Abstract
The role of Th17 responses in airway remodeling in asthma is currently unknown. We demonstrate that both parenteral and mucosal allergen sensitization followed by allergen inhalation leads to Th17-biased lung immune responses. Unlike Th17 cells generated in vitro, lung Th17 cells did not produce TNF-α or IL-22. Eosinophilia predominated in acute inflammation while neutrophilia and IL-17 increased in chronic disease. Allergen-induced tolerance involved Foxp3, Helios and GARP expressing regulatory T cells (Treg) and IL-10/IFN-γ priming. This Treg phenotype was altered in inflamed lungs and abrogated by inhalation of IL-17. Using Th17-deficient mice with genetic disruption of gp130 in T cells, we showed that Th17 cells induce airway remodeling independent of the Th2 response. All-trans retinoic acid administration ameliorated Th17-mediated disease and increased Treg activity, while dexamethasone inhibited eosinophilia but not neutrophilia and enhanced Th17 development in vitro. Targeting the Th17/Treg axis might therefore be therapeutic in neutrophilic and glucocorticoid-refractory asthma.
Keywords: Th17, asthma, regulatory T cell, airway remodeling, neutrophilia
Introduction
The key pathological features of asthma are airway hyperreactivity and remodeling, both of which are generally attributed to activities of allergen-driven Th2 cells and associated allergic inflammation. Based on animal models of acute and chronic allergen exposure, airway remodeling has been mainly associated with chronic lung inflammation while hyperreactivity is most prominent during acute phases of the disease. This suggests prolonged exposure of lung tissue to products of Th2 cells (e.g. IL-131, eosinophils2 or TGF-ß3 leads to gradual remodeling of the tissue and decreased baseline lung function. However, recent studies in infants and young children have shown that remodeling is apparent at the earliest stages of asthma development, suggesting both features develop in parallel4.
Asthmatic inflammation is primarily driven by allergen-specific CD4 T cells which recruit a complex network of innate immune cells into the airways. CD4 T cells can differentiate along 4 distinct pathways after antigen exposure, directed by T-bet (Th1), GATA-3 (Th2), RORγt (Th17) or Foxp3 (Treg) transcription factors. Th17 cytokines have been implicated in the immunopathology of both asthma and chronic obstructive pulmonary disease5, 6. IL-17 (i.e. IL-17A) is a pro-inflammatory cytokine and IL-17-deficient mice develop reduced acute inflammatory responses in a classic mouse asthma model7. Th17 effectors recruit neutrophils into the airway since the IL-17 they produce induces secretion of IL-8, an important neutrophil-recruiting chemokine, from airway epithelial cells8 and smooth muscle9. Moreover, IL-17 stimulates release of IL-6 from human bronchial fibroblasts and G-CSF expression in bronchial epithelial cells, which stimulates neutrophil development and granulopoiesis10.
Adoptive transfer of in vitro-derived Th17 cells into allergen-challenged mice induces airway hyperreactivity and glucocorticoid-resistant inflammation11. On the other hand, IL-17 produced by γδ T cells promotes resolution of pre-established allergic airway inflammation, indicating distinct roles for IL-17 during sensitization and challenge phases12. Furthermore, skewing Th2 inflammation towards Th17 in an acute asthma model inhibited airway hyperreactivity13. However the role of Th17 cells in chronic asthmatic disease and airway remodeling is not known.
Airways harbour many microbes and allergens that do not result in persistent inflammation, and this tolerance is partly mediated by regulatory T cells (Treg). Th17 cells are closely related to the Foxp3+ Treg lineage. Although the majority of Foxp3+ Treg are thymically generated, Foxp3 Treg can be peripherally induced by TGF-ß and IL-2. Conversely, TGF-ß in the presence of IL-6 directs Th17 development. Expression of transcription factor Helios has been proposed as a marker for natural, thymic Treg, with low Helios expression in induced Treg14. Since the balance between Treg and Th17 responses may be controlled by IL-6, the IL-6 pathway represents a potential therapeutic target in inflammatory disease15. Here we characterized Th17 responses in acute and chronic airway inflammation triggered by parenteral or mucosal sensitization and suggest that naturally induced lung Th17 cells have a different phenotype from in vitro polarized cells. We tested experimental disruption of the Th17 response using all-trans retinoic acid (ATRA) treatment or genetic deletion of IL-6R signaling in T cells. Our data establish an important role for Th17 cells in airway remodeling and chronic neutrophilia, independent of allergic inflammation. Targeting the Th17 pathway in neutrophilic asthma is therefore of potential therapeutic benefit.
Results
Primary T cell responses to inhaled allergen induce a mixed Th17/Th2/Treg phenotype
We first examined primary T cell responses to inhaled allergen to characterize responses primed via the lung mucosa. This avoided effects of artificial adjuvants on the response. TCR-transgenic OT-2 CD4 cells (OVA-specific) were labeled with CFSE and transferred into naive recipients given OVA allergen alone intranasally (i.n.; Fig 1A). Within 3 days OT-2 cells were present in mediastinal LN but not lung tissue and had undergone extensive clonal expansion, as demonstrated by CFSE dilution. After 4-6 divisions, some cells had developed into Th17 (IL-17+) and Th2 (IL-4+) effectors but none were Th1-like. This contrasts with in vitro characteristics of OT-2 cells, which have a strong Th1 bias (see Fig 7A). Since allergen alone given i.n. is known to induce tolerance, we performed the same analysis with addition of the mucosal adjuvant TNF-α to the allergen, which is known to induce allergic sensitization16. Primary responses were enhanced (Fig 1B) in terms of both numbers of divisions and proportions of cytokine-producing cells. We also determined whether Treg cells present in OT-2 mice responded to i.n. allergen priming. Foxp3+ cells expanded to the same extent as Foxp3− CD4 cells, and their division was also enhanced by the mucosal adjuvant (Fig 1B).
Figure 1. Acute allergic airway inflammation consists of mixed Th17/Th2 responses.
A&B: CFSE-labelled OT-2 CD4 cells were transferred into B6 mice and primary responses to inhaled OVA (A) or OVA±TNF-α (B) determined by flow cytometry of mediastinal LN after 3 days. Cells were stimulated with PMA+ionomycin before cytokine staining or stained for Foxp3. Data are from n=6 mice pooled from 3 experiments, statistical comparisons are to unstimulated cells (A) or OVA alone (B). C&D: Acute allergic airway inflammation was induced via OVA/alum sensitization or alum alone control and OVA i.n. challenge. BAL T cells (C) or lung T cells (D) were restimulated with anti-CD3/CD28 and cytokine staining in CD4 cells is shown (n=5, similar data were obtained in 3 independent experiments). E: Surface CCR6 and intracellular RORyt staining in CD4 cells as in D. F: Secreted cytokines in BAL supernatants from mice as in C. G&H: Lung Th17 cells are OVA-responsive and distinct in phenotype from in vitro-generated Th17: G shows IL-17 vs TNF-α staining in lung CD4 cells (as in D) stimulated with anti-CD3 or OVA + anti-CD28. H shows Th1 (top) and Th17 (bottom) cells generated in vitro from OT-2 CD4 cells and restimulated with PMA/ionomycin before IL-17, TNF-α and IFN-γ staining. Data are representative of 3 experiments.
Figure 7. Regulation of Th17 responses by glucocorticoid receptor and MEK signaling pathways.
A: In vitro Th17 differentiation from OT-2 CD4 cells is enhanced by dexamethasone (DEX, 10nM) and MEK inhibitor (UO126, 3μg/ml) – upper panel shows intracellular cytokine staining and lower panels show intracellular RORγt staining, means±SEM from 5 (upper panels) or 3 (lower panels) experiments. Statistical comparisons are to TGF-ß/IL-6/IL-23-treated (upper) or control (lower). B: Effect of in vivo DEX treatment on acute (upper graphs) and chronic (lower graphs) airway inflammation induced by OVA+TNF-α mucosal sensitization followed by OVA (50μg) ± DEX (2μg) i.n. challenges. Numbers of BAL cells are shown (n=5). Similar data were obtained in 2 experiments. C: Numbers of BAL Th1, Th2 and Th17 cells as in B. Insufficient T cells were obtained from control mice for cytokine staining. D: Effect of DEX treatment on collagen deposition induced by OVA+TNF-α mucosal sensitization and chronic OVA challenge. Control animals received PBS (n=5).
Acute allergic airway inflammation is dominated by Th17 cells with a phenotype distinct from in vitro-generated Th17
The Th17-priming environment of the lung was confirmed in B6 mice sensitized with OVA/alum and challenged acutely with OVA, the protocol most frequently used as an experimental asthma model (Figs 1C-G). T cells infiltrating the airways contained twice as many IL-17+ CD4 cells as IL-4+ Th2 cells (Fig 1C), and this Th17 predominance was more pronounced in lung CD4 cells (Fig 1D). OVA/alum sensitized animals showed no increase in CD4 cells producing Th1 cytokines or IL-22. Surface expression of the Th17 marker CCR6 and intracellular staining for the Th17 transcription factor RORγt were both increased on lung CD4 T cells (Fig 1E). Furthermore, significant increases in IL-17A, as well as the Th2 cytokine IL-13, were detected in bronchoalveolar lavage (BAL) fluids from mice with acute allergic inflammation, and the Th17-promoting cytokine IL-23 was also increased (Fig 1F). Stimulation of lung cells with OVA+anti-CD28 in vitro also induced IL-17A production in lung CD4 cells, suggesting that the Th17 cells were allergen specific or at least dependent on allergen priming (Fig 1G). Lung Th17 cells clearly did not stain for TNF-α, unlike in vitro generated IL-17+ cells (Fig 1H) which were nearly all TNF-α+ despite having downregulated IFN-γ production under Th17-polarizing conditions. There was also a difference, though not as dramatic, in IL-22 production - a substantial fraction of in vitro Th17 cells produced IL-22 while very few IL-22+ cells were found in the lung. CCR6 expression did not correlate with Th17 development in vitro (not shown).
Chronic allergic airway inflammation is dominated by Th17 responses and neutrophilia irrespective of type of sensitization or route of exposure
Mice were sensitized with OVA combined with either the classic Th2-skewing adjuvant alum, Th17-promoting adjuvant CFA17 or Th1-skewing CASAC (combined adjuvant for synergistic activation of cellular immunity) 18, then examined for lung inflammation induced by short-term or chronic mucosal allergen exposure. Surprisingly, Th17 was the predominant phenotype with all sensitization protocols at the acute stage (Fig 2A). Both alum and CFA sensitization induced mixed eosinophilia/neutrophilia. CASAC induced only neutrophilia with no Th1 cells apparent at the acute stage. After chronic challenge Th17 cells and neutrophilia persisted while fewer eosinophils were present. Irrespective of mode of sensitization, we observed an increase in Th1 cells at the chronic stage. Thus the route and chronicity of allergen challenge were more important than the nature of initial sensitization in directing the response. However to ensure that our results were not influenced by systemic sensitization protocols we compared OVA/alum sensitization with mucosal sensitization via intranasal OVA+TNF-α treatment (Fig 2B-D). Mucosally sensitized mice also developed a Th17-dominated response with mixed eosinophilia/neutrophilia. However eosinophilia was less pronounced and neutrophilia increased progressively as inflammation progressed through subchronic and chronic phases (Fig 2B). Proportions of IL-17+ cells decreased at the chronic stage with OVA/alum and increased with OVA/TNF-α sensitization (Fig 2C). IL-17A in BAL increased substantially at the chronic stage (Fig 2D) in mucosally but not parenterally sensitized mice. OVA/TNF-α resulted in significantly higher IL-17A and IFN-γ but lower IL-13 levels (Fig 2D) compared to OVA/alum. Airway remodeling was observed in both models, as indicated by peribronchiolar collagen deposition and increased airway smooth muscle (shown in supplementary Fig S1), and increased total lung collagen content (Fig 2E). H&E staining (Fig S1) revealed pronounced leukocytic inflammation around the airways at the chronic stage in both models.
Figure 2. Th17 responses persist during airway remodeling induced via parenteral or mucosal sensitization.
A: Numbers of eosinophils, neutrophils and IL-17+ (Th17), IL-4+ (Th2) and IFN-γ+ (Th1) CD4 T cells in the BAL of mice after parenteral sensitization with Th1- (CASAC), Th2- (alum) or Th17-skewing (CFA) adjuvants. Acute (day 13) and chronic (day 53) stages of the response (as described in Methods) are shown. N=5, similar results were obtained in 2 experiments. B-D: Mucosal sensitization with OVA+TNF-α induces an increasingly neutrophilic and IL-17-dominated response compared to parenteral OVA/alum sensitization. B shows eosinophilia & neutrophilia at acute (day 13) sub-chronic (day 33) and chronic stages of disease, C shows % Th1/2/17 cells in BAL CD4 cells. D: levels of cytokines in BAL supernatants. E total lung collagen assay. N=5, representative of 2 experiments. Histological analysis of airway remodeling is shown in supplementary figure S1.
Mucosal inflammation and tolerance to OVA both involve Treg recruitment but Treg from inflamed lungs have low Helios expression and proliferative responses
Since Th17 are closely related to the Foxp3 Treg lineage and RORγt and Foxp3 are reciprocally regulated, we investigated Treg responses in mice developing tolerance or acute lung inflammation to OVA challenge. It is well known that i.n. OVA challenge of unsensitized mice leads to tolerance. However compared to PBS challenge, OVA challenge of mice primed with alum only induced low level T cell infiltration of the airways (Fig 3A). Unlike in sensitized animals, there were no eosinophils or neutrophils recruited (Fig 3A). Suspecting that infiltrating T cells in tolerant mice were Treg, we found increased proportions of Foxp3+ CD4 T cells in the lung but not draining LN of both tolerant and sensitized animals (Fig 3B). Expression of GARP (Glycoprotein-A Repetitions Predominant), the Treg-associated activation marker19, was increased in both lung and LN (Fig 3C).
Figure 3. Respiratory tolerance involves lung Treg recruitment, but Treg in inflamed lung tissue are unresponsive.
A: BAL numbers of inflammatory cells induced by 4 i.n. doses of OVA to unsensitized mice primed with alum only (OVA, i.e. tolerance) or parenterally sensitized mice primed with OVA/alum (OVA/alum, i.e. inflammation). Naïve controls received alum alone then PBS i.n. (PBS). N=5, representative of 3 experiments. B: %Foxp3+ Treg in CD4 cells of lung tissue and LN from mice as in A. C: Surface GARP expression on CD4 cells as in B. D-F: Cells as in B&C were CFSE-labelled and cultured for 4 days in OVA before Foxp3 staining. Proliferation of Foxp3+ CD4 cells was calculated as % divided within the Foxp3+ CD4+ population. D: representative flow cytometry profile, E: pooled % Foxp3+ data from 5 experiments after 4 days culture, n=14. F: pooled % divided data within Foxp3+ CD4+ populations from 5 experiments. G&H: Helios expression expressed as MFI of staining within Foxp3+ CD4 cells from mice as in B, obtained directly ex vivo (G) or after 4 days expansion in OVA (H). I: IL-10 and IFN-γ staining in mediastinal LN as in D cultured for 4 days in OVA and restimulated with PMA+ionomycin. CD4-gated events shown, mean±SEM from 12 mice pooled from 4 experiments.
To determine whether the Treg were active in response to allergen, we CFSE-labeled lung and LN cells and cultured them in OVA for 4 days before CD4/Foxp3 staining. Cells from PBS-challenged mice failed to grow and Foxp3+ cells did not survive (Fig 3D upper panels). Cells from OVA-challenged unsensitized mice proliferated and a much higher proportion expressed Foxp3 after culture (Fig 3D,E&F). Interestingly, cells from inflamed lungs of sensitized mice contained high proportions of Foxp3+ CD4 cells but these Treg had not divided during culture in the presence of allergen. By contrast, Foxp3− CD4 cells from inflamed lung and both Treg and non-Treg from LN of either group divided extensively (Fig 3D,E&F). Since this suggested an impairment in Treg activity in the inflammatory environment of the lung, we also determined Helios expression in the same Treg before and after culture (Fig 3G&H). Helios expression within the Foxp3+ population was generally lower in lung cells than LN, but was increased during tolerance induction. By contrast CD4 Treg from inflamed lung, but not LN, showed low Helios expression. The difference in Helios was most pronounced after culture in allergen (Fig 3H).
Respiratory tolerance involves priming for IL-10 and IFN-γ production in CD4 T cells
We measured the expression of IL-10 by intracellular staining of CD4 cells from OVA-challenged tolerant or sensitized animals. IL-10 staining was not detectable directly ex vivo or in lung cells, but could be revealed after expansion of the draining LN cells in OVA for 4 days (Fig 3I). There was little growth or IL-10 staining in control LN cultures, but CD4 cells from tolerant mice produced IL-10 and IFN-γ with a high proportion staining positive for both. These cytokines were reduced when animals were pre-sensitized. IL-10 could not be co-stained with Foxp3.
IL-17 abrogates respiratory tolerance and enhances epithelial permeability of airways to allergen
We asked whether IL-17 induced by allergen exposure could regulate activity of lung Treg and promote sensitization. Mice were treated i.n. with OVA alone, OVA + IL-17A, or OVA + TNF-α as a positive control for sensitization. After re-challenge with OVA alone it was clear that IL-17 had induced recruitment of eosinophils and greatly increased T cell infiltration (Fig 4A). This was however much less inflammation than induced by TNF-α. Furthermore, the proliferative response of Foxp3+ CD4 Treg (as in Fig 3F) in tolerant mice was abrogated after IL-17 as well as TNF-α exposure (Fig 4B). Priming for IL-10 production in draining LN CD4 cells was also significantly suppressed by IL-17 (Fig 4C). This abrogation of Treg activity could have been due to enhanced allergen uptake, since intranasal instillation of fluorescent-tagged OVA±IL-17 revealed that IL-17 promotes allergen uptake into lung tissue within 6 hours (Fig 4D). Uptake was by both CD11c+ and CD11c− cells, suggesting increased epithelial permeability after IL-17 exposure.
Figure 4. IL-17 abrogates Treg-associated respiratory tolerance and enhances allergen uptake from airways to lung tissue.
A: Numbers of inflammatory cells in BAL of mice treated i.n. with 2 doses of OVA (50@g) ± IL-17 (0.5@g) or TNF-α (1μg), followed by 2 doses of OVA alone. Controls received PBS only. B: Treg activity in mice as in A, assessed by CFSE-labeling and culture for 4 days in OVA prior to Foxp3 staining. Mean % divided ±SEM from 5 animals is shown. C: %IL-10 and IFN-γ staining within CD4 gate from mediastinal LN cultured for 4 days in OVA before PMA+ionomycin restimulation, n=5. D: Uptake of Alexa-488-labelled OVA onto lung tissue cells 6 hours after i.n. challenge of naive mice with 50@g OVA-Alexa488 ± 0.5μg IL-17. Histograms gated on CD11c+ DC/macrophages and CDllc− lung cells are shown, alongside MFIsCSEM from n=3 mice. Statistical comparisons to OVA only group are shown. Results are representative of 2 experiments.
Th17-deficient mice display less airway remodeling after chronic allergen challenge
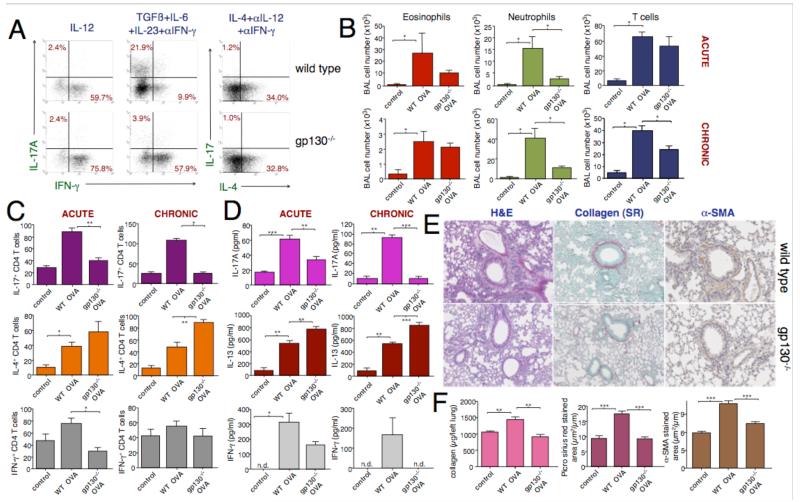
To determine the role of Th17 cells in airway remodeling we obtained CD4-CreXgp130flox/flox mice20, here designated gp130−/−, which have a gp130 deficiency targeted to the T cell lineage. Since gp130 is the signaling component of the IL-6 receptor these mice have T cells unable to respond to IL-6R signaling. Animals with the gp130 mutation but not the CD4-Cre recombinase transgene were used as controls and are referred to as wild-type (WT). We confirmed that CD4 cells from gp130−/− but not controls were deficient in Th17 development in vitro but not in Th1 or Th2 development (Fig 5A). We then compared groups of mice in which acute or chronic allergic airway inflammation had been induced, using the OVA/TNF-α mucosal sensitization model to maximize the chronic neutrophilic response. Lung eosinophilia was not significantly altered in the Th17-deficient strain at the acute or chronic stage, but neutrophilia was profoundly inhibited in Th17-deficient mice (Fig 5B). Levels of BAL T cell infiltration were unchanged in gp130−/− animals at the acute stage but were less than WT mice by the chronic stage (Fig 5C). Numbers of airway Th17 were reduced at both time points, while Th2 cells significantly increased in chronically challenged lungs from the gp130−/− strain. The shift from a Th17 to a Th2 response in gp130−/− was confirmed by detection of lower IL-l7 and higher IL-13 in BAL at both time points (Fig 5D). Although there was a trend for lower IFN-γ in gp130−/−, the Th1 response was negligible in these experiments. Most notably, at the chronic time point, the Th17-deficient animals had greatly reduced signs of airway remodeling as assessed by histology and lung collagen content (Fig 5E&F), despite the continued presence of eosinophils, Th2 cells and IL-13 in the airways of these mice.
Figure 5. Th17 deficiency caused by disrupted T cell IL-6 signaling leads to reduced chronic airway pathology.
A: Cytokine staining in CD4 cells from gp130−/− (lower panels) or control (WT) mice cultured under Th1 (IL-12), Th17 (TGF-ß, IL-6/23, anti-IFN-γ) or Th2 (IL-4, anti-IL-12/IFN-γ) polarizing conditions with anti-CD3+anti-CD28 for 5 days, representative of 3 experiments. B-D: Altered airway inflammation in gp130−/− mice. Groups of gp130−/− or WT mice were mucosally sensitized with OVA/TNF-α and challenged with OVA until day 13 (acute) or day 53 (chronic) time points (n=6, representative of 2 experiments). B: Infiltration of inflammatory cells in BAL; control animals received PBS only. C: Numbers of Th17, Th2 and Th1 CD4 cells in BAL, D: Cytokines in BAL supernatants. E&F: Th17 deficiency abrogates airway remodeling. Chronically challenged gp130−/− or WT groups as in B were assessed by lung histology (E), with staining for collagen and α-SMA quantified in F. F also shows total lung collagen content. N=6, representative of 2 experiments.
Retinoic acid treatment alters the balance between Th17 and Treg activity
We determined whether all-trans retinoic acid (ATRA), the active form of vitamin A, could block Th17-associated pathology or increase Treg activity. We confirmed that ATRA specifically blocks Th17 differentiation in vitro, whilst not affecting Th2 or Th1 development (Fig 6A). ATRA is known to enhance TGF-ß-induced Treg development in vitro21. Here we showed it also enhances growth/expansion of the natural Treg population (Fig 6B). CD4+CD25+ cell growth was enhanced by ATRA resulting in increased yields of Foxp3-staining CD4 cells, while expansion of Foxp3− cells in CD4+CD25− cultures was inhibited. The same effect was observed using the glucocorticoid dexamethasone (DEX, Fig 6B). To determine effects of ATRA in vivo we induced chronic AAI by mucosal sensitization and then commenced i.p. ATRA dosing at 3 different time points (days 26, 35 or 45); mice were killed on day 53. Only prolonged ATRA treatment (day 26 group) significantly inhibited T cell infiltration and neutrophilia (Fig 6C). By contrast ATRA enhanced eosinophilia, most significantly after a shorter period of treatment (Fig 6C). Prolonged ATRA treatment significantly reduced lung collagen content (Fig 6F) but its effect on Th17 cell numbers was not statistically significant and it had no effect on Th2 cells (Fig 6D). The proportion of Foxp3+ CD4 Treg cells in LN was temporarily increased after a week of ATRA treatment (day 45 group, Fig 6E). Although there was no increase in proportion of Foxp3+ Treg in lung tissue in any group, ATRA treatment did significantly increase expression of GARP on lung CD4 cells, indicating increased Treg activation. Thus ATRA treatment alters the balance of Th17 and Treg activity but its beneficial effects may be partially abrogated by enhanced eosinophilia. Finally, airway remodeling appeared to be improved by ATRA treatment from day 26 (Fig 6F), although there was no significant change in quantified smooth muscle α-actin (α-SMA; histological analysis is shown in supplementary Fig S2).
Figure 6. ATRA treatment boosts Treg activity and inhibits chronic Th17/neutrophil-associated airway remodeling.
A: Effect of ATRA on in vitro Th17, Th2 and Th1 development. OT-2 CD4 cells were cultured in polarizing conditions ± different ATRA concentrations for 6 days before washing and restimulation for cytokine staining. MeanMSEM from 3 experiments and expressed as % control (no ATRA). B: Effect of ATRA on CD4 Foxp3+ Treg expansion in vitro. CD25+ and CD25− CD4 cells were cultured with anti-CD3/28 + IL-2, ± ATRA, for 4 days, stained for Foxp3 and numbers of Foxp3+ and Foxp3− cells in each culture counted by flow cytometry; representative of 3 experiments. C-G: Effect of in vivo ATRA treatment, commenced on day 26 (d26), day 35 (d35) or day 45 (d45) of chronic OVA/TNF-α protocol, on airway disease assessed on day 53. C shows eosinophil, neutrophil and T cell infiltration, without (OVA) or with ATRA treatments. N=5, representative of 2 experiments. D: Numbers of Th17, Th2 and Th1 cells in BAL. E: Treg marker expression in CD4 populations from lung and LN cells. F: Total lung collagen assay. Histological analysis of airway remodeling is shown in supplementary Figure S2.
Dexamethasone enhances Th17 differentiation in vitro and fails to inhibit neutrophilia in vivo despite reduced T cell inflammation
Since Th17 cells have been associated with glucocorticoid-resistant airway inflammation11, we determined the effect of DEX on Th17 responses in vitro and in vivo. Addition of DEX to proliferating OT-2 CD4 cells in the presence of Th17-polarizing cytokines (TGF-ß, IL-6 and IL-23) strongly enhanced Th17 differentiation (Fig 7A). Another drug with potent potentiating effects on Th17 development was UO126, an inhibitor of TCR-triggered MEK/ERK signaling (Fig 7A). Th17 cells induced by these drugs also expressed IL-22 (supplementary Fig S3). Addition of both drugs induced the highest levels of Th17 development and intracellular RORyt expression (Fig 7A lower panels). These data indicated that glucocorticoid receptor and MAP kinase pathways are positive and negative regulators of Th17 development respectively. When we examined the effect of in vivo DEX treatment on acute and chronic airway disease initiated by mucosal sensitization, we found that prolonged DEX treatment inhibited lung Th17 recruitment and chronic eosinophilia (Figs 7B&C). However, neutrophilia was not inhibited by DEX. Short-term treatment of acute inflammation showed no significant inhibition by DEX. The increase in lung collagen content associated with airway remodeling was completely abrogated by DEX treatment (Fig 7D). Thus certain aspects of Th17/neutrophil-associated disease, which increase with prolonged allergen exposure, may be refractory to inhibition by glucocorticoids. We therefore measured production of further cytokines IL-17F, IL-22, IL-25 and IL-33 in this model. These cytokines were undetectable in BAL fluid but were secreted by draining LN cells after restimulation with OVA, with the exception of IL-17F which was undetectable (supplementary Fig S3). All cytokines were inhibited by in vivo DEX treatment. The distinct effect of DEX seen in vivo may be attributable to its ability to block expansion of OVA-specific T cells in LN, as shown in Fig S3.
Discussion
Asthma has traditionally been viewed as an allergic disease mediated by Th2 airway infiltration and eosinophilia, but a more complex set of pathologies is now apparent. Poor understanding of asthma sub-phenotypes and over-reliance on a simplified model of acute experimental allergic asthma might explain the poor translation of new asthma therapies to the clinic. While the presence of the Th17 cell in asthma has been postulated for some time, our study is the first to establish a direct role for this pathway in airway remodeling and to examine immunomodulatory strategies for targeting Th17 cells in a chronic disease model. Th17 cells and IL-17 can play beneficial as well as detrimental roles in airway pathology. Predominance of the Th17 response in the lung may reflect that the Th17 phenotype is tailored to meet the needs of the respiratory mucosa, increasing immune surveillance while restricting tissue damage via curtailment of inflammation or cross-regulation of allergic responses12, 13. Nevertheless it is clear from our data that chronic Th17 inflammation contributes to airway remodeling and persistence of allergen sensitivity with increasing neutrophilia. Since IL-17 increases allergen transfer into lung tissue, allergen-driven Th17 cells might also increase susceptibility of lung tissue to infection, resulting in asthma exacerbations 22 or accelerated tissue damage.
In humans, immune responses to inhaled aeroallergens develop in the lung and draining LN. Many animal models bypass this route by using i.p. injections of allergen in alum adjuvant. Some features of this model are inconsistent with human asthma, e.g airway eosinophils can reach 80%23, whereas in patients it is rarely greater than 5%24. The mixed Th17/Th2 response we observed was apparent even in primary T cell responses in draining LN without adjuvant. Tolerance to inhaled OVA in naive mice did induce T cell activity and influx into the lung but this involved Treg and not T effectors. Foxp3+ Treg appear to be generated in respiratory LN in response to allergen, recruited to the lung and then further activated by allergen. We demonstrated priming for IL-10 and IFN-γ production during tolerance, both cytokines known to block allergic inflammation and/or Th17 responses25-27. We could not determine whether the allergen-induced Treg were derived from the natural or peripherally-induced Foxp3+ population. However since they expressed high Helios levels during tolerance and thymic Treg from OT-2 transgenic mice proliferated extensively upon exposure to inhaled OVA, it seems most likely they are derived from natural Treg.
Our novel observations on Treg activity and phenotype in inflamed lungs compared to tolerance may help in development of immunotherapeutic strategies. The inflammatory milieu of the lung appeared to block the ability of Treg to respond to allergen and this state of “paralysis” was associated with reduced Helios expression, a molecule recently associated with suppressive activity28. Although it is possible that the lack of response to OVA was due to a lack of OVA-specificity (i.e. inflamed lungs recruited large numbers of non-specific Treg), OVA-responsive Treg continued to be generated in LN. Perhaps a short-lived inflammatory mediator present in lung tissue but not reaching LN was inhibiting local Treg activity. Reversing this hypothetical state of paralysis in local tissue Treg could potentially assist resolution of established disease.
The OVA/TNF mucosal sensitization model of chronic allergen exposure employed here resulted in less airway remodeling than OVA/alum but may be closer to the human situation and mimic the prominent neutrophilic response seen in some severe asthmatics29. This model recapitulates many clinical features of severe asthma, including closely associated IL-17 and neutrophil levels30, increasing Th17 markers with increasing severity of disease, increased expression of IL-23 and RORyt31, and early airway remodeling32. It therefore provides a potentially useful model for testing new asthma treatments, which can be introduced after initiation of remodeling. Use of OVA rather than house dust mite, which has natural adjuvanticity, allowed us to directly compare tolerance with inflammation in response to the same allergen, revealing that IL-17, like TNF-α, can abrogate tolerance. Activation of epithelium by IL-17 and increased epithelial permeability could expose lung tissue to more allergen-associated or other danger signals in the environment, blocking Treg activity. IL-17 also abrogated priming of CD4 cells for IL-10 and IFN-γ, associated with tolerance. Thus, as well as reciprocal expression of RORyt and Foxp3 in developing allergen-specific T cells, cross-regulation between IL-17 and IL-10 may underlie the inability of sensitized mice to resolve inflammation during persistent allergen exposure. This provides a rationale for therapeutic blockade of both IL-6 and IL-17.
IL-17 has a putative role in acute severe and chronic asthma33, 34. B6 mice demonstrate airway hyperreactivity during acute disease but this is much reduced in the chronic phase. We therefore did not study airway hyperreactivity in chronic disease but focussed on remodeling. We showed that IL-17 is increased alongside remodeling, and that allergen-driven Th17 cells rather than other sources of IL-17 are capable of inducing remodeling. Although some murine DCs express CD4 and could potentially be affected by the mutation in gp130−/− mice, the profound defect we demonstrated in Th17 development is a more likely explanation for the lack of remodeling observed in this strain. Whether Th17 cells induce structural changes directly or via recruitment of neutrophils is not clear since there was a strong correlation between Th17 cells and neutrophilia.
Despite coexistence of Th17 and Th2 cells in inflamed tissues, there is some cross-regulation between Th17 and other subsets, explaining beneficial effects of Th17 development on Th1/Th2-mediated disease. However models where plasticity between Th17 and Th1/Th2 cells are prominent provides evidence for pathogenicity of Th17s. The late appearance of a Th1 response in the lung after chronic allergen challenge seen here (Figure 2) has been observed previously35. It may reflect deviation from a Th17 to Th1 phenotype or be a consequence of remodeling and epithelial dysfunction, leading to a breakdown of mechanisms blocking Th1 responses in the lung. Interestingly it has been proposed that only fully mature Th17, after exposure to IL-23, produce Th1 cytokine TNF-α, and IL-2236. Since neither cytokine was detected in lung Th17 cells but both were produced by in vitro generated cells, in vitro derived Th17 used for adoptive transfer studies11 may be more differentiated and pathogenic than natural lung Th17. Clearly TNF-α production would have a potent sensitizing effect on the airway. Nevertheless, IL-17 alone was sufficient to abrogate respiratory tolerance, probably via activation of epithelial cells that strongly express IL-l7 receptor37.
Th17 deficient animals were skewed towards Th2 immunity compared to wild-type. Therefore a strong Th17 response to allergen may be beneficial in acute disease due to inhibition of more damaging Th2-mediated pathology, as proposed13. However failure to resolve acute Th17 inflammation can clearly lead to long-term tissue damage and remodeling. Since remodeling is such a prominent feature of human asthma, our data support the idea that Th17 pathways could be therapeutic targets in asthma. Natural Treg expansion was enhanced by ATRA, and in vivo ATRA treatment increases proportions of Treg in LN while enhancing their activation (reflected by GARP expression) in the lung. GARP (LRRC32) is an essential receptor for latent TGF-ß on the surface of active Treg19 and is related to suppressive activity38. Treating mice with ATRA during the allergen challenge period ameliorated chronic disease despite exacerbating eosinophilia. This was not associated with an increase in Th2 cells and may have been due to ATRA directly enhancing eosinophil survival39. Our data indicate that IL-17 blockade could ameliorate or exacerbate disease dependent on the type of patient, and like retinoic acids, should be combined with a therapy that blocks Th2 inflammation.
Although DEX enhanced Th17 development in vitro, prolonged treatment with the drug in vivo inhibited Th17-mediated inflammation in chronic disease. This was probably due to the multiple effects of glucocorticoid on diverse cell types including DCs, which could have restricted expansion of allergen-specific T cells and their recruitment to the lung. However the in vitro experiments, in which excess allergen was provided to the cultures, would suggest activity of established Th17 effectors could be resistant to glucocorticoid. Chronic neutrophilia was uninhibited by DEX treatment, in contrast to eosinophilia. This could be due to resistance of Th17 cell activity to DEX inhibition or resistance of neutrophils to DEX-mediated apoptosis40. Although our data do not support the idea that glucocorticoid treatment could exacerbate Th17/neutrophilic components of asthma, they do suggest that other therapies may be indicated for treatment of severe/neutrophilic asthmatics. Most severe, treatment refractory asthma patients have mixed neutrophilia/eosinophilia while around 40% have only neutrophilia41. The latter group may benefit most from Th17-targeted therapies.
Methods
Mice
All experiments were approved by our Institutional Animal Welfare Committee under UK Home Office Regulations. C57BL/6 (B6) mice (Harlan) were used at 4-8 weeks. OT-2 TCR transgenic mice, specific for OVA323-339/I-Ab and CD4-Cre+/−Xgpl30flox/flox mice provided by Dr Werner Müller (University of Manchester) were on a B6 background. Homozygous CD4-Cre+/+Xgp130flox/flox mice, with a deletion of the gp130 IL-6 receptor signaling component (Il6st, IL-6 signal transducer) specifically in T cells, were used as a Th17-deficient strain20 and designated gp130−/−. Control groups in these experiments were CD4-Cre−/−Xgp130flox/flox and designated wild type.
Assessment of primary allergen responses
MACS™-purified CD4 T cells from OT-2 LN + spleen cells were labeled with 5μM CFSE and transferred into the tail vein of B6 recipients given 50μg OVA ± lμg rmTNF-α in 50μl PBS i.n. After 3 days mediastinal LN cells were stimulated with PMA (l0ng/ml) + ionomycin (400ng/ml) + monensin (3μM) for 5 hours before intracellular cytokine staining.
Induction of acute and chronic allergic airway inflammation
For parenteral sensitization protocols, mice were sensitized i.p. with l0μg OVA in 0.2ml alum or complete Freund’s adjuvant or i.d. in CASAC as described42. Chronic challenge groups were immunized on days 0 and 12. Controls received PBS. For mucosal sensitization 10μg OVA+1μg TNF-α was given i.n. on days 0 and 3. Acute challenge mice received 50μg OVA i.n. on days 7, 9-12, and were sacrificed day 13. Chronic groups received 50μg OVA i.n. daily between days 17 and 21, then 3 times a week from day 24 until day 53. All intranasal dosing was given in 50μl PBS/mouse under light inhaled anesthesia (isoflurane). OVA (Sigma, grade V) contained 0.4μg endotoxin per mg OVA protein.
Drug treatments
DEX (Sigma) was mixed with OVA and administered i.n. at 2μg/mouse. All-trans retinoic acid (ATRA, Sigma) in DMSO/olive oil was given i.p. at 100μg/mouse, controls received carrier alone. rmIL-17 was given at 0.5μg IL-17±50μg Alexa488-labelled OVA i.n.
Histopathology and immunostaining of lungs
Paraffin sections were stained with haematoxylin and eosin (H&E) or Picro-sirius red. For α-SMA staining deparaffinized sections were incubated with anti-α-SMA (Abcam, 1/25O) or control rabbit Ig revealed with VECTASTAIN® ABC kit (Vector Labs). Peri-bronchiolar collagen deposition and smooth muscle were quantitated using ImageScope image analysis software (Aperio Technologies) with virtual slides generated by a NanoZoomer 2.0-HT scanner, Hamamatsu Photonics. Circular lines were drawn around the outer and inner bronchiole of 5 bronchioles with a 150-200μm diameter at comparable sites for each slide, using ×400 magnification. The area of specific staining within the airway wall was calculated and divided by the length of the basement membrane for each bronchiole (μm2 per μm).
Collagen assay
Total lung collagen content was measured by Sircol kit (Biocolor Ltd).
Flow cytometry
Antibody staining (0.1μg/sample, eBioscience except anti-IL-22 (Poly5164, BioLegend), anti-IL-4 (BVD6-24G2, Caltag) and anti-CCR6 (29-2L17, BioLegend) was analyzed on a FACScalibur (BD). Inflammatory cells in BAL were identified as in43 with addition of anti-Gr-1 to identify neutrophils (Gr-1+CD11c−CCR3−CD4−CD8−B220−). BAL T cells were separated with anti-CD3/28 Dynabeads and cultured with 3μM monensin for 5 hours. Total cell numbers were calculated by analysis of fixed sample volumes, validated with fluorescent beads. Lung T cells were stimulated with immobilized anti-CD3 (1μg/ml) + anti-CD28 (1μg/ml) + monensin (3μM) or OVA (500μg/ml) + anti-CD28/monensin for 12 hours before intracellular cytokine staining as described44. Intracellular staining protocol for Foxp3 and Helios in CD25+ Treg was as described44.
BAL and LN supernatant ELISAs
ELISAs were performed on BAL fluid or supernatants from mediastinal LN cells cultured (2×l06/ml) for 72 hours with OVA (500μg/ml), as described45 using antibody pairs for IL-17A (R&D), IL-23 (eBioscience), IFN-γ (BD), IL-13 (Arcus), and eBioscience kits for IL-17F, IL-22, IL-25 and IL-33.
In vitro differentiation
Th17 cells were generated from naïve OT-2 CD4+ CD44lo T cells + splenic APC in XVIVO-15 + OVA323-339 (2μg/ml) + hTGFβ (3ng/ml) + IL-1ß (lOng/ml), IL-6 (20ng/ml) + IL-23 (10ng/ml) ± DEX (10nM) or UO126 (Calbiochem, 3μg/ml). Th1 cultures contained IL-12 (10ng/ml) and Th2 IL-4 (10ng/ml) + anti-IL-12/IFN-γ (2.5μg/ml).
Statistical analysis
Data are means ± SEM. Significance between groups was assessed using Student’s t tests except where SDs were significantly different, in which case Mann-Whitney tests were used. In all figures * indicates P<0.05, **: P<0.01, ***: P<0.005.
Supplementary Material
Acknowledgements
This work was funded by Asthma UK grant 07/022. We thank Werner Muller (University of Manchester) for gp130flox/flox mice, Guy’s Hospital tissue bank team for help with histology, and Sejal Saglani and Sun Ying for advice on analysis of airway remodeling.
Footnotes
Disclosure: The authors have no conflicting financial interests.
References
- 1.Kumar RK, Herbert C, Webb DC, Li L, Foster PS. Effects of anticytokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med. 2004;170:1043–1048. doi: 10.1164/rccm.200405-681OC. [DOI] [PubMed] [Google Scholar]
- 2.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 3.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-W antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 4.Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological Features of Asthma Develop in Parallel in House Dust Mite Exposed Neonatal Mice. Am J Respir Cell Mol Biol. 2009;41:281–289. doi: 10.1165/rcmb.2008-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnyder-Candrian S, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doe C, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 8.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanaudenaerde BM, Wuyts WA, Dupont LJ, Van Raemdonck DE, Demedts MM, Verleden GM. Interleukin-17 stimulates release of interleukin-8 by human airway smooth muscle cells in vitro: a potential role for interleukin-17 and airway smooth muscle cells in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22:1280–1283. doi: 10.1016/s1053-2498(02)01234-2. [DOI] [PubMed] [Google Scholar]
- 10.Molet S, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 11.McKinley L, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing γδ T cells. Am J Respir Crit Care Med. 2010;182:464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie AN. Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clin Exp Allergy. 2011;41:1447–1455. doi: 10.1111/j.1365-2222.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- 14.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 16.Piggott DA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of Th17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 18.Wells JW, Cowled CJ, Farzaneh F, Noble A. Combined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunity. J Immunol. 2008;181:3422–3431. doi: 10.4049/jimmunol.181.5.3422. [DOI] [PubMed] [Google Scholar]
- 19.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-ß on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasnacht N, Greweling MC, Bollati-Fogolin M, Schippers A, Muller W. T-cell-specific deletion of gp130 renders the highly susceptible IL-10-deficient mouse resistant to intestinal nematode infection. Eur J Immunol. 2009;39:2173–2183. doi: 10.1002/eji.200838710. [DOI] [PubMed] [Google Scholar]
- 21.Mucida D, et al. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett NW, et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung TT, et al. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 24.Lex C, et al. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- 25.Akbari O, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, et al. Effect of interferon-γ on allergic airway responses in interferon-γ-deficient mice. Am J Respir Crit Care Med. 2002;166:451–456. doi: 10.1164/rccm.200202-095OC. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Zabransky DJ, et al. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006;368:780–793. doi: 10.1016/S0140-6736(06)69288-X. [DOI] [PubMed] [Google Scholar]
- 30.Bullens DM, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 32.Saglani S, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 33.Linden A. Role of interleukin-17 and the neutrophil in asthma. Int Arch Allergy Immunol. 2001;126:179–184. doi: 10.1159/000049511. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ramli W, et al. Th17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 35.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 37.McAllister F, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan DV, et al. Signal peptide cleavage is essential for surface expression of a regulatory T cell surface protein, leucine rich repeat containing 32 (LRRC32) BMC Biochem. 2011;12:27. doi: 10.1186/1471-2091-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueki S, et al. Retinoic acids are potent inhibitors of spontaneous human eosinophil apoptosis. J Immunol. 2008;181:7689–7698. doi: 10.4049/jimmunol.181.11.7689. [DOI] [PubMed] [Google Scholar]
- 40.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 41.Wenzel SE, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 42.Wells JW, Choy P, Lloyd CM, Noble A. Suppression of allergic airway inflammation and IgE responses by a class I restricted allergen peptide vaccine. Mucosal Immunol. 2009;2:54–62. doi: 10.1038/mi.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–121. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Giorgini A, Noble A. Blockade of chronic graft-versus-host disease by alloantigen-induced CD4+CD25+Foxp3+ regulatory T cells in non-lymphopenic hosts. J Leukoc Biol. 2007;82:1053–1061. doi: 10.1189/jlb.0407227. [DOI] [PubMed] [Google Scholar]
- 45.Thomas MJ, Noble A, Sawicka E, Askenase PW, Kemeny DM. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J. Immunol. 2002;168:216–223. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.