2 μg of atRA/day during the first week (Fig. 3A). The total cumulative release over a 2-wk time period was 12 μg, of which 92% of the release occurred in the first week. HPLC-MS analysis performed on the supernatant collected from the cumulative release experiments revealed that, when compared with the standard HPLC spectrum for all retinoic acid isomers (Fig. 3B, inset), atRA was the sole isomer released from the atRA-POC membrane (Fig. 3B).
Fig. 3.
atRA-eluting periadventitial poly(1,8 octanediolcitrate) (atRA-POC) discs released only atRA for 2 wk. A: release profile demonstrates that atRA-POC discs continued to release atRA for 2 wk with maximum release of 12 μg. B: comparison of high-performance liquid chromatography curves for various isomers of retinoic acid (RA, inset) with that of the supernatant from atRA-POC discs reveals that atRA is the primary isomer released.
atRA-POC membranes significantly inhibit the formation of neointimal hyperplasia after arterial injury.
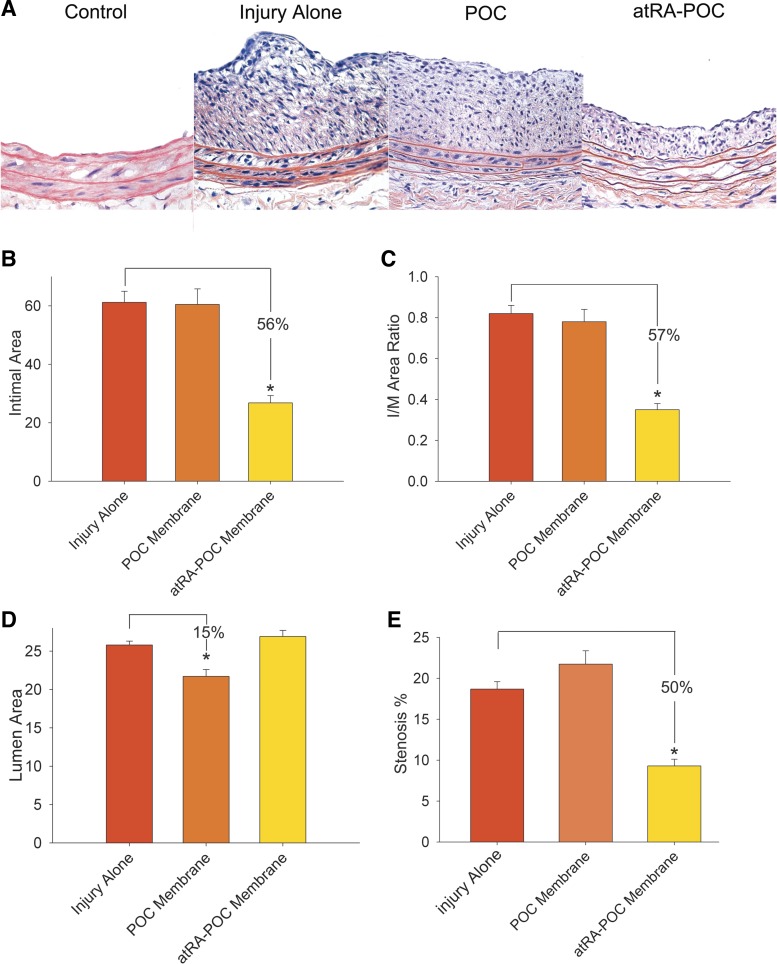
Balloon injury resulted in the formation of neointimal hyperplasia as confirmed by representative (H and E)-stained cross-sections (Fig. 4A). Only the arteries wrapped with atRA-POC showed a significant decrease in neointimal hyperplasia (Fig. 4A). Morphometric analysis of all samples revealed a 56% decrease in intimal area and 57% reduction in the I/M, in animals treated with the atRA-POC membrane compared with injury alone (P < 0.001, Fig. 4, B and C). Consistent with the inhibition of neointimal hyperplasia, we observed a 50% reduction in percent stenosis compared with injury alone (P < 0.001, Fig. 4E). We also observed a 15% decrease in luminal area and a corresponding 15% reduction in circumference in the animals receiving POC membranes alone (P < 0.001, Fig. 4D) compared with injury alone.
Fig. 4.
atRA-POC membranes significantly inhibited the formation of neointimal hyperplasia. A: representative hematoxylin and eosin staining of rat common carotid cross-sections. Quantification of the intimal area (B) and intima-to-media (I/M) area ratio (C) revealed significant decreases in injured rats treated with atRA-POC membranes, compared with injury alone (*P < 0.001). Quantification of lumen area (D) and percent stenosis (E) revealed that treatment with atRA-POC membranes decreased percent stenosis, whereas treatment with POC membranes decreased lumen area.
Treatment with atRA-POC membranes had no effect on proliferation or apoptosis at 2 wk.
To evaluate the effect of atRA-POC membranes on proliferation, we assessed rat CCA cross-sections stained for Ki67. Qualitative grading of these samples demonstrated no differences between treatment groups in the intimal, medial, or adventitial layers (data not shown). Similarly, assessment of carotid cross-sections for apoptosis using TUNEL revealed no differences in all layers (data not shown). These findings are not unexpected because the carotid arteries were harvested 2 wk after injury, a time point when little proliferation and apoptosis are still present.
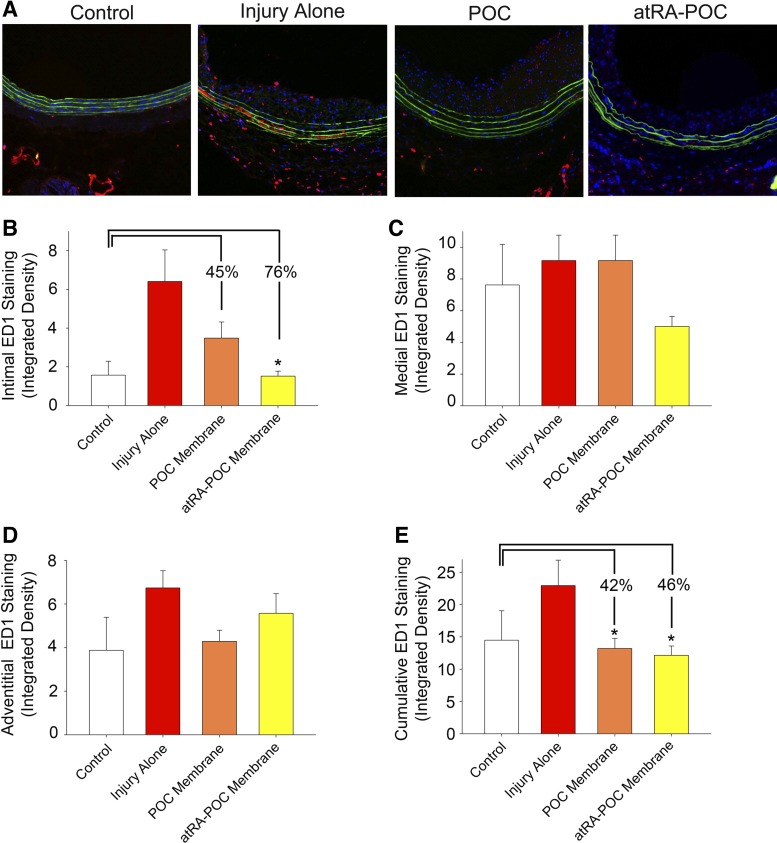
Treatment with atRA-POC membranes decreased macrophage infiltration and VCAM-1 staining in the intimal layer.
To assess the biocompatibility of the atRA-POC membranes, an assessment of macrophage infiltration was performed. Figure 5A shows representative ED1 staining of rat CCA cross-sections. Quantification of the measured integrated density of the positive staining revealed a 76% decrease in macrophage infiltration in the intimal layer in animals treated with the atRA-POC compared with injury alone (Fig. 5B, P = 0.003, n = 6). Although the decrease in intimal macrophage infiltration with the POC membrane did not reach statistical significance (P = 0.057), we observed a 46% and 42% significant decrease in cumulative macrophage infiltration in animals receiving either the atRA-POC and POC membranes compared with injury alone (Fig. 5E, P < 0.03, n = 6).
Fig. 5.
Treatment with atRA-POC membranes decreased macrophage infiltration in the intima layer and cumulatively, whereas treatment with POC membranes decreased cumulative macrophage infiltration. A: representative ED1 staining of rat common carotid artery cross-sections harvested at 2 wk following arterial injury. Quantification of the integrated density of the positive ED1 staining in the intima (B), media (C), adventitia layers (D), and cumulatively (E). Treatment with atRA-POC and POC membranes resulted in a 76% (*P < 0.003) and 45% (P < 0.057) decrease in ED1 intimal staining and a 46% and 42% (P < 0.03) decrease in the cumulative ED1 staining, respectively, compared with injury alone; n = 6 arteries/treatment group, 4 sections per artery. Red color represents ED1-positive staining.
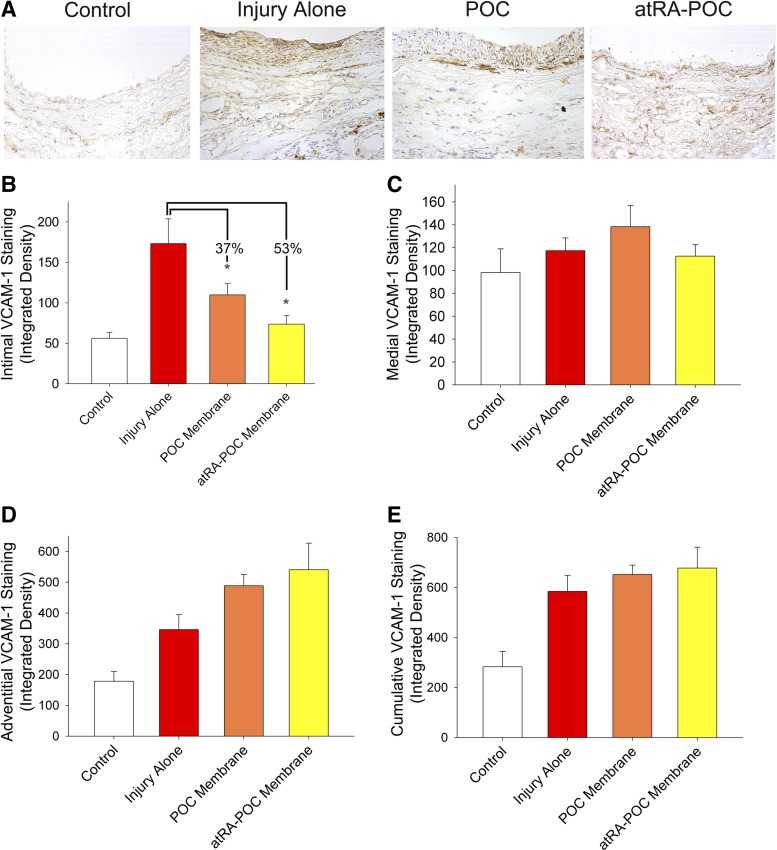
Figure 6A shows representative VCAM-1 staining of rat CCA cross-sections. Quantification of the measured integrated density revealed a 53% and 37% decrease in VCAM-1 intimal staining in animals treated with atRA-POC (P = 0.001, n = 6) and POC membranes (P = 0.018, n = 6), respectively, compared with injury alone.
Fig. 6.
Treatment with atRA-POC and POC membranes decreased vascular cell adhesion molecule 1 (VCAM-1) staining in the intimal layer. A: representative VCAM-1 staining of rat common carotid artery cross-sections harvested 2 wk following arterial injury. Quantification of the integrated density measured VCAM-1 staining in the intima (B), media (C), adventitia layers (D), and cumulatively (E). Treatment with atRA-POC and POC membranes resulted in a 53% (*P < 0.001) and 37% (*P < 0.018) decrease in integrated density in VCAM-1 intimal staining, respectively, compared with injury alone; n = 6 arteries/treatment group, 4 sections per artery. Brown color represents VCAM-1-positive staining.
DISCUSSION
Herein we show, for the first time, that localized periadventitial delivery of atRA via an atRA-POC membrane inhibits the development of neointimal hyperplasia in a rat carotid balloon injury model. Our in vitro studies suggest that atRA modulates proliferation in both a cell-specific and dose-dependent manner, without inducing apoptosis or cell death. For VSMC, a biphasic effect on [3H]-thymidine incorporation was observed; treatment with atRA at lower concentrations resulted in a slight increase in [3H]-thymidine incorporation, whereas higher concentrations showed a dose-dependent decrease in [3H]-thymidine incorporation. Although a similar pattern was observed for both VSMC and AF, the statistically significant difference in VSMC at lower concentrations suggests that atRA may exert cell-specific effects. In vitro evaluation of the atRA-POC membrane demonstrated that atRA was successfully loaded onto the membrane and released atRA slowly, mostly over the first week. In vivo experiments revealed a significant reduction in neointimal formation, as evidenced by reduced intimal area, I/M area ratio, and percent stenosis at 2 wk in animals treated with the atRA-POC periadventitial membrane compared with injury alone. atRA-POC membrane had no effect on proliferation or apoptosis at the 2-wk time point. Compared with injury alone, atRA-POC membranes resulted in a decrease in macrophage infiltration in the intimal layer with a corresponding decrease in VCAM-1 staining. In addition, both the POC and atRA-POC membranes decreased the cumulative macrophage infiltration compared with injury alone. Combined, these data confirm our hypothesis and strongly support the use of localized delivery of atRA to inhibit the formation of neointimal hyperplasia.
There has been considerable in vitro investigation exploring the use of atRA as an antiproliferative agent. As in previous in vitro studies, we demonstrated that atRA inhibits VSMC proliferation as measured by [3H]-thymidine incorporation, but the effects on proliferation and the atRA concentrations at which inhibition occurred differed. Whereas others have reported that treatment with atRA (10 nmol/l to 2 μmol/l) inhibited rat VSMC proliferation, we found a bimodal pattern, with atRA stimulating proliferation at lower concentrations (1–10 μmol/l) and inhibiting proliferation at higher concentrations (50–200 μmol/l) (15, 23, 24). Results of flow cytometry found that these differences were not attributable to an increase in cell death or apoptosis. Surprisingly, our analysis of total live cell number did not detect any significant decrease in cell number. Considering that the doubling time for rat VSMC and AF has been reported to be 19–33 h and that our experimental time points were 18–24 h, it is probable that we would have detected a decrease in cell number if longer time points were used (19). Use of [3H]-thymidine incorporation investigates cells that have incorporated thymidine into their new DNA but not necessarily divided; hence, this assay detects a decrease in the number of actively proliferating cells, not overall cell number. These findings support the notion that atRA is acting primarily as an antiproliferative agent rather than a cytotoxic agent. Prior in vivo investigation also supports our finding of a dose-dependent effect of atRA, as positive remodeling only occurred in animals receiving 10 mg/kg but not those receiving a lower (5 mg/kg) or higher dose (24 mg/kg) (7).
Several factors could account for the difference between our in vitro experiments and previously published results. Whereas others investigated the effect of atRA on platelet-derived growth factor (PDGF)-stimulated VSMC proliferation (23), we investigated the effects of atRA on proliferation in the absence of PDGF. Similarly, whereas one group investigated the effect of atRA on the proliferation kinetics of VSMC over 20 days (15), we evaluated the effect of atRA after a 24-h period. Although others observed inhibition at much lower concentrations of atRA, this decrease in proliferation was not apparent until day 5 (15). Another possible reason for these differences is that our study utilized [3H]-thymidine incorporation to assess proliferation, whereas others used electronic cell counting to evaluate cell number (15). Finally, we used rat VSMC from passages 3–9 for all in vitro proliferation experiments, whereas others used rat VSMC from passages 10–30 (23) or human aortic VSMC from passages 21–22 (15). Cells from these later passages most likely do not resemble the physiology of primary cells, as they have likely transformed over time. In addition, the doubling time for human aortic VSMC has been estimated to be 70–85 h, which is two to three times that of rat VSMC (19).
Our findings were similar to prior in vivo investigations utilizing either systemic or local endovascular delivery methods, but there were some notable differences. Studies employing a balloon withdrawal carotid artery injury model demonstrated that daily oral supplementation of 25 mg/kg of atRA for 14–21 days after injury inhibited neointimal formation by decreasing both intimal area and the I/M area ratio by ∼45% (7, 23). Similarly, treatment with atRA-POC membrane, which released a maximum 12 μg over 2 wk in vitro, resulted in a 56% reduction in intimal area and a 57% reduction in the I/M. Although other studies documented an increase in lumen area of 35% and an increase in arterial circumference of 15% with atRA administration 14–21 days after injury (7, 23), we did not observe a significant increase in luminal area or arterial circumference in animals treated with the atRA-POC membranes at 2 wk. However, we did observe a similar decrease in percent stenosis (50%) in animals treated with the atRA-POC membranes, as reported in the literature (44%) (7). More importantly, our results surpass the outcome observed in another study that utilized local endovascular delivery. Herdeg et al. (13) delivered 10 ml of atRA (10 μmol/l) via catheter infusion after carotid balloon injury in an atherosclerotic rabbit model and did not show a significant decrease in intimal area formation or percent stenosis. However, the authors did report a statistically significant increase in total vessel diameter. In contrast, we observed a 50% decrease in percent stenosis at 2 wk in animals treated with atRA delivered via the periadventitial POC membrane. Evaluation of proliferation and apoptosis at the 2-wk time point atRA-POC membranes had no impact on proliferation or apoptosis. This was most likely due to the very early time course of cellular apoptosis and proliferation after arterial injury (2).
The increased efficacy of atRA-POC membranes in vivo over systemic and other localized delivery methods described in prior literature can be accounted for. When delivered systemically, a much larger amount of atRA is required to generate useful tissue concentrations locally attributable to the volume of distribution. Furthermore, systemically delivered atRA is quickly bound by albumin and retinol-binding proteins in the serum and is then rapidly transferred to tissue stores in the liver (3). As we were able to demonstrate significant reduction in neointimal formation where another localized delivery method did not, the mode of local delivery may have played a role. Whereas Herdeg et al. (13) used endoluminal delivery to generate high localized concentrations, this was limited to a one-time dose of atRA delivery over an average of only 9.5 min and would be flushed out once blood flow is restored. Periadventitial delivery has the advantage that it avoids the binding of atRA to carrier proteins and allows atRA to diffuse directly into the arterial wall. Even if there is some distribution of atRA to tissue stores, continued release from the atRA membrane exposes the injured area to a source of atRA for a prolonged period of time vs. a one-time dosing. Although continued inhibition of neointimal hyperplasia may require longer treatment times, the release profile of the atRA-POC membrane can be modified by changing the ratio of citrate and 1,8-octanediol. Moreover, the efficacy of atRA at inhibiting neointimal hyperplasia suggests that this agent might be useful in other biomaterials, such as coatings for indwelling catheters or synthetic grafts to improve biocompatibility and long-term patency rates.
Another difference between published work and our results was in the impact that atRA has on aspects of vessel remodeling. Positive remodeling is associated with luminal and circumferential enlargement. Negative remodeling, which is one of the major contributors to restenosis after balloon angioplasty, reflects vessel contraction and may be mediated by the differentiation of AF from a secretory to contractile phenotype (myofibroblasts), resulting in an overall increase in proliferation of myofibroblasts (5). These myofibroblasts alter the matrix collagen composition and orientation in the adventitia and eventually undergo rapid apoptosis, resulting in scar-like tissue that contracts permanently and leads to decreased vessel diameter (5). In agreement with previously published work (7, 13, 23), we did not observe any evidence of negative remodeling. As atRA exerts effects on both cell differentiation and AF proliferation, it is likely that atRA delivery in our model is acting by preventing AF proliferation and preserving AF differentiation, preventing their transition to a myofibroblast phenotype. The effect of atRA on positive remodeling is not as clear. Whereas previous work showed significant increases in both lumen area and overall vessel circumference (7, 13, 23), we only found a trend toward an increase in these parameters. However, the reported effects on remodeling were concentration dependent in the report of DeRose et al. (7) and only observed at serum concentration levels of 92 ng/ml, a level that may not have been achieved with the current formulation of the atRA-POC membrane.
Another factor regarding the mode of delivery that may have influenced the effect of atRA on remodeling is the presence of the periadventitial membrane. Although we did not observe any significant increases in lumen area or circumference in animals treated with atRA-POC membrane, we did observe an unexpected 15% reduction in both lumen area and circumference in animals treated with the POC membrane. This suggests that the presence of the membrane around the vessel could create a physical limitation to the overall circumference by preventing vasodilation, which is known to have a major impact on both luminal area and vessel circumference (7, 23). It is important to note that, even if the physical presence of the membrane prevents increases in overall vessel circumference, this could be attenuated by further decreasing the elastic modulus of the membrane or increasing its degradation rate (33).
To begin to establish the biocompatibility of the atRA-POC membranes, carotid cross-sections were stained for ED1 to assess changes in macrophage infiltration, as these cells are important in both foreign body reaction and long-term biocompatibility (1). We observed a decrease in macrophage infiltration in the intimal layer of animals treated with atRA-POC membranes and a decrease in the cumulative macrophage infiltration in animals treated with either POC and atRA-POC membranes. Interestingly, in our initial investigation of POC, we demonstrated that POC-coated ePTFE grafts had less macrophage infiltration into the intima and graft material compared with control ePTFE grafts (32). To establish a potential mechanism for this relative decrease in macrophage infiltration, we performed VCAM-1 staining. Compared with injury alone, VCAM-1 intimal staining in the atRA-POC and POC treatment groups exhibited a similar pattern to our ED1 staining, suggesting that the decrease in macrophage infiltration may be related to the decrease in VCAM-1 expression. These data may suggest that atRA, POC, or one of its biodegradable components exerts an anti-inflammatory effect. Prior work by Zhang et al. (35) also demonstrated that treatment with atRA resulted in decreased intimal monocyte and macrophage infiltration in a rat cardiac allograft vasculopathy model. Although inflammation does play a role in the arterial injury response, it is time dependent. Immediately following arterial injury, platelets and inflammatory cells adhere to the site of injury, and inflammatory cells begin to migrate into the tissue (11). These cells release cytokines and growth factors that stimulate VSMC and fibroblast proliferation and migration (6). Studies have demonstrated that administration of anti-inflammatory agents inhibit the development of neointimal hyperplasia (34). Lacking a clear mechanism and time course, the impact of this anti-inflammatory effect of atRA-POC on intimal formation is unclear but warrants further investigation. Given the large volume of data on the antiproliferative effects of atRA on VSMC both in vitro and in vivo, this remains the most likely mechanism by which atRA-POC membranes decrease neointimal hyperplasia. What can be concluded from our ED1 and VCAM-1 investigations is that this anti-inflammatory effect of atRA-POC and POC membranes is favorable and suggests that the material is biocompatible (1).
There are admittedly some limitations of our study. Regarding the in vitro work, the effect of proliferation of atRA was examined only for a period of 18–24 h, which may not have been an adequate time point for cell doubling time. We also did not use any additional growth factors to promote proliferation above that of serum. Although this is a simplified environment, both our in vitro and in vivo investigations demonstrate that, similar to prior studies, atRA is an effective antiproliferative agent that effectively inhibits neointimal hyperplasia in vivo. Regarding the in vivo work, one weakness in our study is the inability to precisely quantify the amount of atRA delivered to the artery. Another limitation is that we characterized the effect of atRA on proliferation and apoptosis at the 2-wk time point. It has been established in the literature that, to characterize proliferation and apoptosis after arterial injury, early time points ranging from 1 h to 7 days should be utilized. The 2-wk time course is traditionally utilized to assess neointimal formation. Given that our primary end point of interest was to evaluate neointimal hyperplasia, with the existing published literature, we did not pursue the earlier time points. A final limitation and an area of future investigation is to elucidate the anti-inflammatory mechanisms of the atRA-POC and POC membranes, as this could greatly impact their applications to other biomaterial approaches.
In summary, we have demonstrated that atRA-POC membranes effectively inhibit neointimal formation. Furthermore, we demonstrated the biocompatibility of atRA-POC membranes, as evidenced by decreased inflammation. Combined, these findings support localized delivery of atRA as a therapy to inhibit the formation of neointimal hyperplasia after vascular injury that is associated with open cardiovascular procedures.
GRANTS
This work was supported by funding from the National Institutes of Health (K08HL084203, M. Kibbe; T32 HL094293-01, E. Gregory; 1R43HL096217-01A1), the Society for Vascular Surgery Foundation, the Northwestern Memorial Foundation Collaborative Development Initiative (the Center for Limb Preservation), and by the generosity of Mrs. Hilda Rosenbloom and Mrs. Eleanor Baldwin.
DISCLOSURES
Drs. Ameer, Webb, and Kibbe have ownership in VesselTek BioMedical, the recipient of grant 1R43HL096217-01A1.
AUTHOR CONTRIBUTIONS
Author contributions: E.K.G., A.R.W., J.M.-V., and M.E.F. performed experiments; E.K.G., A.R.W., J.M.-V., and M.E.F. analyzed data; E.K.G. and A.R.W. interpreted results of experiments; E.K.G. and A.R.W. prepared figures; E.K.G. drafted manuscript; E.K.G., A.R.W., J.M.-V., G.A.A., and M.R.K. edited and revised manuscript; E.K.G., A.R.W., J.M.-V., M.E.F., G.A.A., and M.R.K. approved final version of manuscript; A.R.W., G.A.A., and M.R.K. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Lynnette Dangerfield for administrative support and Edwards Lifesciences for providing the Fogarty balloon catheters.
REFERENCES
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 20: 86–100, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelini A, Visonà A, Calabrese F, Pettenazzo E, Yacoub A, Valente M, Bonandini EM, Jori G, Pagnan A, Thiene G. Time course of apoptosis and proliferation in vascular smooth muscle cells after balloon angioplasty. Basic Appl Myol 12: 33–41, 2002. [Google Scholar]
- 3.Cifelli CJ, Ross AC. All-trans-retinoic acid distribution and metabolism in vitamin A-marginal rats. Am J Physiol Gastrointest Liver Physiol 291: G195–G202, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 49: 327–333, 1983. [PubMed] [Google Scholar]
- 5.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 31: 2391–2396, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg 81: 1254–1269, 1994. [DOI] [PubMed] [Google Scholar]
- 7.DeRose JJ, Madigan J, Umana JP, Prystowsky JH, Nowygrod R, Oz MC, Todd GJ. Retinoic acid suppresses intimal hyperplasia and prevents vessel remodeling following arterial injury. Cardiovasc Surg 7: 633–639, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Douer D, Zickl LN, Schiffer CA, Appelbaum FR, Feusner JH, Shepherd L, Willman CL, Bloomfield CD, Paietta E, Gallagher RE, Park JH, Rowe JM, Wiernik PH, Tallman MS. All-trans retinoic acid and late relapses in acute promyelocytic leukemia: Very long-term follow-up of the North American Intergroup Study I0129. Leuk Res 37: 795–801, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favennec L, Cals MJ. The biological effects of retinoids on cell differentiation and proliferation. J Clin Chem Clin Biochem 26: 479–489, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Fenaux P, Wang ZZ, Degos L. Treatment of acute promyelocytic leukemia by retinoids. Curr Top Microbiol Immunol 313: 101–128, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Fingerle J, Johnson R, Clowes AW, Majesky MW, Reidy MA. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci USA 86: 8412–8416, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamon M, Bauters C, McFadden EP, Wernert N, Lablanche JM, Dupuis B, Bertrand ME. Restenosis after coronary angioplasty. Eur Heart J 16, Suppl I: 33–48, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Herdeg C, Oberhoff M, Baumbach A, Schroeder S, Leitritz M, Blattner A, Siegel-Axel DI, Meisner C, Karsch KR. Effects of local all-trans-retinoic acid delivery on experimental atherosclerosis in the rabbit carotid artery. Cardiovasc Res 57: 544–553, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Holmes DR., Jr In-stent restenosis. Rev Cardiovasc Med 2: 115–119, 2001. [PubMed] [Google Scholar]
- 15.Johst U, Betsch A, Wiskirchen J, Schober W, Vonthein R, Rinkert N, Kehlbach R, Claussen CD, Duda SH. All-trans and 9-cis retinoid acids inhibit proliferation, migration, and synthesis of extracellular matrix of human vascular smooth muscle cells by inducing differentiation in vitro. J Cardiovasc Pharmacol 41: 526–535, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem 80: 1702–1708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg 47: 173–182, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, Aalami OO, Jiang Q, Webb AR, Yang J, Carroll T, Ameer GA. Citric acid-based elastomers provide a biocompatible interface for vascular grafts. J Biomed Mater Res A 93: 314–324, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Kirschenlohr HL, Metcalfe JC, Weissberg PL, Grainger DJ. Adult human aortic smooth muscle cells in culture produce active TGF-β. Am J Physiol Cell Physiol 265: C571–C576, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg 27: 357–362, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Klinkert P, Schepers A, Burger DH, van Bockel JH, Breslau PJ. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg 37: 149–155, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Marks DS, Vita JA, Folts JD, Keaney JFJ, Welch GN, Loscalzo J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J Clin Invest 96: 2630–2638, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, Blaner WS. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation 98: 1219–1227, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Miano JM, Topouzis S, Majesky MW, Olson EN. Retinoid receptor expression and all-trans retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation 93: 1886–1895, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, Aalami OO, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Kibbe MR. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med 44: 73–81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham NA, Morrison A, Schwock J, Aviel-Ronen S, Iakovlev V, Tsao MS, Ho J, Hedley DW. Quantitative image analysis of immunohistochemical stains using a CMYK color model. Diagn Pathol 2: 8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 125: e2–e220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano MC, Vavra AK, Jen M, Hogg ME, Murar J, Martinez J, Keefer LK, Ameer GA, Kibbe MR. Poly(diol-co-citrate)s as novel elastomeric perivascular wraps for the reduction of neointimal hyperplasia. Macromol Biosci 11: 700–709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 6: 345–364, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Tsihlis ND, Murar J, Kapadia MR, Ahanchi SS, Oustwani CS, Saavedra JE, Keefer LK, Kibbe MR. Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury. J Vasc Surg 51: 1248–1259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiegman PJ, Barry WL, McPherson JA, McNamara CA, Gimple LW, Sanders JM, Bishop GG, Powers ER, Ragosta M, Owens GK, Sarembock IJ. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: a favorable effect on vessel remodeling. Arterioscler Thromb Vasc Biol 20: 89–95, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA. Modulating expanded polytetrafluoroethylene vascular graft host response via citric acid-based biodegradable elastomers. Adv Mater 18: 1493, 2006. [Google Scholar]
- 33.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials 27: 1889–1898, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Chen J, Yang J, Xu CW, Pu P, Ding JW, Jiang H. Resveratrol attenuates oxidative stress induced by balloon injury in the rat carotid artery through actions on the ERK1/2 and NF-kappa B pathway. Cell Physiol Biochem 31: 230–241, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Wu Q, Shui C. All-trans retinoic acid attenuates cardiac allograft vasculopathy in rats. Transplant Proc 42: 1895–1898, 2010. [DOI] [PubMed] [Google Scholar]