Abstract
Introduction
The goals of scale-up of antiretroviral therapy (ART) have expanded from prevention of morbidity and death to include prevention of transmission. Morbidity and mortality risk are associated with CD4 counts; transmission risk depends on plasma viral load (VL). This study aimed to describe CD4 count and VL distributions among HIV-infected individuals in a South African township to gain insights into the potential impact of ART scale-up on community HIV transmission risk.
Methods
A random sample of 10% of the adult population was invited to attend an HIV testing service. Study procedures included a questionnaire, HIV testing, CD4 count and VL testing.
Results
1144 (88.0%) of 1300 randomly selected individuals participated in the study. 260 tested positive, giving an HIV prevalence of 22.7% (95%CI:20.3-25.3). A third of all HIV-infected individuals (33.5%, 95%CI:27.8-39.6) reported taking ART. The median CD4 count was 417 cells/μl (IQR:285-627); 33 (12.7%, 95%CI:8.9-17.4) had a CD4 count of ≤200 cells/μL. VL measurements were available for 219 (84.2%) individuals and were undetectable in 72 (33.9%), >1500 copies/ml in 127 (58.0%) and >10,000 copies/ml in 96 (43.8%). Of those reporting they were receiving ART, 30.4% had a VL>1500 copies/ml compared to 58.0% of those reporting they were not receiving ART.
Conclusion
A small proportion of those living with HIV in this community had a CD4 count <200 cells/μl; more than half had a VL high enough to be associated with considerable transmission risk. A substantial proportion of HIV-infected individuals remained at risk of transmitting HIV even after starting ART.
Introduction
Of an estimated 34 million people living with HIV in 2010, 67% of them were in sub-Saharan Africa [1]. Enormous progress has been made in scale-up of antiretroviral treatment (ART) in the region. The initial focus of ART scale-up was on reducing morbidity and mortality [2] and so, with resources being scarce, eligibility for ART was initially defined as WHO stage 4 disease and/or a CD4 count ≤200 cell/μl. Great success has been seen at both an individual level [3,4] and at a population level [5,6,7] in reducing morbidity and mortality. However, less than half (47%) of people who need ART using a CD4 count threshold of ≤ 350 cells/μl are thought to be receiving it [8]. Increasing evidence from randomized trials [9] and cohort studies [10] has shown the benefit of starting ART at higher CD4 count thresholds rather than delaying treatment. Thus, revised World Health Organization (WHO) guidelines for resource-limited settings in 2010 recommended that ART be started at CD4 counts ≤350 cells/μl [11,12]. The debate around when to start ART has intensified with the additional benefit of early ART for prevention of HIV transmission emerging as another key consideration.
HIV RNA level is the single most important predictor of HIV transmission [13,14,15]. A study from Rakai, Uganda, reported that there were no instances of HIV transmission between sero-discordant couples when serum HIV RNA levels in the infected partner were ≤1500 copies/ml [13]. These results led to increased interest in the potential benefits of ART in HIV prevention [16,17,18,19,20]. The HPTN 052 trial [21] of sero-discordant couples found that the risk of HIV infection in the sero-negative partner was reduced by 96% when the HIV infected partner received ART. Several observational studies have shown similar results [22]. Thus, the goal of ART scale up has expanded from preventing death and morbidity to include prevention of infection (treatment as prevention, “TasP”).
The impact of ART on population morbidity and mortality depends on the CD4 count distribution in treated individuals and thus the population CD4 count reflects population health. In contrast, the impact of ART on HIV incidence in a population depends on reductions in population viral load (VL) as shown in Vancouver [23] and San Francisco [24]. Thus, the population VL is a proxy for the average transmission risk in a given population. The impact of ART on these population-based biomarkers is influenced by HIV test uptake, linkage to and retention in ART care as well as adherence to treatment [25]. Most previous studies on CD4 distributions in African populations were done prior to widespread access to ART [26,27,28,29,30]. Similarly, the impact of ART scale-up on population viral load in communities in sub-Saharan Africa is unkown. Viral load data in sub-Saharan Africa are available from ART cohorts, but data on population viral load are lacking.This study describes CD4 count and viral load distributions among HIV-infected individuals using data from a community-based HIV sero-prevalence study in a South African township [31].
Methods
Study community
The study was conducted in a peri-urban township in the greater area of Cape Town, with a population of approximately 17,000 people and a measured adult HIV prevalence of 23% in 2010 [31]. The community is served by a single public-sector primary care clinic, which provides ART. A hospital provides all secondary care for the population. The hospital also provides ART for some HIV-infected individuals from the community.
ART provision began in 2004. Between 2005 and 2008 ART services were partly provided according to the Antiretroviral Treatment Protocol of the Western Cape [32] and partly through a study funded by the National Institutes of Health (NIH) [33]. Patients enrolled in the NIH-funded study could access ART with a CD4 count below 350 cells/μl or WHO stage 3 disease whereas eligibility in the provincial programme was defined by a CD4 count of 200 cells/μl or WHO stage 4 disease. The NIH-funded study completed enrollment at the end of 2006 after which all new patients were treated under the provincial ART program guidelines.
Community-based cross-sectional survey
A population-based survey estimating HIV prevalence, CD4 count and viral load distributions was conducted between September and December 2010 and has been described in detail elsewhere [31]. In brief a house-to-house enumeration of the community in August 2010 provided a database of 12520 residents 15 years or older of whom 1300 residents were randomly selected for inclusion in the study (10% of the community). Simple random sampling was performed using Stata 11.0 (Stata Corp. LP, College Station, TX, United States of America). Each adult resident in the community had an equal chance of being selected for the survey. The census 2010 data were used as a sampling frame. Field workers invited the selected individuals to attend a mobile HIV testing service. Field workers visited households of selected individuals up to 5 times to encourage participation. No study procedures were performed in people’s homes. Consent, questionnaires, HIV testing and blood drawing for CD4 count testing and viral load testing were performed at the mobile HIV testing service.
Mobile HIV testing service
The mobile HIV testing service used in this study has been described elsewhere [34]. In brief, this nurse-run and counselor-supported unit provided free HIV counseling and testing services in combination with free screening for other chronic conditions (hypertension, diabetes and obesity) and TB. HIV testing was performed according to the Provincial Government of the Western Cape guidelines [32]. The mobile testing service was parked in front of the primary school in the centre of the community. It operated on weekdays and weekends as well as after hours to ensure that individuals with regular work had an opportunity to participate.
Participants could choose one of three options to receive their result: i) to test and receive their HIV result together with screening for chronic diseases, ii) to provide blood and not receive their HIV result, but undergo screening for chronic diseases or iii) to only provide blood and not receive their HIV result or other screening. Individuals who consented to rapid HIV testing and tested positive were subsequently staged according to the WHO staging manual and underwent a point of care CD4 count test (Alere™Pima™ CD4 Analyser, Waltham, MA, USA) using venous blood samples. All participants were compensated for transport and time with ZAR 70 (approximately 9.6 US dollars) gift vouchers.
Data collection, management and analysis
Age, sex, nationality, migration history and previous HIV testing experience were recorded via a short questionnaire. All participants were asked whether or not they were taking ART. All individuals who tested positive for HIV had a point of care CD4 count test using venous blood. A second venous EDTA blood sample was taken and stored at −20°C for viral load measurement at the National Health Laboratory Service in Cape Town. All blood samples with undetectable viral loads from individuals who reported not receiving ART were tested in the laboratory for HIV antibody and antigen using two different Enzyme-linked immunosorbent assays (ELISAs) (Abott Architect HIV Ag/Ab Combo assay, 4th generation, Siemens Enzygast anti HIV-1/2 Plus 3rd generation).
Ethical approval for viral load measurement was obtained in mid September 2010 for individuals more than 17 years of age. Thus, viral load was not measured for the participants enrolled in the study at the beginning of September and for minors. Data were double entered and verified in EpiData version 3.1.
Data were analysed using Stata 11.2 (StataCorp, College Station, USA). Proportions and confidence intervals were calculated for categorical variables, and medians and interquartile ranges (IQR) for continuous variables. χ2 test for trend was performed for viral load categories across different CD4 strata. Coverage was calculated as number of patients receiving ART divided by the number needing treatment and the latter included not only those in need (at different CD4 thresholds) who are untreated but also those who had started and were retained on treatment. A multivariate logistic regression analysis was performed to assess the association between viral load >1500 copies/ml and explanatory variables such as age, sex, and recent migration.
Average HIV transmission rate
We estimated the potential average annual transmission rate in the community following the approach of Auvert et al [27], which is to weight the annual HIV transmission rates in different viral load categories (as estimated in a recent systematic review [35]) by the proportions of HIV-positive individuals in different viral load categories in our population. In addition, we performed sensitivity analyses in which the transmission rates were replaced with transmission rates from the Rakai study [13] and transmission rates in the Partners in Prevention study [36]. A sensitivity analysis was also conducted to assess the effect of assuming that HIV-positive individuals who know they are HIV-positive have 76% less unprotected sex than undiagnosed individuals, based on studies comparing frequencies on unprotected sex in HIV-diagnosed and undiagnosed individuals in developing countries [66]-[69].
Ethics
Written informed consent was obtained from all individuals participating in the survey. Data collection and analysis was approved by the Human Research Ethics Committee (Faculty of Health Science) University of Cape Town and the Ethics Committee of the London School of Hygiene and Tropical Medicine.
Results
Participation and HIV prevalence
Of 1300 individuals randomly selected from the community, 1144 (88.0%) participated and their characteristics are shown in Supplemental Digital Content Table 1. Individuals who did not participate in the study were older (median age 31; IQR 27-38) and more likely to be men (76.2%) compared to individuals who participated in the study. Overall 260 participants tested HIV-positive, (22.7% 95%CI 20.3-25.3). Just over half (54.6%, 95%CI 48.3-60.8) of the HIV-infected individuals reported knowing their serostatus (Supplemental Digital Content Table 1) and of these, 87 reported that they were receiving ART (61.3%, 95%CI 52.7-69.3).
ART coverage
Overall 87 of the 260 HIV-infected individuals reported receiving ART (33.5%, 95%CI 27.8-39.6). ART coverage was 43.9% (95%CI 36.9-51.1), 55.4% (95%CI 47.3-63.3) and 77.0% (95%CI 68.1-84.4) for CD4 thresholds <500 cell/μl, <350 cells/μl and <200 cells/μl respectively.
Coverage was significantly lower in men compared to women. The proportion of HIV-infected individuals receiving ART was 38.7% in women and 23.0% in men (p=0.01). Coverage at CD4 thresholds <500 cell/μl, <350 cells/μl and <200 cells/μl was 51.5%, 63.2% and 81.7% in women compared to 29.4%, 39.2% and 64.5% in men.
CD4 count distribution
The median CD4 count of HIV-infected individuals was 417 cells/μl (IQR 285-627). The median CD4 count was 404 cells/μl (IQR 277-621) in those not yet receiving ART and 440 cells/μl (IQR 295-627) in those who were receiving ART. Overall, 12.7%, 51.0% and 36.3% of the population had a CD4 cell count of <200, 200-500 and >500 cells/μl, respectively (Table 1). There was no significant difference in the CD4 counts in individuals on ART and not on ART.
Table 1. CD4 counts among those who tested HIV-positive.
| CD4 count | Total (N=219) | On ART (N=79) | Not yet on ART (N=140) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | |
| ≤200 cells/μl | 33 | 13.0 | 0.09; 0.18 | 10 | 11.5 | 0.06; 0.20 | 22 | 13.6 | 0.09; 19.8 |
| 201-350 cells/μl | 72 | 28.5 | 0.23; 0.34 | 26 | 29.9 | 0.21; 0.41 | 44 | 27.2 | 0.20; 0.35 |
| 351-500 cells/μl | 60 | 23.7 | 0.19; 0.29 | 19 | 21.8 | 0.14; 0.32 | 41 | 25.3 | 0.19; 0.33 |
| >500 cells/μl | 88 | 34.8 | 0.29; 0.41 | 32 | 36.8 | 0.27; 0.48 | 55 | 34.0 | 0.27; 0.42 |
Viral load distribution
Viral load measurements were available for 219 (84.2%) of the 260 HIV-infected individuals. Comparing individuals with missing and available viral load data, they were of similar age and gender and there was no difference in the proportion who knew their HIV-serostatus or who were receiving ART.
A total of 72 (33.9%) individuals had an undetectable viral load, 127 (58.0%) had a viral load of >1500 copies/ml and 96 (43.8%) had a viral load of >10,000 copies/ml (Table 2). The proportion of individuals with a viral load >50,000 copies/ml was highest among those with CD4 counts <200 cells/μL (55.2%), and lower among those with CD4 counts 200-349 cells/μL (35.1%), 350-499 cells/μL (23.5%) or >500 cells/μL (14.6%, test for trend p<0.001).
Table 2. Plasma viral load measurements among those who tested HIV-positive (data available for 219 participants).
| Viral load | Total (N=219) | On ART (N=79) | Not yet on ART (N=140) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | |
| <50 copies/ml | 72 | 32.9% | 26.7; 39.5 | 48 | 60.8% | 49.1; 71.6 | 24 | 17.2% | 11.3; 24.4 |
| 50-1500 copies/ml | 20 | 9.1% | 5.7; 13.8 | 7 | 8.8% | 3.6; 17.4 | 13 | 9.3% | 5.0; 15.4 |
| 1501-10000 copies/ml | 31 | 14.2% | 9.8; 19.5 | 9 | 11.4% | 5.3; 20.5 | 22 | 15.7% | 10.1; 22.8 |
| 10001-50000 copies/ml | 36 | 16.4% | 11.8; 22.0 | 7 | 8.9% | 3.6; 17.4 | 29 | 20.7% | 14.3; 28.4 |
| >50000 copies/ml | 60 | 27.4% | 22.6; 33.8 | 8 | 10.1% | 4.5; 19.0 | 52 | 37.2% | 29.1; 45.7 |
We next examined viral load measurements according to reported ART status. More than one third (39.2%) of individuals who said they were taking ART had a detectable viral load, substantially less than the corresponding proportion in individuals who reported that they were not yet taking ART (82.9%; p<0.01). Similarly, of those reporting being on ART, 30.4% had a viral load >1500 copies/ml, compared to 73.6% in those reporting that they were not on ART (p<0.01).
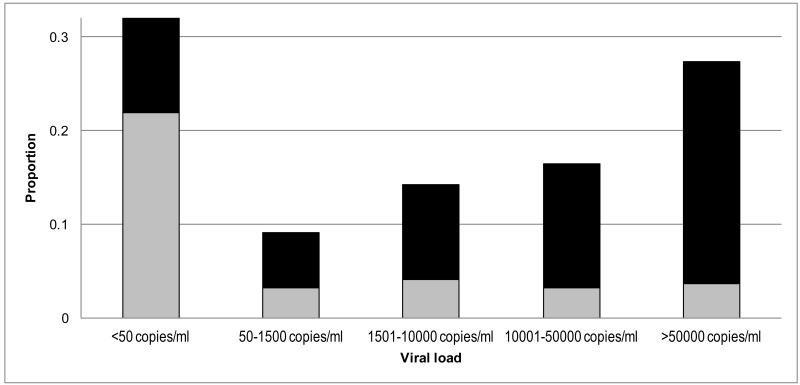
Overall 58.0% of all HIV-infected individuals had a viral load >1500 copies/ml, but only a small proportion was attributable to individuals who reported receiving ART compared to individuals not yet on ART (Figure 1). More men (69.3%) had a viral load >1500 copies/ml compared to women (52.1%, p=0.01). The proportion of individuals with viral loads >1500 copies/ml decreased with increasing age: 91.3%, 59.7%, 50.0% and 52.9% had a viral load >1500 copies/ml among individuals <25, 25-29, 30-34 and ≥35 years respectively (p<0.01). More individuals living in the township for less than 3 years had a viral load >1500 copies/ml (71.4%) compared to individuals living in the township for more than 3 years (54.8%, p=0.05). A multivariate logistic regression model including age, gender, recent immigration, ART status and CD4 count showed that female sex (adjusted OR[aOR]=0.46; 95%CI 0.23-0.93), older age, reported receipt of ART (aOR=0.16; 95%CI 0.09-0.32) and a CD4 count >200 cells/μl (aOR=0.39; 95%CI 0.15-1.05) all decreased the risk of having a viral load >1500 copies/ml (Table 3). Recent immigration did not increase the risk in the multivariate analysis.
Figure 1. Viral load distribution by self-reported treatment status.
Proportions of individuals receiving ART are displayed in grey, Proportions of individuals not yet receiving ART are displayed in black.
Table 3. Predictors of VL Plasma Levels >1500 copies/ml (data available for 219 participants).
| Predictors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
|
|
|||||
| Odds ratio | 95%CI | Odds ratio | 95%CI | ||
| Gender | Male | 1 | |||
| Female | 0.48 | 0.27; 0.87 | 0.46 | 0.23; 0.93 | |
|
| |||||
| Age (years) | <25 | 1 | |||
| 25-29 | 0.14 | 0.03; 0.66 | 0.17 | 0.03; 0.88 | |
| 30-34 | 0.10 | 0.02; 0.45 | 0.08 | 0.01; 0.42 | |
| >35 | 0.11 | 0.02; 0.49 | 0.08 | 0.02; 0.43 | |
|
| |||||
| Resident in the community | >3 years | 1 | |||
| <3 years | 2.06 | 0.99; 4.29 | 0.91 | 0.37; 2.21 | |
|
| |||||
| Reported ART status | Not on ART | 1 | |||
| On ART | 0.16 | 0.09; 0.29 | 0.18 | 0.09; 0.32 | |
|
| |||||
| CD4 count (cells/μl) | ≤200 | 1 | |||
| >200 | 0.48 | 0.20; 1.14 | 0.39 | 0.15; 1.05 | |
HIV transmission risk
The potential average annual rate of HIV transmission from HIV-positive individuals to their susceptible partners, based on average transmission rates from the systematic review [35], was 0.045, 39% lower than the average transmission rate of 0.074 estimated in the Orange Farm community prior to the availability of ART (Table 4). The average annual transmission rate in individuals on ART was estimated to be 0.022, 63% lower than the average annual transmission rate in untreated individuals in the same community (0.058), and 76% lower than the average annual HIV transmission rate that existed in ART-eligible individuals in Orange Farm prior to ART rollout (0.088). In the sensitivity analysis, the 63% difference in potential transmission rate when comparing adults on ART to adults not on ART changed relatively little when using the transmission rates in Rakai (59%) and the transmission rates in the Partners in Prevention study (65%). However, in the sensitivity analysis in which HIV-diagnosed individuals were assumed to engage in less unprotected sex, the average transmission rate in individuals on ART was 88% lower than that in individuals not on ART. In this scenario, it was estimated that an HIV screening intervention that reduced the fraction of undiagnosed HIV-positive adults by 50% would reduce the average HIV transmission rate by 30%.
Table 4. Average annual HIV transmission rate in the study community compared to previous results from a study in Orange Farm township, South Africa [27].
| Viral load (copies/ml) | ||||||
|---|---|---|---|---|---|---|
| <400 | 400-3,499 | 3,500-9,999 | 10,000-49,999 | 50,000+ | Total | |
| Annual HIV transmission rate [13] | 0 | 0.04 | 0.12 | 0.14 | 0.23 | - |
| % of HIV-positive population in the study community | 36.5% | 10.1% | 9.6% | 16.4% | 27.4% | 100% |
| Not on ART | 20.0% | 10.0% | 12.1% | 20.7% | 37.1% | 100% |
| On ART | 65.8% | 10.1% | 5.1% | 8.9% | 10.1% | 100% |
| % of HIV-positive population in Orange Farm | 3.1% | 8.2% | 12.2% | 25.5% | 51.1% | 100% |
| With CD4 <200 cells/μl | 0.0% | 0.0% | 0.0% | 20.6% | 79.4% | 100% |
| Weighted HIV transmission rate in the study community | 0.001 | 0.002 | 0.004 | 0.013 | 0.025 | 0.045 |
| Not on ART | 0.000 | 0.002 | 0.005 | 0.017 | 0.034 | 0.058 |
| On ART | 0.001 | 0.002 | 0.002 | 0.007 | 0.009 | 0.022 |
| Weighted HIV transmission rate in Orange Farm | 0.000 | 0.002 | 0.005 | 0.021 | 0.046 | 0.074 |
| With CD4 <200 cells/μl | 0.000 | 0.000 | 0.000 | 0.017 | 0.072 | 0.088 |
Discussion
This study describes the population HIV viral load and CD4 count distribution in a defined community in Africa during ART scale-up. A high proportion of HIV-infected individuals (58%) had a viral load >1500 viral copies/ml, which is known to be associated with a high risk of transmission [13]. This proportion was highest (73.6%) among those reporting not receiving ART and yet almost one third of those reporting being on ART had viral loads above this threshold. Thus, despite high HIV test uptake in this community (71.0%) and high ART coverage at an eligibility threshold of <200 CD4 cells/μl (77.0%), more than half of those living with HIV remain at high risk of transmitting the virus. In contrast, only a relatively small proportion of individuals (12.7%) had very low CD4 cell counts <200 cells/μl that would be associated with substantial risk of morbidity and mortality.
The concept of “community viral load” has been explored in Canada and the US using routine clinic viral loads to estimate the population viral load distributions [23,24,38,39]. However, population viral load may be underestimated as those individuals who are undiagnosed or not accessing care are excluded, especially in developing countries, where the proportion of undiagnosed HIV remains high [8,31] and viral load measurements are not routinely available. Thus, our approach of using a randomly selected community-based sample is more likely to be representative of the true population viral load.
A recent study from rural Uganda in a lower prevalence setting (adult HIV prevalence 7.8%) showed similar results [37]. Almost half of the HIV diagnoses were previously undiagnosed (47%) and more than a third (38%) of the HIV-infected individuals were receiving ART. Among individuals infected with HIV, 37% had an undetectable viral load, 49% had a viral load >10000 copies/ml, but only 17% had a CD4 count <200 cells/μl.
The overall average annual rate of HIV transmission from HIV-positive individuals to their susceptible partners in this community was estimated to be 0.045. This was 39% lower than estimated in the Orange Farm community prior to the availability of ART [27], and this impact is a reflection of the impact of ART on population viral load. The transmission rate in individuals on ART was estimated to be 63% lower than in untreated individuals in the same community. These results are in contrast to the results from the HPTN 052 trial showing a 96% reduction in transmission in individuals on ART compared to individuals not on ART [21]. A meta-analysis of observational studies estimated an 84% decreased risk of infection comparing individuals receiving ART with individuals not receiving ART [22]. Randomized controlled trials and observational studies conducted in well resourced research clinics might overestimate adherence and thus the effectiveness of ART. However, single time-point viral loads might not be representative of the viral load prevailing over time.
A limitation of our analysis is that it is based on the theoretical HIV transmission rates that would be expected if transmission rates were the same as those reported in a recent systematic review of transmission rates in untreated individuals [35]. Several studies have shown that condom usage in individuals receiving ART is increased [40,41,42,43] and by ignoring this difference in behaviour our analysis is likely to underestimate the benefits of ART, relative to untreated individuals. In the sensitivity analysis in which we assumed HIV-diagnosed individuals had a lower frequency of unprotected sex, the difference in infectiousness between treated and untreated individuals increased from 63% to 88%.
It is of concern that one third (n=24, 30.4%) of individuals reporting that they were taking ART had a viral load ≥ 1500 copies/ml in this community. Possible explanations include poor adherence, recent ART initiation and misclassification of ART status. Only 3 of the 24 individuals with viral loads >1500 copies/ml reported receiving ART for a duration of <6 months. ART status was determined by self-report and it is possible that some individuals reported receiving ART even though they were not. The proportion of viraemic individuals in this study is in contrast to cross-sectional studies conducted in sub-Saharan African ART cohorts reporting a prevalence of viraemia of 15% among patients receiving ART for 12 months or longer [44,45,46]. A possible explanation for the marked difference in the proportion of viraemic individuals in this population-based study and clinic-based cross-sectional studies is the Hawthorne effect. Patients who attend clinics know they will be asked about their adherence and assessed for treatment failure. This might result in improved adherence in the days and weeks prior to their clinic appointment. Furthermore patients with missing viral loads in clinical cohorts are more likely to be the ones with poor adherence. Viral load measurements obtained outside a clinic setting are therefore more likely to reflect reality. Woman and individuals aged 25+ were more likely to have viral loads <1500 copies/ml both in univariate analysis and after adjusting for ART status and CD4 count. This is entirely consistent with ART cohort studies from sub-Saharan Africa showing reduced adherence in men [47,48] and young adults [49,50,51] and higher risk of viraemia in men [52,53].
A considerable proportion (n=24, 17.2%) of individuals who reported that they were not being treated with ART had an undetectable viral load. Blood samples of these individuals were re-tested in the laboratory using two different ELISAs and these effectively ruled out false positive rapid HIV test results. One explanation for the apparent discrepancy in treatment status and viral load results is that these 24 individuals might actually have been taking ART. Treatment history relied on self-report and was not verified with clinic records or measurement of ARVs in plasma, which is a limitation of this study. It is also possible that some of these individuals are so called HIV controllers defined as HIV-infected individuals who continually present with undetectable viral loads [54]. A recent study from Uganda classified 1.4% of 637 HIV-seroconverters as HIV controllers [55]. Furthermore, a study investigating population viral load in rural Uganda showed that 10% of individuals without any evidence of ARVs in their plasma had viral load measurements below the limits of quantification [37].
The median CD4 cell count in this population was 417 cells/μl. Only 13% of the HIV-infected population had a CD4 count <200 cells/μl; levels below this threshold are associated with high mortality and morbidity [4,29]. Current CD4 count is one of the strongest predictors of HIV-associated mortality and morbidity [4,29,56,57]. ART-associated reductions in mortality correlate with increases in CD4 counts [56]. HIV programs aim to diagnose and treat individuals before CD4 counts reach a critical level of <200 cells/μl. The results of this survey are encouraging, as the majority of HIV-infected individuals in this community had a CD4 count >200 cells/μl. This shows that high levels of coverage impact on population CD4 health.
A third of the HIV-infected individuals in this population received ART. Coverage at CD4 thresholds of <200 cells/μl and <350 cells/μl was 77.0% and 55.4% respectively. Our finding that coverage was significantly lower in men is consistent with studies showing that HIV-infected men are less likely to access treatment [58,59], have an increased risk for loss to follow-up in the pre-treatment period [60] and present in the more advanced stages of HIV disease [61]. Our results are also in line with national estimates of ART coverage [62]. Closing the coverage gap in men needs to be a priority to tackle health related gender inequalities, improve overall health in men and decrease transmission from men to women.
The participation rate in this survey was 88%, and non-response might have resulted in biased estimates. Other population-based HIV sero-prevalence surveys from sub-Saharan Africa reported similar absenteeism and refusal rates [63,64,65]. The CD4 count distribution in individuals on ART measured in this survey was similar to the actual distribution of the ART cohort at the time of the survey. Thus the survey sample seems to be representative, at least among individuals receiving ART. The study was conducted in a single community; the findings may therefore only be generalised to similar settings with comparable levels of service delivery and HIV prevalence.
In conclusion, this study highlights the high proportion of HIV-infected individuals with viral loads >1500 copies/ml. It also shows that only a small proportion of the HIV-infected population had very low CD4 cell counts <200 cells/μl which are associated with high risk of morbidity and mortality. This suggests that the effectiveness of current therapeutic guidelines as implemented in the field seems to be aimed towards decreasing morbidity and mortality, but falls short in preventing HIV transmission. In view of the recent data regarding the individual and public health benefit of immediate ART more efforts are needed to expand HIV testing and access to ART as a mechanism to fully realize the potential of “TasP”.
Supplementary Material
Acknowledgment
The authors thank the research and clinic staff and participants for their contributions.
Sources of Funding: KK (087262/Z/08/Z) and SDL(088590/Z/09/Z) are funded by the Wellcome Trust, London, UK. RW is funded by IEDEAA (5U01AI069924-02, CEPAC (5 R01 AI058736-02). LGB is funded by the NIH CIPRA (1U19AI53217).
Footnotes
Conflict of interest: None declared.
References
- 1.World AIDS Day Report 2011. UBAIDS; Geneva, Switzerland: [last accessed 1//6/2012]. 2011. http://issuu.com/unaids/docs/worldaidsday_report_2011/1. [Google Scholar]
- 2.Treating 3 million by 2005: making it happen: the WHO strategy: the WHO and UNAIDS global initiative to provide antiretroviral therapy to 3 million people with HIV/AIDS in developing countries by the end of 2005 / Treat 3 Million by 2005. World Health Organization; Geneva: [last accessed 26/6/2012]. 2003. http://data.unaids.org/Publications/External-Documents/who_3by5-strategy_en.pdf. [Google Scholar]
- 3.Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med. 2009;30:685–699. viii. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 5.Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd S, Molesworth A, Dube A, Banda E, Jahn A, et al. Population-level reduction in adult mortality after extension of free anti-retroviral therapy provision into rural areas in northern Malawi. PLoS One. 2010;5:e13499. doi: 10.1371/journal.pone.0013499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahy M, Stover J, Stanecki K, Stoneburner R, Tassie JM. Estimating the impact of antiretroviral therapy: regional and global estimates of life-years gained among adults. Sex Transm Infect. 2010;86(Suppl 2):ii67–71. doi: 10.1136/sti.2010.046060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global HIV/AIDS response: epidemic update and health sector progress towards Universal Access. World Health Organization; Geneva, Switzerland: [last accessed 3/6/2012]. 2011. http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf. [Google Scholar]
- 9.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 11.Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach (2010 version) World Health Organization; Geneva: [last accessed 14/8/2010]. 2010. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed] [Google Scholar]
- 12.South African antiretroviral treatment guidelines. South African National AIDS Council/Department of Health; Pretoria: [last accesses 25/05/2010]. 2010. http://www.sanac.org.za/documents/2010%20ART%20Guideline-Short.pdf. [Google Scholar]
- 13.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 14.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 15.Kumarasamy N, Venkatesh KK, Srikrishnan AK, Prasad L, Balakrishnan P, et al. Risk factors for HIV transmission among heterosexual discordant couples in South India. HIV Med. 2010;11:178–186. doi: 10.1111/j.1468-1293.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 16.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 17.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. Aids. 2010;24:729–735. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachariah R, Harries AD, Philips M, Arnould L, Sabapathy K, et al. Antiretroviral therapy for HIV prevention: many concerns and challenges, but are there ways forward in sub-Saharan Africa? Trans R Soc Trop Med Hyg. 2010;104:387–391. doi: 10.1016/j.trstmh.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Granich R, Kahn JG, Bennett R, Holmes CB, Garg N, et al. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost-effectiveness 2011-2050. PLoS One. 2012;7:e30216. doi: 10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallett TB, Baeten JM, Heffron R, Barnabas R, de Bruyn G, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med. 2011;8:e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anglemyer A, Rutherford GW, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2011:CD009153. doi: 10.1002/14651858.CD009153.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnighausen T, Tanser F, Dabis F, Newell ML. Interventions to improve the performance of HIV health systems for treatment-as-prevention in sub-Saharan Africa: the experimental evidence. Curr Opin HIV AIDS. 2012;7:140–150. doi: 10.1097/COH.0b013e32834fc1df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connelly D, Veriava Y, Roberts S, Tsotetsi J, Jordan A, et al. Prevalence of HIV infection and median CD4 counts among health care workers in South Africa. S Afr Med J. 2007;97:115–120. [PubMed] [Google Scholar]
- 27.Auvert B, Males S, Puren A, Taljaard D, Carael M, et al. Can highly active antiretroviral therapy reduce the spread of HIV?: A study in a township of South Africa. J Acquir Immune Defic Syndr. 2004;36:613–621. doi: 10.1097/00126334-200405010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehle TM, Shisana O. Estimates of eligibility for antiretroviral treatment (ART) and projected ART impact on AIDS mortality among South African educators. Sahara J. 2005;2:304–310. doi: 10.1080/17290376.2005.9724855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes CB, Wood R, Badri M, Zilber S, Wang B, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 30.Kelly P, Zulu I, Amadi B, Munkanta M, Banda J, et al. Morbidity and nutritional impairment in relation to CD4 count in a Zambian population with high HIV prevalence. Acta Trop. 2002;83:151–158. doi: 10.1016/s0001-706x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 31.Kranzer K, van Schaik N, Karmue U, Middelkoop K, Sebastian E, et al. High prevalence of self-reported undiagnosed HIV despite high coverage of HIV testing: a cross-sectional population based sero-survey in South Africa. PLos One. 2011;6:e25244. doi: 10.1371/journal.pone.0025244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Western Cape Department of Health . The Western Cape Antiretroviral Programme. Provincial Government of the Western Cape: Western Cape Department of Health; Cape Town: [last accessed 2/2/2011]. 2006. http://web.uct.ac.za/depts/epi/artrollout/ [Google Scholar]
- 33.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Schaik N, Kranzer K, Wood R, Bekker LG. Earlier HIV diagnosis - are mobile services the answer? S Afr Med J. 2010;100:671–674. doi: 10.7196/samj.4162. [DOI] [PubMed] [Google Scholar]
- 35.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. Aids. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 36.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, et al. Assessment of Population-Based HIV RNA Levels in a Rural East African Setting Using a Fingerprick-Based Blood Collection Method. Clin Infect Dis. 2013;56:598–605. doi: 10.1093/cid/cis881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castel AD, Befus M, Willis S, Griffin A, West T, et al. Use of the community viral load as a population-based biomarker of HIV burden. Aids. 2012;26:345–353. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 39.Wood E, Kerr T, Marshall BD, Li K, Zhang R, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moatti JP, Prudhomme J, Traore DC, Juillet-Amari A, Akribi HA, et al. Access to antiretroviral treatment and sexual behaviours of HIV-infected patients aware of their serostatus in Cote d’Ivoire. Aids. 2003;17(Suppl 3):S69–77. doi: 10.1097/00002030-200317003-00010. [DOI] [PubMed] [Google Scholar]
- 41.Dia A, Marcellin F, Bonono RC, Boyer S, Bouhnik AD, et al. Prevalence of unsafe sex with one’s steady partner either HIV-negative or of unknown HIV status and associated determinants in Cameroon (EVAL ANRS12-116 survey) Sex Transm Infect. 2010;86:148–154. doi: 10.1136/sti.2008.035147. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesh KK, de Bruyn G, Lurie MN, Mohapi L, Pronyk P, et al. Decreased sexual risk behavior in the era of HAART among HIV-infected urban and rural South Africans attending primary care clinics. Aids. 2010;24:2687–2696. doi: 10.1097/QAD.0b013e32833e78d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. Aids. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 44.Muwonga J, Edidi S, Butel C, Vidal N, Monleau M, et al. Resistance to antiretroviral drugs in treated and drug-naive patients in the Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2011;57(Suppl 1):S27–33. doi: 10.1097/QAI.0b013e31821f596c. [DOI] [PubMed] [Google Scholar]
- 45.El-Khatib Z, Ekstrom AM, Ledwaba J, Mohapi L, Laher F, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. Aids. 2010;24:1679–1687. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johannessen A, Naman E, Kivuyo SL, Kasubi MJ, Holberg-Petersen M, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC Infect Dis. 2009;9:108. doi: 10.1186/1471-2334-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in Sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS Res Ther. 2009;6:21. doi: 10.1186/1742-6405-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Khatib Z, Katzenstein D, Marrone G, Laher F, Mohapi L, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One. 2011;6:e17518. doi: 10.1371/journal.pone.0017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS One. 2010;5:e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nozaki I, Dube C, Kakimoto K, Yamada N, Simpungwe JB. Social factors affecting ART adherence in rural settings in Zambia. AIDS Care. 2011;23:831–838. doi: 10.1080/09540121.2010.542121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunutsor S, Evans M, Thoulass J, Walley J, Katabira E, et al. Ascertaining baseline levels of antiretroviral therapy adherence in Uganda: a multimethod approach. J Acquir Immune Defic Syndr. 2010;55:221–224. doi: 10.1097/QAI.0b013e3181e255ec. [DOI] [PubMed] [Google Scholar]
- 52.Kipp W, Alibhai A, Saunders LD, Senthilselvan A, Kaler A, et al. Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care. 2010;22:271–278. doi: 10.1080/09540120903193625. [DOI] [PubMed] [Google Scholar]
- 53.Barth RE, Tempelman HA, Moraba R, Hoepelman AI. Long-Term Outcome of an HIV-Treatment Programme in Rural Africa: Viral Suppression despite Early Mortality. AIDS Res Treat. 2011;2011:434375. doi: 10.1155/2011/434375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Laeyendecker O, Redd AD, Lutalo T, Gray RH, Wawer M, et al. Frequency of long-term nonprogressors in HIV-1 seroconverters From Rakai Uganda. J Acquir Immune Defic Syndr. 2009;52:316–319. doi: 10.1097/QAI.0b013e3181bc08f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–342. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Remien RH, Chowdhury J, Mokhbat JE, Soliman C, Adawy ME, et al. Gender and care: access to HIV testing, care, and treatment. J Acquir Immune Defic Syndr. 2009;51(Suppl 3):S106–110. doi: 10.1097/QAI.0b013e3181aafd66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson LF. Access to antiretroviral treatment in South Africa, 2004 - 2011. Southern African Journal of HIV Medicine. 2012;13:22–27. doi: 10.4102/sajhivmed.v18i1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marston M, Harriss K, Slaymaker E. Non-response bias in estimates of HIV prevalence due to the mobility of absentees in national population-based surveys: a study of nine national surveys. Sex Transm Infect. 2008;84(Suppl 1):i71–i77. doi: 10.1136/sti.2008.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amornkul PN, Vandenhoudt H, Nasokho P, Odhiambo F, Mwaengo D, et al. HIV prevalence and associated risk factors among individuals aged 13-34 years in Rural Western Kenya. PLoS One. 2009;4:e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziraba AK, Madise NJ, Matilu M, Zulu E, Kebaso J, et al. The effect of participant nonresponse on HIV prevalence estimates in a population-based survey in two informal settlements in Nairobi city. Popul Health Metr. 8:22. doi: 10.1186/1478-7954-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- 67.Sherr L, Lopman B, Kakowa M, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. Aids. 2007;21:851–860. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]
- 68.Muller O, Sarangbin S, Ruxrungtham K, et al. Sexual risk behaviour reduction associated with voluntary HIV counselling and testing in HIV infected patients in Thailand. AIDS Care. 1995;7:567–572. doi: 10.1080/09540129550126227. [DOI] [PubMed] [Google Scholar]
- 69.Mwangi M, Bunnell R, Nyoka R, et al. Unsafe sex among HIV-infected adults in Kenya: results of a nationally representative survey. J Acquir Immune Defic Syndr. 2011;58:80–88. doi: 10.1097/QAI.0b013e3182251001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.