Abstract
Cardiac tissue from female rainbow trout demonstrates a sex-specific preference for exogenous glucose and glycolysis, impaired Ca2+ handling, and a greater tolerance for hypoxia and reoxygenation than cardiac tissue from male rainbow trout. We tested the hypothesis that dichloroacetate (DCA), an activator of pyruvate dehydrogenase, enhances cardiac energy metabolism and Ca2+ handling in female preparations and provide cardioprotection for hypoxic male tissue. Ventricle strips from sexually immature fish with very low (male) and nondetectable (female) plasma sex steroids were electrically paced in oxygenated or hypoxic Ringer solution with or without 1 mM DCA. In the presence of 5 mM glucose, aerobic tissue from male trout could be paced at a higher frequency (1.79 vs. 1.36 Hz) with lower resting tension and less contractile dysfunction than female tissue. At 0.5 Hz, DCA selectively reduced resting tension below baseline values and lactate efflux by 75% in aerobic female ventricle strips. DCA improved the functional recovery of developed twitch force, reduced lactate efflux by 50%, and doubled citrate in male preparations after hypoxia-reoxygenation. Independent of female sex steroids, reduced myocardial pyruvate dehydrogenase activity and impaired carbohydrate oxidation might explain the higher lactate efflux, compromised function of the sarcoplasmic reticulum, and reduced mechanical performance of aerobic female tissue. Elevated oxidative metabolism and reduced glycolysis might also underlie the beneficial effects of DCA on the mechanical recovery of male cardiac tissue after hypoxia-reoxygenation. These results support the use of rainbow trout as an experimental model of sex differences of cardiovascular energetics and function, with the potential for modifying metabolic phenotypes and cardioprotection independent of sex steroids.
Keywords: cardiac, dichloroacetate, glucose, hypoxia, lactate, rainbow trout, sex differences
biological sex and sex steroid hormones affect cardiovascular function and disease in humans and other mammals (9, 46). For example, premenopausal women are more resistant to ischemic heart disease than men of similar age (45), and the female advantage is lost after the onset of menopause (42). Not surprisingly, sex steroids have been hypothesized to help define sex differences in the heart and outcomes associated with cardiac disease. Although estrogen is generally considered cardioprotective and testosterone is viewed as detrimental to mammalian heart function, a mechanistic understanding of sex steroid benefits and impacts on pathophysiological outcomes is lacking (9).
Experimental studies using rodent models have also provided evidence for sex-related differences in myocardial responses to ischemia and reperfusion. Hearts from young adult female rats are generally less susceptible to postischemic contractile dysfunction than age-matched male hearts (2, 8, 47), and experimental removal of systemic estrogen via ovariectomy is associated with poor recovery of contractile recovery after an ischemic insult (74). Specific mechanisms for sex-based differences in cardiovascular responses to acute ischemic events remain to be defined but may be modulated by systemic levels of estrogen (16), possibly through actions on cardiomyocyte Ca2+ handling (57). Sex differences in cardiac excitation-contractile (E-C) coupling, sarcoplasmic reticulum (SR) Ca2+ release, and Ca2+ transients have been reported in normal rat ventricular myocytes, with female myocytes having lower E-C coupling gain, less SR Ca2+ release, and smaller Ca2+ transients than male myocytes (26).
Sex differences in preischemic and postischemic contractile function may also be directly related to myocardial substrate utilization and different patterns of glucose catabolism. In mammals, exogenous glucose provides beneficial effects on electromechanical cardiac function during and after recovery from hypoxia (19). Glycolysis is elevated in female nonhypertrophied hearts compared with male nonhypertrophied hearts, whereas glucose oxidation is lower in female versus male hearts (59). The extent to which glucose is metabolized oxidatively versus nonoxidatively appears to be a key determinant of postischemic myocardial function, with the recovery of heart function after ischemia inversely related to rates of nonoxidative glycolysis (60). Moreover, stimulation of glucose oxidation and/or reduction of glycolysis improve function of ischemia-reperfused hypertrophied rat hearts (58, 71).
The majority of laboratory animal studies on sex-dependent differences in the cardiovascular system have used rodents; however, there is a growing appreciation that sex differences in the cardiovascular system are not confined to mammals. Using fish as experimental models can provide novel insights because of their evolutionary significance, diverse physiological ecology, and cardiac plasticity (32, 55). With almost 30,000 species, fishes show the highest diversity among vertebrates, and mammals and fishes share common morphological, physiological, and pathological features in their cardiovascular systems. Rainbow trout (Oncorhynchus mykiss) are of particular interest because these fish exhibit 1) selective hypertrophy of the single ventricle associated with increasing androgens (18) and development of systemic hypertension in males during sexual maturation (15), 2) coronary arteriosclerosis in both sexes (23), 3) proportional growth of cardiac capillaries in male rainbow trout in response to physiological ventricular hypertrophy (13), and 4) hyperplastic cardiac growth that accompanies somatic growth (24) and therefore a sustained capacity for cardiac regeneration (5). Cardiac function in healthy rainbow trout requires aerobic metabolism for ATP production, and the rainbow trout heart is well endowed with mitochondria (14). Similar to mammalian cardiac muscle, the glycolytic pathway is also important in the hypoxia-intolerant rainbow trout heart to support mechanical performance under aerobic conditions (35).
Our recent studies have highlighted metabolic and functional sex differences in hearts of rainbow trout that are similar to mammalian sex differences, and yet the differences between male and female rainbow trout may not be tied directly to circulating sex steroids. Cardiac tissue from sexually immature female rainbow trout prefers aerobic glycolysis and exogenous glucose for ATP production compared with cardiac tissue from male rainbow trout (7), which stores more endogenous glycogen (37) and can release more Ca2+ from their sarcoplasmic reticulum (SR) (7). Female ventricle strips do not maintain contractile force or resting tension in vitro under aerobic conditions as well as male preparations, whereas male preparations do not preserve contractile function as well as female preparations after hypoxia and reoxygenation (7). Inhibition of the glycolytic pathway during aerobic incubations of rainbow trout ventricle strips results in incomplete relaxation, increased resting force, and decreases in twitch force (22, 35). However, compared with the mammalian heart, little is known about the relationship between anaerobic and aerobic energy metabolism in fish hearts. Alterations in myocardial glucose oxidation via mitochondrial pyruvate dehydrogenase [PDH; Enzyme Commission classification no. (EC) 1.2.4.1], the rate-limiting enzyme for glucose oxidation, might explain fundamental sex differences in ventricular energy metabolism and contractile function in rainbow trout.
One pharmacological strategy to improve mammalian cardiac function during aerobic reperfusion after ischemia and increase cardiac efficiency has been to stimulate PDH with dichloroacetate (DCA, an inhibitor of PDH kinase, EC 2.7.11.2), and increase the rates of myocardial glucose and pyruvate oxidation while decreasing lactate production and fatty acid oxidation (44, 49, 66, 68). To explore the potential for using sexually immature rainbow trout as an experimental model of sex-related differences in cardiovascular physiology, we reexamined the functional limitations of isolated cardiac tissue from female rainbow trout and tested the hypothesis that acute stimulation of PDH with DCA normalizes Ca2+ homeostasis in aerobic female preparations and increases hypoxia tolerance and cardioprotection in male preparations.
MATERIALS AND METHODS
Experimental animals.
Ten-month-old sexually immature male and female rainbow trout weighing ∼250–300 g were obtained from Clear Springs Foods (Buhl, ID), transported to the Aquatics Research Facility at Idaho State University (Pocatello, ID), and held in 1,000-liter circular tanks containing dechlorinated, filtered, UV-sterilized water at 14 ± 1°C. Fish were fed commercial trout pellets (45% protein, 20% carbohydrate, and 20% fat, dry weight basis), 1% of body weight every other day between 1500 and 1700 hours, exposed to a constant 12:12-h light-dark photoperiod, and held for 1–2 wk before experiments. All experiments were conducted in accordance with the National Institutes of Health guidelines (NIH Pub. No. 78-23, 1978), and were approved by the Animal Welfare Committee of Idaho State University.
Ventricle strip preparation for contractile and biochemical measurements.
Individual fish of unknown sex were netted rapidly and euthanized by blunt trauma to the head. Ventricles were excised, weighed, and placed in ice-cold, freshwater teleost Ringer solution modified from Hoar and Hickman (40) containing (in mM) 111 NaCl, 5 KCl, 0.5 NaH2PO4, 10 NaHCO3, 1.5 CaCl2, 1.0 MgSO4, and 5.0 glucose and equilibrated with 99.5% O2-0.5% CO2 at pH 7.6 at 14°C. Unless noted otherwise, chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were of analytic grade. The concentrations provided represent final concentrations during incubations. A portion (∼50 mg) of each ventricle was also frozen immediately using aluminum clamps precooled in liquid nitrogen and stored at −80°C. The sex of each fish was determined by visual or microscopic examination of the gonads, and the gonads were weighed.
Uniform strips (weighing ∼15–25 mg, 4–5 mm long × 0.7–1.0 mm wide) containing both compact and spongy layers were cut from each ventricle using a single-edge razor blade. Each strip was vertically mounted, tied at its base and apex with 3-0 surgical silk, and attached to an isometric force transducer (model ISDB-4, World Precision Instruments, Sarasota, FL) between platinum electrodes. Strips were suspended in 25-ml water-jacketed organ baths maintained at 14°C and containing oxygenated (99.5% O2-0.5% CO2) Ringer solution for 60 min (equilibration period). Po2 was measured in tissue baths using a microrespiration cell (model RC200, Strathkelvin, Glasgow, UK) and calibrated Clark-type electrode (model 1302). Each ventricle strip was used for only one experiment. Ventricle strips were field stimulated with a voltage that elicited full contraction (40–60 V, 2- to 3-fold higher than threshold) at a physiological frequency (0.5 Hz) with 5-ms square wave pulses (Grass S88 Stimulator, Grass Medical Instruments, Quincy, MA). The length of each strip was increased gradually to a value where maximum isometric force production was achieved (Lmax), and muscle length was then reduced to 90% Lmax to avoid damage to the preparation. After equilibration, contracting ventricle strips in selected experiments were exposed to fresh Ringer solution without 5 mM glucose for another 60 min (incubation period) to determine the specific effects of glucose and/or pharmacological compounds. Unlike isolated mammalian papillary muscle, which has a parallel fiber arrangement, the ventricle strip from rainbow trout has a heterogeneous fiber orientation. Measurements of isometric twitch force [F; or developed force (in mN)] and resting tension [RT (in mN)] in rainbow trout ventricle strips were therefore normalized to percentages of baseline values obtained after the initial stabilization period, which is consistent with other laboratories (6, 36, 64). Baseline F, set as 100% original twitch force, was established after 60 min and defined as time 0. Contractile performance was assessed by measuring F, RT, time to peak force (tp), and time to 80% relaxation (t0.8r) using a data-acquisition system (BioPac MP100, BIOPAC, Santa Barbara, CA) and software (AcqKnowledge, version 3.8.2, BIOPAC). We also measured the postrest potentiation (PRP) of F at the end of selected experiments to assess SR Ca2+ storage and subsequent release (21).
Maximum contraction frequencies under aerobic conditions.
To increase the energy demands of cardiac tissue and establish limits for mechanical performance, oxygenated ventricle strips from male and female rainbow trout were electrically paced at increasing stimulation frequencies. After equilibration in Ringer solution with 5 mM glucose at 0.5 Hz for 60 min, preparations were exposed to fresh Ringer solution with or without glucose and allowed to contract for another 60 min. Baseline F was set as 100% after 120 min and established the beginning time for experiments. Strips were then stimulated at 0.2–2.0 Hz, with frequency changed every 15 min. Frequency thresholds were defined as the minimum frequency at which mechanical alternans (i.e., alterations of large and small contractions) was observed. We also examined whether the SR was affected at different contraction frequencies by pretreating ventricle strips with ryanodine (10 μM) for 15 min after the equilibration period, before frequency testing, and PRP was measured at each frequency. A previous study (34) on rainbow trout cardiac tissue demonstrated that this concentration of ryanodine reduces the contribution of the SR to E-C coupling. The exposure time of tissue to ryanodine before PRP measurements was 75 min for 0.2 Hz and between 150 and 180 min for the highest stimulation frequencies.
Acute effects of DCA on cardiac performance during different oxygenation states.
Although it is unlikely that DCA will gain extensive clinical use, it has been a valuable research tool for elucidating the benefits of increased pyruvate oxidation on ischemic and reperfused mammalian hearts (67). Based on preliminary experiments using 0.25–2 mM DCA and 15- to 60-min incubation times (n = 4; data not shown), we chose a DCA concentration of 1 mM and preincubation time of 15 min to maximize DCA effectiveness in rainbow trout cardiac tissue at the lowest possible concentration and exposure time. After equilibration, ventricle strips were either pretreated with DCA dissolved in Ringer solution to stimulate PDH or sodium cyanide [NaCN; 2 mM in Ringer solution, pH adjusted to 7.6 with HCl (38)] to maximally stimulate anaerobic glycolysis for 15 min followed immediately by the addition of 5 mM glucose. While the present study did not focus on the impacts of exogenous glucose on cardiac function, glucose was used in conjunction with DCA for comparative purposes and metabolic insights. Controls remained either glucose free or received 5 mM glucose and contracted for another 60 min under aerobic conditions. We also evaluated the acute effects of DCA or NaCN on ventricle strips exposed to hypoxia (99.5% N2-0.5% CO2, Po2: 10–20 mmHg) for 30 min followed by reoxygenation for 30 min. All experiments with hypoxia and reoxygenation were conducted with 5 mM glucose present. At the end of experiments, a sample of Ringer solution was collected, deproteinized [1:1 (vol/vol) with 6% HClO4], and ventricle strips were freeze clamped at the temperature of liquid N2. Samples were stored at −80°C until media lactate (30) and tissue citrate (17) were measured. The release of lactate from the perfused rainbow trout heart (4), much like the rat heart (43), parallels rates of nonoxidative glycolysis. Conversely, an increase in cytosolic citrate from mitochondria can inhibit glycolysis in rat hearts (33).
Circulating sex steroids.
Heparinized blood samples (10 U/ml) were taken from the caudal vessels immediately after euthanasia, and plasma concentrations of testosterone, 11-ketotestosterone (11-KT), and 17β-estradiol (E2) were determined by radioimmunoassy following the procedure of Sower and Schreck (65) as modified by Fitzpatrick et al. (29). The sensitivities of the assays were as follows: 0.63 ng/ml for E2 and testosterone and 1.26 ng/ml for 11-KT. All plasma samples were kept frozen at −80°C, and a single set of steroid extractions and assays was conducted.
Statistical analysis.
Data are expressed as means ± SE of absolute values or percent changes (F, RT and PRP). One independent data point per ventricle strip was taken for all measurements. Variables reflecting cardiac performance (F, RT, tp, and t0.8r) were recorded every 5 min after equilibration and were averaged for five consecutive waveforms. Comparisons between sexes were made using Student's t-test (physical characteristics, lactate efflux, and tissue citrate), and effects of sex and treatment were analyzed using univariate ANOVA (DCA and NaCN experiments) or repeated-measures ANOVA (frequency trials) with a Tukey-Kramer post hoc test using SAS software (version 9.1.3, Cary, NC). Data were log transformed when normality was not achieved. The corresponding figures and tables reflect nontransformed raw data for increased clarity. P values of <0.05 were considered statistically significant.
RESULTS
Physical and hormonal characteristics of experimental animals.
Body mass, fork length, ventricle mass, relative ventricle mass, gonad mass, and gonadosomatic index (GSI) were not different between male and female rainbow trout (Table 1). The small size of testes and ovaries (GSI < 0.5%) suggested that male and female rainbow were sexually immature. This observation was confirmed by measurements of circulating sex steroids. For a subset of female rainbow trout (n = 12), testosterone, 11-KT, and E2 were undetectable (<1.3 ng/ml) in plasma. Male rainbow trout (n = 15) had very low levels of plasma testosterone (2.9 ± 0.6 ng/ml) and 11-KT (4.1 ± 0.9 ng/ml) and undetectable E2.
Table 1.
Physical characteristics of experimental rainbow trout
| Variables | Male Rainbow Trout | Female Rainbow Trout |
|---|---|---|
| Number of fish/group | 34 | 44 |
| Body mass, g | 319 ± 15 | 294 ± 12 |
| Fork length, cm | 28.8 ± 0.5 | 28.3 ± 0.4 |
| Ventricle mass, mg | 332 ± 24 | 298 ± 14 |
| Relative ventricle mass, % | 0.104 ± 0.004 | 0.101 ± 0.003 |
| Gonad mass, g | 1.38 ± 0.61 | 0.43 ± 0.06 |
| Gonadosomatic index, % | 0.38 ± 0.16 | 0.14 ± 0.02 |
Values are means ± SE. Relative ventricle mass = (ventricle mass/body mass) × 100; gonadosomatic index = (gonad mass/body mass) × 100. Physical characteristics were not different between male and female rainbow trout.
Male ventricle strips can be paced at a higher frequency than female ventricle strips with less dysfunction.
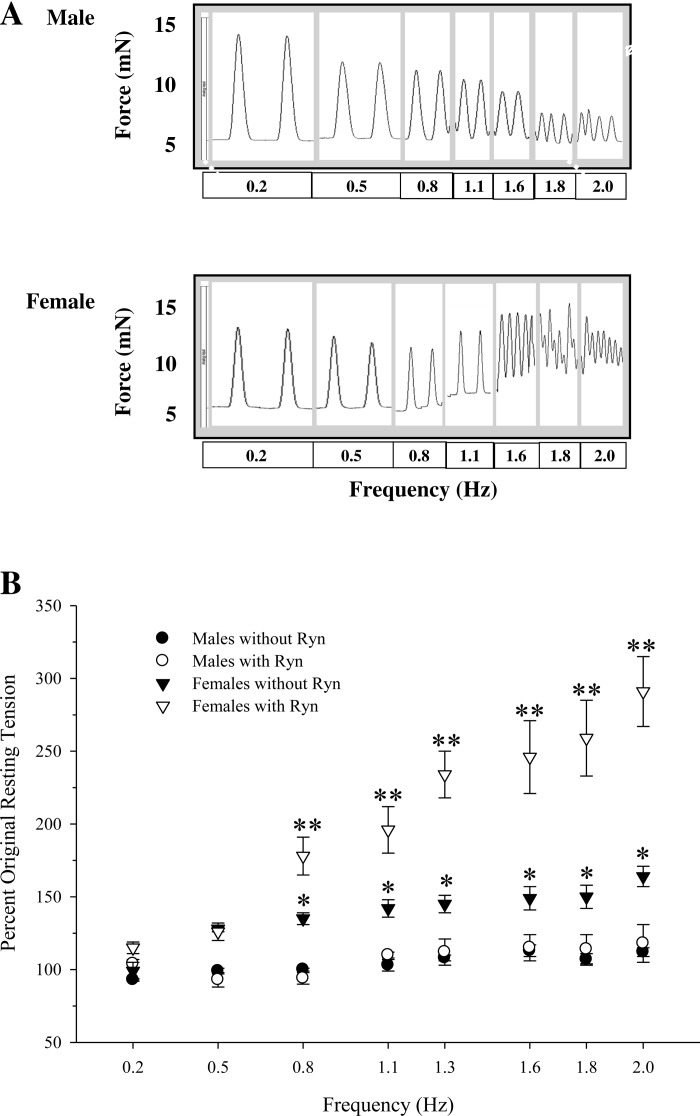
As shown in Fig. 1A and Table 2, F decreased at 0.5 Hz compared with 0.2 Hz and as stimulation frequency was increased above 0.8 Hz (P < 0.001 by repeated-measures ANOVA), illustrating the well-established negative force-frequency relation in fishes (62). The frequency threshold for mechanical alternans was higher for male preparations (1.79 ± 0.04 Hz) versus female preparations (1.36 ± 0.02 Hz, P = 0.02 by repeated-measures ANOVA). RT was selectively increased in female ventricle strips with increasing stimulation frequency (P < 0.01 by repeated-measures ANOVA; Fig. 1B), and pretreatment with ryanodine further increased RT in female preparations (P < 0.01 by repeated-measures ANOVA). For male and female ventricle strips, PRP increased with increasing stimulation frequencies, and male ventricle strips had higher PRP values at 0.2 and 0.5 Hz than female ventricle strips (P < 0.05 by repeated-measures ANOVA). For both sexes, ryanodine reduced PRP at 0.2 Hz and 0.5 Hz (P < 0.05 by repeated-measures ANOVA) and increased t0.8r at frequencies of ≥0.8 Hz (P < 0.05 by repeated-measures ANOVA), providing indirect evidence for SR activity at different contraction rates. tp decreased as stimulation frequency increased (P < 0.05 by repeated-measures ANOVA). t0.8r was shorter for male preparations at 0.2 and 0.5 Hz (P < 0.05 by repeated-measures ANOVA).
Fig. 1.
A: effects of varying contraction frequencies (0.2–2.0 Hz) on isometric twitch force (F) and resting tension in ventricle strips from male and female rainbow trout. The original recording of twitch force production in trout ventricle strips from one fish of both sexes showed a decrease in absolute twitch force and increase in resting tension (female fish only) at higher frequencies. The temporal scale at each frequency was varied to capture at least two contraction cycles. After the equilibration period (60 min at 0.5 Hz), ventricle strips from both sexes contracted for 15 min (or until steady performance at a specific frequency was observed) at each frequency tested. All ventricle strips contracted at increasing pacing frequencies until we observed mechanical dysfunction. B: effects of pretreatment with ryanodine (Ryn; 10 μM) on ventricle strips from both sexes. Values are means ± SE; n = 6–7 independent data points for male and female preparations. *P < 0.05 and **P < 0.01 vs. the initial value.
Table 2.
Contractile variables from male and female ventricle strips paced at increasing frequencies with or without Ryn (10 μM) pretreatment
| Pacing Frequency |
|||||||
|---|---|---|---|---|---|---|---|
| 0.2 Hz | 0.5 Hz | 0.8 Hz | 1.1 Hz | 1.3 Hz | 1.6 Hz | 1.8 Hz | |
| F, % | |||||||
| Male ventricle strips | |||||||
| Without Ryn | 113 ± 6 | 91 ± 5* | 101 ± 8 | 83 ± 8* | 73 ± 12* | 63 ± 7* | 40 ± 6† |
| With Ryn | 101 ± 3† | 93 ± 6* | 106 ± 4 | 78 ± 8* | 67 ± 11* | 59 ± 8* | 36 ± 8† |
| Female ventricle strips | |||||||
| Without Ryn | 111 ± 4 | 95 ± 7* | 111 ± 9 | 76 ± 7* | 59 ± 6* | ||
| With Ryn | 101 ± 2† | 91 ± 9* | 112 ± 11 | 78 ± 8* | 53 ± 7* | ||
| PRP, mN | |||||||
| Male ventricle strips | |||||||
| Without Ryn | 1.18 ± 0.20‡ | 1.76 ± 0.10§ | 1.67 ± 0.20* | 3.53 ± 1.18* | 4.80 ± 1.08* | 5.78 ± 1.27* | 5.98 ± 1.37* |
| With Ryn | 0.20 ± 0.09† | 0.29 ± 0.18‡ | 0.98 ± 0.29* | 1.76 ± 0.78* | 4.71 ± 1.27* | 5.20 ± 1.18* | 5.78 ± 1.57* |
| Female ventricle strips | |||||||
| Without Ryn | 0.69 ± 0.10 | 0.98 ± 0.20 | 1.18 ± 0.20* | 4.02 ± 0.59* | 4.61 ± 0.59* | ||
| With Ryn | 0.20 ± 0.20† | 0.10 ± 0.10‡ | 1.08 ± 0.20* | 2.16 ± 1.18* | 3.73 ± 1.67* | ||
| tp, ms | |||||||
| Male ventricle strips | |||||||
| Without Ryn | 485 ± 25 | 375 ± 11* | 360 ± 12* | 355 ± 14* | 350 ± 5† | 315 ± 4† | 280 ± 3† |
| With Ryn | 470 ± 18 | 370 ± 8* | 355 ± 9* | 340 ± 18* | 330 ± 6† | 300 ± 3† | 260 ± 3† |
| Female ventricle strips | |||||||
| Without Ryn | 475 ± 10 | 380 ± 15* | 370 ± 13* | 370 ± 8* | 340 ± 5† | ||
| With Ryn | 470 ± 15 | 375 ± 20* | 370 ± 15* | 370 ± 9* | 330 ± 8† | ||
| t0.8r, ms | |||||||
| Male ventricle strips | |||||||
| Without Ryn | 340 ± 8‡ | 320 ± 5§ | 280 ± 3* | 265 ± 2* | 245 ± 6* | 205 ± 3† | 180 ± 4† |
| With Ryn | 335 ± 16‡ | 305 ± 12§ | 295 ± 4*‡ | 275 ± 3*‡ | 260 ± 3*‡ | 230 ± 5†‡ | 200 ± 3†‡ |
| Female ventricle strips | |||||||
| Without Ryn | 360 ± 7 | 370 ± 11 | 290 ± 8* | 260 ± 3* | 260 ± 4* | ||
| With Ryn | 375 ± 12 | 365 ± 10 | 310 ± 6*‡ | 275 ± 4*‡ | 285 ± 8*‡ | ||
Values are expressed as either percentages or absolute means ± SE. Ryn, ryanodine; F, isometric twitch force; PRP, postrest potentiation; tp, time to peak force; t0.8r, time to 80% relaxation. Ventricle strips from male (n = 6) and female (n = 7) rainbow trout were subjected to increasing stimulation frequencies after the equilibration period (0.5 Hz for 60 min). Each ventricle strip contracted at a particular frequency for 15 min or until steady performance was observed. The frequency threshold (observation of mechanical alternans) occurred at a higher frequency in male (1.79 ± 0.04 Hz) than female (1.36 ± 0.02 Hz, P = 0.02) preparations. Data were not recorded beyond threshold frequency in both sexes. The percent F for each frequency was calculated with respect to absolute Fmax (in mN) for both sexes (5.39 ± 0.98 in male preparations and 5.88 ± 0.88 in female preparations) at 0.5 Hz during the 60-min equilibration period. The exposure time of tissue to Ryn before PRP measurements was 75 min for 0.2 Hz and between 150 and 180 min for the highest stimulation frequencies.
P < 0.05 or **P < 0.01 vs. 0.2 Hz for each sex;
P < 0.01 vs. controls (no Ryn) within each sex;
P < 0.05 vs. female preparations within each treatment at a given frequency.
Exogenous glucose and DCA provide sex-specific benefits for resting tension and contractile performance after hypoxia and reoxygenation.
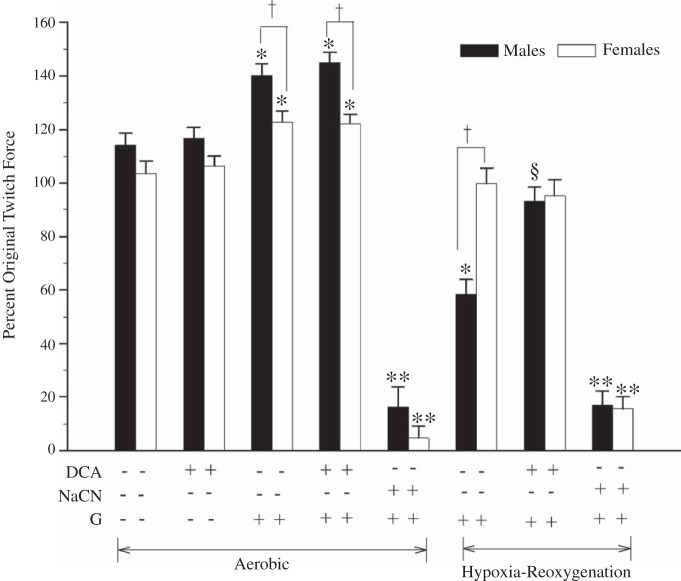
Male ventricle strips that received glucose and contracted at 0.5 Hz under aerobic conditions had increased F (P = 0.031 by ANOVA; Fig. 1), increased tp (P < 0.001 by ANOVA; Table 3), lower t0.8r (P < 0.001 by ANOVA; Table 3), and higher PRP values compared with glucose-free controls and female preparations under identical conditions (P < 0.001 by ANOVA; Table 3). Exogenous glucose also increased tp in female ventricle strips compared with glucose-free controls (P < 0.001 by ANOVA; Table 3). Pretreatment of aerobic ventricle strips with DCA in the absence of glucose did not affect F in either sex (P = 0.58 by ANOVA; Fig. 2). However, DCA lowered RT below baseline values (P < 0.001 by ANOVA; Table 3), reduced t0.8r (P < 0.001 by ANOVA; Table 3), and increased tp (P < 0.001 by ANOVA; Table 3) in female ventricle strips compared with glucose-free controls. In the absence of exogenous glucose, DCA also increased PRP in both sexes (P < 0.001 by ANOVA; Table 3). The addition of DCA to exogenous glucose did not affect F compared with glucose alone in both sexes (P = 0.20 by ANOVA; Fig. 2) or PRP in male rainbow trout (P = 0.90 by ANOVA; Table 3). On the other hand, the combination of DCA and glucose increased PRP in female preparations (P < 0.01 by ANOVA; Table 3) to values identical to male preparations and increased tp in male preparations compared with glucose controls (P < 0.001 by ANOVA; Table 3).
Table 3.
Contractile variables from rainbow trout ventricle strips treated with dichloroacetate and NaCN under aerobic conditions
| Contractile Variables |
|||||
|---|---|---|---|---|---|
| Number of Samples/Group | Resting tension, % | PRP, % | tp, ms | t0.8r, ms | |
| Glucose-free group | |||||
| Male ventricle strips | 23 | 104 ± 2* | 134 ± 9 | 379 ± 6* | 385 ± 4* |
| Female ventricle strips | 26 | 126 ± 2 | 128 ± 8 | 312 ± 6 | 416 ± 4 |
| Glucose-treated group | |||||
| Male ventricle strips | 22 | 101 ± 2 | 169 ± 3*† | 390 ± 6* | 330 ± 4† |
| Female ventricle strips | 24 | 99 ± 2 | 151 ± 4† | 350 ± 6† | 325 ± 5† |
| DCA-treated group | |||||
| Male ventricle strips | 9 | 106 ± 2* | 164 ± 6*† | 380 ± 5* | 381 ± 4 |
| Female ventricle strips | 7 | 80 ± 2† | 147 ± 6† | 362 ± 5† | 384 ± 4† |
| DCA + glucose-treated group | |||||
| Male ventricle strips | 9 | 105 ± 2* | 175 ± 6 | 410 ± 5*‡ | 336 ± 4 |
| Female ventricle strips | 7 | 83 ± 2‡ | 171 ± 6‡ | 365 ± 5 | 334 ± 4 |
| NaCN + glucose-treated group | |||||
| Male ventricle strips | 6 | 141 ± 8‡ | ND | ND | ND |
| Female ventricle strips | 6 | 155 ± 5‡ | ND | ND | ND |
Values are expressed as either percentages or absolute means ± SE. Treatments included dichloroacetate (DCA; 1 mM) or sodium cyanide (NaCN; 2 mM). Contractile values for controls (glucose-free and 5 mM glucose-treated groups) were combined under their respective variables from all experiments. Values from each experiment were compared to their respective controls for the statistical analysis. PRP, tp, and t0.8r from the NaCN + glucose-treated group were not detected (ND).
P < 0.05 vs. female ventricle strips;
P < 0.01 vs. the glucose-free control group;
P < 0.01 vs. the glucose-treated control group for respective contractile variables within each sex.
Fig. 2.
Effects of pretreatment with dichloroacetate (DCA; 1 mM) or sodium cyanide (NaCN; 2 mM) on F under aerobic conditions and after hypoxia-reoxygenation. Control strips either remained glucose free (Gf) or received 5 mM glucose (G). The presence (+) and absence (−) of specific compounds in the incubation medium are shown. Absolute Fmax (in mN) was not significantly different between male (6.18 ± 0.78) and female (5.88 ± 1.08) preparations during the 60-min equilibration period. Values are means ± SE; n = 6–9 independent data points for male and female preparations. *P < 0.05 and **P < 0.01 vs. controls; †P < 0.05 and §P < 0.01 vs. male preparations with glucose and no DCA under hypoxia-reoxygenation.
Hypoxia for 30 min and NaCN treatment to inhibit aerobic metabolism reduced F in ventricle strips from both sexes (P < 0.001 by ANOVA; Fig. 2) to values <20% of original values. NaCN treatment also increased RT by 40–55% compared with controls (P < 0.001 by ANOVA; Table 3). After reoxygenation of hypoxic ventricle strips for 30 min, male preparations that received DCA and glucose recovered F better than glucose controls (P < 0.001 by ANOVA; Fig. 2). Conversely, DCA treatment did not increase F after reoxygenation in female preparations that received glucose (P = 0.99 by ANOVA; Fig. 2).
Sex-specific effects of DCA on lactate efflux and tissue citrate concentration.
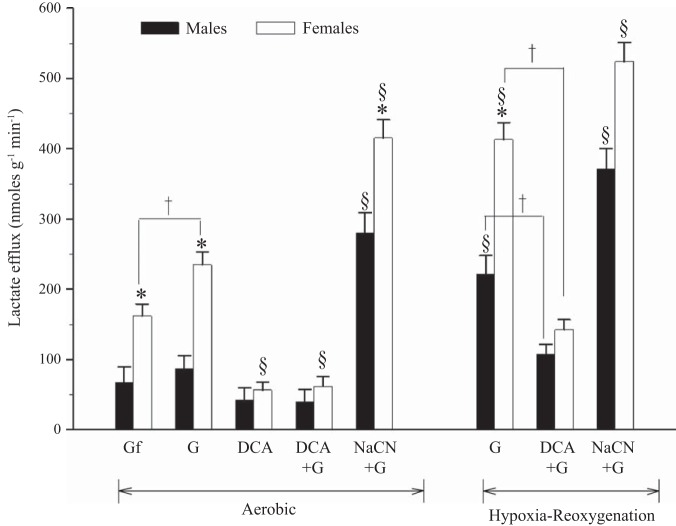
Lactate efflux was higher in female ventricle strips exposed to aerobic conditions and hypoxia compared with male preparations under identical conditions (P < 0.01 by ANOVA; Fig. 3). Pretreatment with DCA decreased lactate efflux in aerobic female preparations compared with controls (P < 0.01 by ANOVA; Fig. 3). Under hypoxia, DCA also reduced lactate efflux in ventricle strips from female and male rainbow trout (P < 0.01 by ANOVA; Fig. 3). In contrast, pretreatment with NaCN increased lactate efflux in both sexes (female > male) under all oxygenation states (P < 0.001 by ANOVA; Fig. 3).
Fig. 3.
Effects of pretreatment with DCA (1 mM) or NaCN (2 mM) on lactate efflux under aerobic conditions and after hypoxia-reoxygenation. Control strips remained glucose free or received 5 mM glucose. Values are means ± SE; n = 6–9 independent data points per treatment for male and female preparations. *P < 0.05 vs. male preparations under identical treatment conditions; §P < 0.01 vs. controls within each sex; †P < 0.05 within each sex.
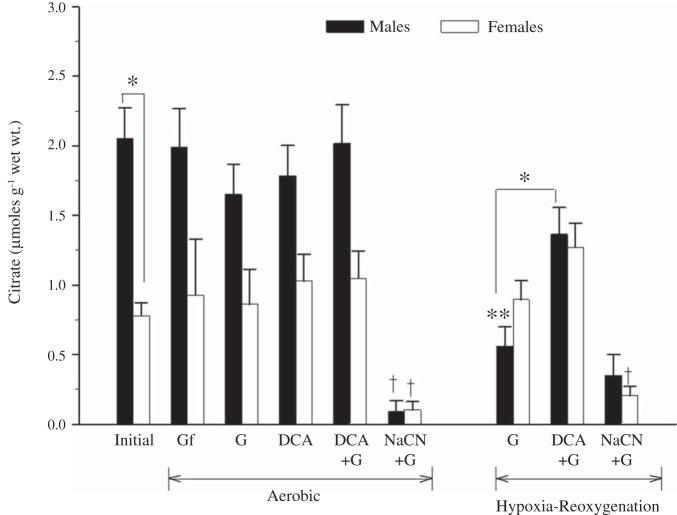
The initial concentration of citrate was 2.7-fold higher in ventricles from male rainbow trout compared with female rainbow trout (P < 0.01 by ANOVA; Fig. 4). During aerobic incubations, DCA with or without exogenous glucose did not affect tissue citrate levels in either sex (P = 0.60 by ANOVA; Fig. 4). Citrate was reduced in male ventricle strips after hypoxia-reoxygenation in the absence of DCA compared with the initial aerobic value (P < 0.01 by ANOVA; Fig. 4), and DCA increased citrate in the presence of glucose (P < 0.05 by ANOVA; Fig. 4). On the other hand, male and female ventricle strips pretreated with NaCN exhibited significant reductions in citrate under aerobic conditions (P < 0.001 by ANOVA; Fig. 4) and after hypoxia-reoxygenation (P < 0.001 by ANOVA; Fig. 4).
Fig. 4.
Effects of pretreatment with DCA (1 mM) or NaCN (2 mM) on citrate levels in initial samples of ventricular tissue and ventricle strips from both sexes under aerobic conditions and after hypoxia-reoxygenation. Control strips either remained glucose free or received 5 mM glucose. Values are means ± SE; n = 6–9 independent data points per treatment for for male and female preparations. *P < 0.05 and **P < 0.01 vs. the initial value; †P < 0.001 vs. glucose controls under a similar oxygenation state.
DISCUSSION
The possibility of metabolic optimization in a nonmammalian heart was studied for the first time in male and female rainbow trout. This study specifically investigated acute effects of DCA on sex differences in cardiac performance and energy metabolism. The results extend previous data suggesting that the metabolic phenotype of female rainbow trout hearts differs from that of male rainbow trout hearts before sexual maturation (7). Similar to the mammalian heart, acute treatment of rainbow trout ventricle strips with DCA improved mechanical and metabolic function under O2-limiting conditions and recovery. Our data also indicate that there are significant sex differences in DCA effects and that these effects involve glycolytic and oxidative pathways for ATP production and Ca2+ homeostasis by the SR.
The use of sexually immature rainbow trout as an experimental model for sex differences in myocardial physiology provides a novel opportunity to elucidate determinants of phenotypic sex differences and myocardial plasticity in the absence of circulating sex steroids. Similar to mammals, sex steroids act as endogenous inducers of sexual maturation in fishes: E2 in female fish and androgens in male fish (28). However, there is strong correlation between GSI and plasma androgen levels in male and female rainbow trout (61). In female rainbow trout, peak levels of E2 (∼20 ng/ml) are achieved ∼1 mo before ovulation, whereas testosterone and 11-KT peak ∼1 wk before ovulation at ∼200 and 20 ng/ml, respectively (62). In closely related coho salmon (Oncorhynchus kisutch), plasma concentrations of testosterone and 11-KT rise in male coho salmon during spermatogenesis, with 11-KT levels (150 ng/ml) exceeding testosterone levels (∼60 ng/ml) (29).
In the present study, 10-mo-old, 300-g rainbow trout had either very low levels of plasma androgens (male fish: <5 ng/ml) or nondetectable levels of sex steroids (female fish). These data confirm our assessment of gonadal development via GSI values and support the maturation history of rainbow trout from Clear Springs Foods. Male and female rainbow trout typically reach sexual maturity at 2 yr of age and a body weight of ∼900–1,300 g when reared in 14°C freshwater. However, some male rainbow trout are precocious and reach sexual maturity at 1 yr. This characteristic probably explains the low but detectable levels of testosterone and 11-KT in male rainbow trout sampled in the present study. An important extension of the present study will be to assess functional and metabolic differences in cardiac physiology during sexual maturation, with increased plasma levels of E2 in female rainbow trout and increased androgens in both male and female rainbow trout.
Although ventricle strips from male and female rainbow trout developed similar F values (∼ 6 mN) under control conditions (aerobic, 0.5-Hz stimulation frequency) with glucose as the sole exogenous substrate (present study and Ref. 7), sex differences in contractile function occur under increasing energy demands due to elevated stimulated frequencies. The frequency threshold for mechanical dysfunction was lower in female versus male preparations, and RT was also selectively increased in female ventricle strips. Similar to the present results, rainbow trout of unknown sex acclimated and tested at 17°C, respectively, exhibited a negative force-frequency relationship and incomplete mechanical restitution at pacing frequencies of >1.25 Hz (1). Shiels et al. (62) also reported an increased diastolic Ca2+ and a frequency-dependent reduction in Ca2+ current, which likely explain the increased RT in female rainbow trout and negative force-frequency relationship, respectively, in both sexes observed in the present study.
In adult mammals, the SR is the major source of contraction-activation Ca2+ and the major Ca2+ sink during relaxation (10). Similar to developing mammals, the majority of Ca2+ required for myocardial contraction in fish comes from extracellular sources across the sarcolemma, and the functionality/role of the SR in the regulation of beat-to-beat ventricular contraction in most fish has been questioned (63, 69). Consistent with the present results, others (63) have shown in rainbow trout cardiac tissue that the inhibitor of SR function ryanodine only reduces force of contraction at low contraction frequencies and cannot eliminate PRP. Although ryanodine was only effective at reducing PRP at 0.2 and 0.5 Hz in both sexes (Table 2), ryanodine was very effective at increasing RT at higher frequencies (≥0.8 Hz) in female preparations (Fig. 1B), suggesting that SR uptake of Ca2+ may be more important and limiting at high contraction frequencies. The lower contraction frequency threshold, higher RT, longer t0.8r, reduced PRP values, and negative impacts of ryanodine on RT of female versus male ventricle strips further highlight the sex differences in contractile capabilities and myocardial Ca2+ handling under aerobic conditions. Cardiomyocytes from female rats have lower E-C coupling gain, less SR Ca2+ release, and smaller Ca2+ transients than cardiomyocytes from male rats (26). In the absence of ovarian hormones, sexually mature female rats also show suppressed cardiac SR Ca2+ uptake and sarco(endo)plasmic reticulum Ca2+-ATPase activity (12). Whether the absence of E2 and other sex steroids in female rainbow trout compromises SR function in a manner similar to ovarectomized rats is a distinct possibility and worth additional research.
Consistent with a previous study in mammals (19), exogenous glucose increased contractile force in fully oxygenated cardiac tissue from sexually immature male and female rainbow trout (Fig. 2). Previous studies (7, 22) from our group have demonstrated that the addition of 5 mM glucose, but not pyruvate or lactate, to aerobic female ventricle strips normalizes RT. Farrell et al. (25) also showed that 10 mM exogenous glucose increases maximum cardiac output and power output in rainbow trout hearts (sex not mentioned) that were perfused in situ. Given the results from the present and previous studies on rainbow trout (7, 22, 25, 35), it appears that glycolytically derived ATP from glucose is an important requirement for maximum cardiac performance and the maintenance of diastolic relaxation. We cannot assume, however, that exogenous glucose is the only substrate for lactate production or pyruvate oxidation in the rainbow trout heart. It is clear, however, that under the present experimental conditions used, endogenous glycogen cannot support the glycolytic energy requirements for maintaining resting tension in aerobic female cardiac tissue. Our finding that ventricular preparations from rainbow trout release lactate under “fully” oxygenated conditions (buffer Po2 > 600 mmHg) is consistent with previous studies for this species (7, 31) and suggests that ATP turnover from glycolysis and anaerobic lactate production was occurring, especially in female rainbow trout (7). However, the observed lactate efflux from ventricle strips during oxygenated conditions (∼100–250 nmol·min−1·g−1; Fig. 3) were only 2–6% of values reported for perfused rainbow trout hearts under anoxic conditions at 15°C [4.1 μmol·min−1·g−1 (54)]. Assuming that total energy production equals 2 μmol ATP·min−1·g−1 of contracting ventricular tissue under aerobic conditions (53), and 1 molecule of ATP is produced per lactate molecule, glycolysis could account for 5% of ATP production in male rainbow trout and 12% in female rainbow trout. Whether lactate efflux from oxygenated ventricle strips in the present study reflects hypoxic regions in preparations and possible sex differences in thickness of the ventricular compact layer, myoglobin content, mitochondrial function, and/or capacity for glycolytic flux will require further investigation. In addition, more definite studies of potential sex differences in the contributions of anaerobic versus aerobic metabolism for myocardial ATP production and mechanical performance might help explain the beneficial effects of exogenous glucose in female rainbow trout and DCA for male rainbow trout.
DCA, unlike exogenous glucose, did not impact F in aerobic ventricle strips from rainbow trout (Fig. 2), suggesting that the stimulation of carbohydrate oxidation, per se, does not increase Ca2+-mediated activation of contractile proteins. DCA pretreatment, by itself or in combination with exogenous glucose, decreased RT in aerobic female ventricle strips, and DCA was much more effective than glucose (Table 3). By decreasing lactate efflux in aerobic female ventricle strips by 75% (Fig. 3), DCA appears to reduce RT by a different mechanism than exogenous glucose, which increased lactate efflux by ∼50% (Fig. 3). We posit that both exogenous glucose and DCA stimulate glycolytic activity and ATP production. It is conceivable that exogenous glucose increases glycolytic flux in female rainbow trout cardiomyocytes by stimulating facilitated glucose uptake, elevating intracellular glucose and using a feedforward mechanism, whereas DCA enhances glycolysis to a greater degree by increasing PDH activity, pyruvate oxidation, and reducing lactate production. Although exogenous glucose increased lactate efflux during aerobic conditions, it is also possible that intracellular pyruvate levels were also increased, stimulating PDH activity and therefore glucose oxidation and ATP production. DCA has been shown to stimulate both glycolysis and glucose oxidation in the aerobic mammalian heart (67). Ultimately, the reason for impaired pyruvate oxidation in female cardiac tissue or sex differences in DCA effects during aerobic conditions are unclear but may be related to higher activities of competing enzyme lactate dehydrogenase (EC 1.1.1.27) in female versus male rainbow trout (7) and, therefore, lower levels of intracellular pyruvate or reduced glycogenolysis (7).
Independent of its metabolic effects, DCA may also open voltage-dependent K+ channels, causing membrane hyperpolarization and decreasing the concentration of intracellular Ca2+ (48). These actions would promote cardiomyocyte relaxation and help explain the striking reduction of resting tension in DCA-treated female ventricle strips (Table 3). However, if DCA opens voltage-dependent K+ channels in rainbow trout cardiomyocytes, it is not clear why DCA treatment did not affect male preparations in a similar manner.
Under aerobic conditions, DCA pretreatment and exogenous glucose also elevated PRP in male and female ventricle strips contracted at 0.5 Hz compared with glucose-free controls (Table 3). This finding points to the SR and Ca2+ handling as regulatory targets for the actions of glucose and DCA. In the mammalian heart, it has been proposed that glycolytically derived ATP is preferentially used for Ca2+ reuptake into the SR and maintenance of diastolic relaxation (41, 72). Both glucose and DCA could be stimulating glycolytic production of ATP, which supports myocardial SR function and increased storage of Ca2+ in rainbow trout. It is noteworthy that the combination of exogenous glucose and DCA selectively increased PRP in female preparations beyond the independent effects of glucose and DCA, implying separate and additive mechanisms of DCA in female cardiac tissue.
Other mechanisms may explain the observed link between DCA-stimulated PDH activity, alterations in myocardial energy metabolism, and enhanced intracellular Ca2+ homeostasis in rainbow trout cardiac tissue. For example, glycolytically produced ATP also reduces intracellular Na+ by enhances Na+ efflux via Na+/K+-ATPase (20). This, in turn, would potentially reduce reverse-mode Na+/Ca2+ exchange activity and Ca2+ overload in the female cardiomyocyte, reducing RT. We also cannot discount the possibility that the cytosolic redox state and balance between cytosolic and mitochondrial redox potential (73) could be important in the rainbow trout heart. Ultimately, the determination of whether DCA pretreatment and exogenous glucose provide similar benefits for increasing glycolytic activity and maintaining cytoplasmic ATP levels cardiac tissue from female rainbow trout will require further investigation. Future studies are also needed to determine whether DCA enhances cardiomyocyte SR Ca2+ uptake and/or Ca2+ release and alters the utilization of endogenous glycogen or triglyceride in the aerobic rainbow trout heart.
The detrimental effects of hypoxia, ischemia, and reperfusion on the mammalian heart are well known and provide key insights to the pathology and treatment of human ischemic heart disease (39, 70). Fish live in water with variable O2 content and display different degrees of myocardial hypoxia tolerance. Rainbow trout, much like mammals, possess a heart that rapidly fails under O2-limiting conditions (4). Similar to our present and previous results for ventricle strips (7), when the rainbow trout heart is exposed to hypoxic conditions in situ (Po2 < 5 mmHg), contractile performance declines and anaerobic glycolysis and lactate efflux can increase >10- and 35-fold, respectively (4). Lactate also increases in the mammalian myocardium and blood during hypoxia secondary to cardiac failure or ischemia (3). High concentrations of lactate are known to exert deleterious effects on the recovery of the heart after reperfusion (50). Interestingly, male rats are more susceptible to postischemic contractile dysfunction than female rats (2, 8, 47), and male rainbow trout preparations do not preserve contractile function as well as female rainbow trout preparations after hypoxia and reoxygenation (present study and Ref. 7).
Common therapeutic strategies have been to increase glucose use and glycolytic ATP production by the diseased heart (19) and use DCA for metabolic therapy (11, 67). Similar to the mammalian heart, acute treatment of ventricle strips from male rainbow trout with DCA improved mechanical and metabolic function under O2-limiting conditions and recovery (Figs. 2–4). The recovery of heart function after ischemia has been inversely correlated with rates of nonoxidative glycolysis (60), and stimulation of glucose oxidation and/or reduction of glycolysis improve the function of ischemia-reperfused hypertrophied rat hearts (58, 71). Our results showing decreased lactate efflux in DCA-treated ventricle strips from male and female rainbow trout (Fig. 3) suggest that DCA enhanced myocardial oxidation of pyruvate, even at relatively low Po2 values (10–20 mmHg). In male rainbow trout, the fact that citrate, a key intermediate in substrate fuel partitioning, was increased by DCA in the presence of exogenous glucose after hypoxia-reoxygenation may provide a mechanism for reducing glycolysis at the levels of phosphofructokinase (33) and reflect increased mitochondrial oxidation of fatty acids (56). Interestingly, the reported citrate concentrations for rainbow trout are either much higher (52) or at the upper range (51) compared with mammalian cardiac values. Overall, both lactate and citrate appear to be markers of metabolic sex differences and related to the beneficial effects of DCA in sexually immature rainbow trout. However, simple measurements of metabolite concentrations in tissue or in the medium surrounding tissue may not accurately reflect intracellular metabolite fluxes. A more detailed assessment of glycolytic flux and oxidation of exogenous glucose, glycogen, and intracellular triglyceride would help to better define cardiac sex differences and the mechanisms by which DCA improves Ca2+ handling and hypoxia tolerance in rainbow trout.
Finally, we cannot be sure that the sex-based differences observed for rainbow trout at Clear Springs Foods are identical in other strains of rainbow trout or rearing facilities. A previous study (27) has demonstrated significant intraspecific differences in myocardial hypoxia tolerance for rainbow trout. It is also important to note that our conclusions are based solely on in vitro preparations that do not incorporate a full complement of metabolic energy sources and circulating hormones. Thus, while the present study provides evidence for conserved metabolic and functional properties between rainbow trout and mammalian hearts, it remains to be determined whether our findings in vitro can translate to sex differences in mammalian cardiac function or be used in the development of a comparative model to study human cardiovascular disease.
GRANTS
This work was supported in part by National Institutes of Health Grant P20-RR-016454 (INBRE Program), the National Science Foundation (NSF)-Idaho EPSCoR Program, NSF Awards EPS-0447689 and IOB-517669, the MSTI/MSMRI Research Institute of St. Luke's Regional Medical Center, the Department of Biological Sciences, and the Graduate Student Research and Scholarship Committee at Idaho State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.K.B. and K.J.R. conception and design of research; P.K.B. performed experiments; P.K.B. and K.J.R. analyzed data; P.K.B. and K.J.R. interpreted results of experiments; P.K.B. and K.J.R. prepared figures; P.K.B. and K.J.R. drafted manuscript; P.K.B. and K.J.R. edited and revised manuscript; P.K.B. and K.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express appreciation to Dr. Carl Schreck for helpful comments and Dr. Grant Feist in Dr. Scheck's laboratory for conducting sex steroid assays. The authors also acknowledge Dr. Teri Peterson for statistical insights and recommendations and Clear Springs Foods for technical assistance.
Present address of P. K. Battiprolu: Department of Metabolic Disorders, Amgen Incorporated, South San Francisco, CA 94568.
REFERENCES
- 1.Aho E, Vornanen M. Contractile properties of atrial and ventricular myocardium of the heart of rainbow Oncorhynchus mykiss: effects of thermal acclimation. J Exp Biol 202: 2663–2677, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Alves MG, Oliveira PJ, Carvalho RA. Substrate selection in hearts subjected to ischemia/reperfusion: role of cardioplegic solutions and gender. NMR Biomed 24: 1029–1037, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Apstein CS, Deckelbaum L, Hagopian L, Hood WB. Acute cardiac ischemia and reperfusion–contractility, relaxation, and glycolysis. Am J Physiol Heart Circ Physiol 235: H636–H648, 1978. [DOI] [PubMed] [Google Scholar]
- 4.Arthur PG, Keen JE, Hochachka PW, Farrell AP. Metabolic state of the in situ perfused trout heart during severe hypoxia. Am J Physiol Regul Integr Comp Physiol 263: R798–R804, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Ausoni S, Sarore S. From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J Cell Biol 184: 357–364, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey JR, Barter T, Driedzic WR. Maintenance of resting tension in the American eel (Anguilla rostrata L.) is dependent upon exogenous fuel and the sarcoplasmic reticulum. J Exp Zool 286: 707–717, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Battiprolu PK, Harmon KJ, Rodnick KJ. Sex differences in energy metabolism and performance of teleost cardiac tissue. Am J Physiol Regul Integr Comp Physiol 292: R827–R836, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bell JR, Porrello ER, Huggins CE, Harrap SB, Delbridge LM. The intrinsic resistance of female hearts to an ischemic insult is abrogated in primary cardiac hypertrophy. Am J Physiol Heart Circ Physiol 294: H1514–H1522, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Bell JR, Bernasochi GB, Varma U, Raaijmakers AJ, Delbridge LM. Sex and sex hormones in cardiac stress–mechanistic insights. J Steroid Biochem Mol Biol 137: 124–135, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Cardiac excitation-contraction coupling. Nature 473: 36–39, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Bersin RM, Stacpoole PW. Dicloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J 134: 841–855, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291: H1101–H1108, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Clark JJ, Clark RJ, McMinn JT, Rodnick KJ. Microvascular and biochemical compensation during ventricular hypertrophy in male rainbow trout. Comp Biochem Physiol B 139: 695–703, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Clark RJ, Rodnick KJ. Morphometric and biochemical characteristics of ventricular hypertrophy in male rainbow trout (Oncorhynchus mykiss). J Exp Biol 201: 1541–1552, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Clark RJ, Rodnick KJ. Pressure and volume overloads are associated with ventricular hypertrophy in male rainbow trout. Am J Physiol Regul Integr Comp Physiol 277: R938–R946, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Curl CL, Wendt JR, Canny BJ, Kotsanas G. Effects of ovariectomy and 17β-oestradiol replacement on [Ca2+] in female rat cardiac myocytes. Clin Exp Pharmacol Physiol 30: 489–494, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Dagley S. Citrate-UV spectrophotometric determination. In: Methods of Enzymatic Analysis (2nd ed.), edited by Bergmeyer HV. New York: Academic, 1965, p. 1562–1565. [Google Scholar]
- 18.Davie PS, Thorarensen H. Heart growth in rainbow trout in response to exogenous testosterone and 17-α-methyltestosterone. Comp Biochem Physiol A 117: 227–230, 1997. [Google Scholar]
- 19.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 99: 578–588, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Dizon J, Burkhoff D, Tauskela J, Whang J, Cannon P, Katz J. Metabolic inhibition in the perfused rat heart: evidence for glycolytic requirement for normal sodium homeostasis. Am J Physiol Heart Circ Physiol 274: H1082–H1089, 1998. [DOI] [PubMed] [Google Scholar]
- 21.El-Sayed MF, Gesser H. Sarcoplasmic reticulum, potassium, and cardiac force in rainbow trout and plaice. Am J Physiol Regul Integr Comp Physiol 257: R599–R604, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Farrar RS, Battiprolu PK, Pierson NS, Rodnick KJ. Steroid-induced cardiac contractility requires exogenous glucose, glycolysis and the sarcoplasmic reticulum in rainbow trout. J Exp Biol 209: 2114–2128, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Farrell AP. Coronary arteriosclerosis in salmon: growing old or growing fast? Comp Biochem Physiol A 132: 723–735, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Farrell AP, Hammons AM, Graham MS, Tibbits GF. Cardiac growth in rainbow trout, Salmo gairdneri. Can J Zool 62: 523–536, 1988. [Google Scholar]
- 25.Farrell AP, MacLeod KR, Scott C. Cardiac performance of the trout (Salmo gairdneri) heart during acidosis: effects of low bicarbonate, lactate and cortisol. Comp Biochem Physiol A 91: 271–277, 1988. [Google Scholar]
- 26.Farrell SR, Ross JL, Howlett SE. Sex differences in mechanisms of cardiac excitation coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 299: H36–H45, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Faust HA, Gamperl AK, Rodnick KJ. All rainbow trout (Oncorhynchus mykiss) are not created equal: intra-specific variation in cardiac hypoxia tolerance. J Exp Biol 207: 1005–1015, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Feist G, Schreck CB. Brain-pituitary-gonadal axis during early development and sexual differentiation in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 102: 394–409, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick MS, Van Der Kraak G, Schreck CB. Plasma profiles of sex steroids and gonadotropin in coho salmon (Oncorhynchus kisutch) during final maturation. Gen Comp Endocrinol 62: 437–451, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Fleischer WR. Enzymatic methods for lactic and pyruvic acid. In: Standard Methods in Clinical Chemistry, edited by MacDonald RP. New York: Academic, 1970, p. 24–59. [Google Scholar]
- 31.Gamperl AK, Todgham AE, Parkhouse WS, Dill R, Farrell AP. Recovery of trout myocardial function following anoxia: reconditioning in a non-mammalian model. Am J Physiol Regul Integr Comp Physiol 281: R1755–R1763, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Gamperl AK, Farrell AP. Cardiac plasticity in fishes: environmental influences and intraspecific differences. J Exp Biol 207: 2539–2550, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes, and starvation. Nature 200: 169–170, 1963. [DOI] [PubMed] [Google Scholar]
- 34.Gesser H. Cardiac force-interval relationship, adrenaline and sarcoplasmic reticulum in rainbow trout. J Comp Physiol B 166: 278–285, 1996. [Google Scholar]
- 35.Gesser H. Mechanical performance and glycolytic requirements in trout ventricular muscle. J Exp Zool 293: 360–367, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Hansen SP, Gesser H. Extracellular Ca2+, force, and energy state in cardiac tissue of rainbow trout. Am J Physiol Regul Integr Comp Physiol 271: R946–R954, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Harmon KJ, Bolinger MT, Rodnick KJ. Carbohydrate energy reserves and effects of food deprivation in male and female rainbow trout. Comp Biochem Physiol A Mol Integr Physiol 158: 423–431, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Hartmund T, Gesser H. ATP, creatine phosphate, and mechanical activity in rainbow trout myocardium under inhibition of glycolysis and cell respiration. J Comp Physiol B 160: 691–697, 1991. [Google Scholar]
- 39.Hearse DJ, Garlick PB, Humphrey SM. Ischemic contracture of the myocardium: mechanism and prevention. Am J Cardiol 39: 986–993, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Hoar WS, Hickman CP., Jr A Laboratory Companion for General and Comparative Physiology. Englewood Cliffs, NJ: Prentice Hall, 1967. [Google Scholar]
- 41.Jeremy RW, Koretsune Y, Marban E, Becker LC. Relationship between glycolysis and calcium homeostasis in postischemic myocardium. Circ Res 70: 1180–1190, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Jousilahti P, Vartianen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14,786 middle-aged men and women in Finland. Circulation 99: 1165–1172, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Leong HS, Grist M, Parsons H, Wambolt RB, Lopaschuk GD, Brownsey R, Allard MF. Accelerated rates of glycolysis in the hypertrophied heart: are they a methodological artifact? Am J Physiol Endocrinol Metab 282: E1039–E1045, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation 91: 2071–2079, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 353: 89–92, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol 71: 1–18, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Lujan HI, Dicarlo SE. Sex differences to myocardial ischemia and β-adrenergic receptor blockade in conscious rats. Am J Physiol Heart Circ Physiol 294: H1523–H1529, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation 105: 244–250, 2002. [DOI] [PubMed] [Google Scholar]
- 49.McVeigh JJ, Lopaschuk GD. Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol Heart Circ Physiol 259: H1079–H1085, 1990. [DOI] [PubMed] [Google Scholar]
- 50.Mochizuki S, Neely JR. Energy metabolism during reperfusion following ischemia. J Physiol 76: 805–812, 1980. [PubMed] [Google Scholar]
- 51.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 44: 662–667, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Opie LH, Tuschmidt R, Bricknell O, Girardier L. Role of glycolysis in maintenance of the action potential duration and contractile activity in isolated perfused rat heart. J Physiol 76: 821–829, 1980. [PubMed] [Google Scholar]
- 53.Overgaard J, Gesser H. Force development, energy state and ATP production of cardiac muscle from turtles and trout during normoxia and severe hypoxia. J Exp Biol 207: 1915–1924, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Overgaard J, Stecyk JA, Gesser H, Wang T, Farrell AP. Effects of temperature and anoxia upon the performance of in situ perfused trout hearts. J Exp Biol 207: 655–665, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Powers DA. Fish as model systems. Science 246: 352–358, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Randle PJ. Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc 54: 317–327, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Ross JL, Howlett SE. Age and ovariectomy abolish beneficial effects of female sex on rat ventricular myocytes exposed to simulated ischemia and reperfusion. PLOS ONE 7: 338425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saeedi R, Grist M, Wambolt RB, Bescond-Jacquet A, Lucien A, Allard MF. Trimetazidine normalizes postischemic function of hypertrophied rat hearts. J Pharmacol Exp Ther 314: 446–454, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Saeedi R, Wambolt RB, Parsons H, Antler C, Leong HS, Keller A, Dunaway GA, Popov KM, Allard MF. Gender and post-ischemic recovery of hypertrophied rat hearts. BMC Cardiovasc Disord; 10.1186/1471-2261-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schonekess BO, Allard MF, Lopaschuk GD. Recovery of glycolysis and oxidative metabolism during postischemic reperfusion of hypertrophied rat hearts. Am J Physiol Heart Circ Physiol 271: H798–H805, 1996. [DOI] [PubMed] [Google Scholar]
- 61.Schreck CB. Evaluation of diel variation in androgen levels of rainbow trout, Salmo gairdneri. Copeia 4: 865–868, 1972. [Google Scholar]
- 62.Scott AP, Sumpter JP, Hardiman PA. Hormone changes during ovulation in the rainbow trout (Salmo gairdneri Richardson). Gen Comp Endocrinol 49: 128–134, 1983. [DOI] [PubMed] [Google Scholar]
- 63.Shiels HA, Farrell AP. The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200: 1607–1621, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Shiels HA, Vornanen M, Farrell AP. The force-frequency relationship in fish hearts–a review. Comp Biochem Physiol A Mol Integr Physiol 132: 811–826, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Sower SA, Scheck CB. Steroid and thyroid hormones during sexual maturation of coho salmon (Oncorhynchus kisutch) in sea water or fresh water. Gen Comp Endocrinol 47: 42–53, 1982. [DOI] [PubMed] [Google Scholar]
- 66.Stanley WC, Hernandez LA, Spires D, Bringas J, Wallace S, McCormack JG. Pyruvate dehydrogenase activity and malonyl CoA levels in normal and ischemic swine myocardium: effects of dichloroacetate. J Mol Cell Cardiol 28: 905–914, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 33: 243–257, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi M, Wilson C, Hunter CA, Pehowich DJ, Clanachan AS, Lopaschuk GD. Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am J Physiol Heart Circ Physiol 280: H1762–H1769, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Tibbits GF, Philipson KD, Kashihara H. Characterization of myocardial Na+-Ca2+ exchange in rainbow trout. Am J Physiol Cell Physiol 262: C411–C417, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Varma N, Morgan JP, Apstein CS. Mechanisms underlying ischemic diastolic dysfunction: relation between rigor, calcium homeostasis, and relaxation rate. Am J Physiol Heart Circ Physiol 284: H758–H771, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol 36: 1378–1385, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Weiss J, Hilderbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest 75: 436–447, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White LT, O'Donnell Griffin J, Lewandowski ED. Cytosolic redox state mediates postischemic response to pyruvate dehydrogenase stimulation. Am J Physiol Heart Circ Physiol 277: H626–H634, 1999. [DOI] [PubMed] [Google Scholar]
- 74.Zhai P, Eurell TE, Cotthaus R, Jeffrey EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol 279: H2766–H2775, 2000. [DOI] [PubMed] [Google Scholar]