Abstract
Rats with lesions of the perirhinal cortex, and a control group, were required to find a platform in one corner of a white rectangle, and in the reflection of this corner in a black rectangle. Test trials revealed that these groups were able to integrate information regarding the shape of the pool and the color of its walls (black or white) in order to identify the correct location of the platform. A clear effect of the perirhinal cortex lesions was, however, revealed using an object recognition task that involved the spontaneous exploration of novel objects. The results challenge the view that the perirhinal cortex enables rats to solve discriminations involving feature ambiguity.
Keywords: Perirhinal cortex, spatial discrimination, object recognition memory, geometry
Despite being closely connected, the hippocampus and the perirhinal cortex appear to play rather different roles in memory. There is abundant evidence to show, for example, that damage to the hippocampus can impair profoundly performance on a variety of spatial tasks, whereas damage to the perirhinal cortex has a more transient, less severe influence on spatial behavior (see Aggleton, Kyd, & Bilkey, 2004, for a review). This does not mean that the perirhinal cortex plays no role to play in spatial behavior. A series of experiments has shown that this region is involved in what has been referred to as “object-in-place” memory (Gaffan & Parker, 1996), “what-where” memory (Eacott & Gaffan, 2005), or “object-in-context” memory (Norman & Eacott, 2005), all of which allow animals to remember the location in which a particular object can be found.
An example of object-in-context memory was provided by Dix and Aggleton (1999). Rats were exposed to two identical objects, A1 and A2, in one context and a different pair of identical objects, B1 and B2, in a second context. They were then shown A1 and B1 in the first context where it was found that significantly more time was spent exploring B1 than A1. For this outcome to have occurred, animals must have learned about the relationship between the objects and contexts in which they were presented. The importance of the perirhinal cortex for this learning was subsequently revealed in a study by Norman and Eacott (2005) who found that when rats with lesions of this region were given the task just described, they failed to show enhanced exploration of the object that was presented in a different context. Such an outcome can be understood if it is accepted that the perirhinal cortex is responsible for integrating information about objects and where they occur (see also Barker & Warburton, 2008; Gaffan & Parker, 1996).
An experiment by Iordanova, Burnett, Aggleton, Good and Honey (2009) indicates that the perirhinal cortex may not just be involved with forming memories about the location of physical objects, it may also be involved in allowing animals to learn where auditory cues are experienced. Sham rats were presented with an auditory cue, X, in Context A and an auditory cue, Y, in Context B (the two contexts were different conditioning chambers). They then received aversive conditioning with X but not Y before being returned to the contexts in the absence of the auditory stimuli. The tests revealed stronger fear in A than B, which indicates that the rats had retained a record of which cue occurred in which context, and that this record mediated the transfer of the conditioned response from the cue used for aversive conditioning to the context in which the cue occurred. When a group with lesions of the perirhinal cortex was trained and tested in the same manner, the level of contextual fear during the test trial was the same in contexts A and B. This outcome was attributed by Iordanova et al. (2009) to the lesions impairing the knowledge about which cue occurred in which context.
The foregoing results can be understood by referring to the theory of Bussey and Saksida (2002, see also Bussey, Saksida, and Murray, 2005) who argued that the perirhinal cortex is important for constructing representations of conjunctions of stimuli. In support of this claim, Buckley and Gaffan (1998; see also Bussey et al., 2002) demonstrated that monkeys with lesions to the perirhinal cortex have considerable difficulty solving biconditional discriminations but not simple discriminations. In the latter, the outcome of a trial is indicated by the presence or absence of individual stimuli, whereas in the former, individual stimuli signal reinforcement and nonreinforcement with equal probability, and the trial outcome is indicated by different combinations of these features. Presumably, it was an inability to form representations of these combinations that made it difficult for the lesioned monkeys to solve the biconditional discrimination. It is thus possible that lesions in the experiments by Norman and Eacott (2005) and Iordanova et al. (2008) were effective because they prevented animals from integrating information about the contexts and the objects or stimuli that occurred in them. If this is correct then lesions of the perirhinal cortex should disrupt the solution of any spatial task, provided the solution depends on appreciation of the significance of a combination of stimuli rather than individual stimuli.
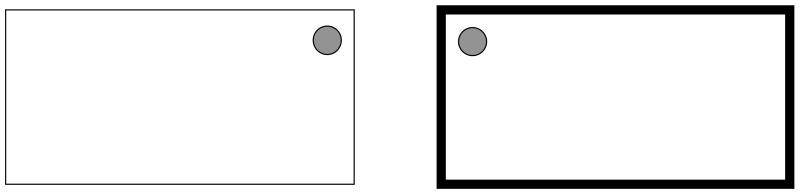
In order to test this prediction, rats in Experiment 2 were trained to find a platform in one corner of a rectangular arena (see Figure 1) which on some trials was constructed from white walls and on others from black walls. Rats were required to swim to one corner to find the platform in the white rectangle, and to the reflection of this corner in the black rectangle (see Figure 1). In order to swim directly to the platform in both environments it would be insufficient to focus on the geometric properties of the walls, or their color. Instead it is necessary to learn to approach a corner created by a short wall to the left of a long wall in the black arena (for the example shown in Figure 1), and to go to the other type of corner in the white arena. This discrimination, therefore, can be regarded as a biconditional one of the form AW+ AB− CB+ CW−, where A is a corner with certain geometric properties, C is the mirror image of this corner, B is black and W is white. It was anticipated that normal rats would be able to solve this task, the question of interest was how animals with lesions to the perirhinal cortex would react to it. According to the proposals of Bussey and Saksida (2002), it is possible that these animals would be unable to swim directly to the platform in both pools.
Figure 1.
Diagram of the apparatus used for spatial discrimination. Thick lines represent black walls, thin lines represent white walls, filled circles represent a submerged platform.
During the first 24 sessions of the experiment, rats were trained in the two rectangles in different sessions. Test trials at the end of this stage revealed that both groups had solved the discrimination. The task was then made more demanding by training the rats for two trials in each rectangle in each of the subsequent 13 sessions. The results from the subsequent test trials were similar to those from the first phase, as they failed to reveal an effect of the perirhinal lesions. It is possible that this outcome was a consequence of the lesions being unsatisfactory and having no impact on the function of the perirhinal cortex. Accordingly, a further phase of the experiment was conducted in order to assess the effectiveness of the lesions using a different behavioral task.
Ennaceur and Delacour (1988) have shown that if normal rats are allowed to explore one object and then a short while later are shown the same object accompanied by a novel object, then they will spend more time exploring the novel than the familiar one. This effect, however, is not observed in rats with perirhinal lesions (Ennaceur, Neave, & Aggleton, 1996; Norman & Eacott, 2004). In view of these findings, both groups in the second phase of the experiment were given a test of spontaneous object recognition. They were placed in one compartment of the apparatus shown in Figure 2, and allowed to explore a single object. The rats were then allowed access to the opposite compartment, for the first test trial, where two objects were placed over two wells, each baited with a single pellet of food. One of the objects was identical to the object that had just been presented – the now familiar object, and one of the objects was novel. After being allowed to collect the food and to explore the objects for up to a minute, the rats were allowed to return to the original compartment, for a second test trial, where there was again two objects. One object was identical to the object that had been novel in the previously visited compartment, and the other object had not been exposed before. Testing continued in this manner for a further 18 trials. On the basis of previous work (Albasser, Poirier, & Aggleton, 2009), we expected the sham group on each trial to spend more time exploring the novel than the familiar object. If the lesions of the perirhinal cortex had been effective then this preferential exploration will be less marked in the perirhinal group.
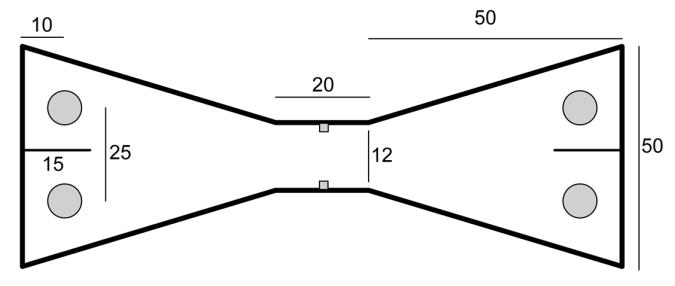
Figure 2.
A plan of the apparatus used for the spontaneous object recognition test of Experiment 2. The numbers represent dimensions in cm, and the filled circles depict the location of the food wells where the objects were placed
Experiment 1
Method
Subjects
Twenty-four adult male Hooded Lister rats (Rattus norvegicus) supplied by Harlan Olac, UK were used for the experiment. They were approximately six months old at the start of the experiment. All the rats were housed in pairs, in a room that was illuminated between 8 am and 8 pm. Food and water were available ad libitum in the home cages throughout the experiment. Animal husbandry and experimental procedures were conducted in accordance with the “Principles of laboratory animal care” (NIH publication No. 85-23, revised 1985) and the UK Animals (Scientific Procedures) Act (1986). Following the surgery, all rats participated in an experiment in which they were exposed to auditory stimuli and shocks in standard conditioning chambers before they were used for the present experiment. There were 12 rats with bilateral lesions of the perirhinal cortex - the perirhinal group – and 12 rats with sham lesions - the sham group.
Apparatus
The experiment was conducted in a rectangular arena contained within a circular pool that was 2 m in diameter and 0.6 m deep. The circular pool was white, made from fiberglass and mounted on a platform 0.6 m above the floor in the middle of a room which was 4.0 m × 3.0 m and 2.3 m high. The pool was filled to a depth of 27 cm with water that was rendered opaque by the addition of 0.5 l of white opacifier E308 (Roehm and Haas, UK, Ltd, Dewsbury). The water was changed daily and its temperature was 25°C (+/−2 C°). A video camera with a wide angled lens was fixed 1.75 m above the center of the pool. The lens of the camera was situated 25 cm above a 30-cm diameter hole in a white circular panel with a diameter of 2 m which was suspended from the ceiling. The image from the camera was relayed to a monitor and recording equipment. The rats’ movements were analysed using Watermaze software (Morris and Spooner, 1990). In the circular panel above the pool were eight 45-W spot lights, 22.5 cm in diameter, which were arranged at equal distances in a circle with a diameter of 1.6 m. The spotlights were illuminated throughout the experiment. The escape platform, which was made from clear Perspex, was 10 cm in diameter and was mounted on a column. The surface of the platform was composed of a series of concentric ridges. The column stood on the floor of the pool and the platform surface was 2.5 cm below the surface of the water. A light-blue, 1.5 m high curtain hanging from the ceiling was drawn completely around the pool, and fell 25 cm beyond the pool’s edge. The room was additionally illuminated by four, 1.53-m strip lights that were attached end to end in pairs on opposite walls of the room, running parallel to the floor and 75 cm above the floor. There was a sliding door in the center of one of the walls without a strip light. The door was open throughout the experiment and allowed access to an adjacent room where the experimenter remained throughout each trial, and where it was possible to observe the experiment on a monitor.
Four Perspex boards, 0.59 m high and 2 mm thick, were suspended vertically in the pool from bars which extended over the edge of the pool to form a white, rectangular arena. Two boards were 1.8 m in length and two were 0.9 m. The center of the platform was 25 cm from the appropriate corner on a line that bisected the corner. For trials in the white rectangle the four boards were white, and for trials in the black rectangle they were black.
The apparatus for the object recognition task was a chamber made from black Perspex in the shape of a bow tie (see Figure 2). The chamber was 120 cm long, 50 cm high, and its maximum width was 50 cm. Objects could be placed in either end of the chamber, in four test compartments created by partitions, 15 cm wide and 50 cm high, that were fixed to the midline of the end walls. To move from one side of the apparatus to the other, rats has to travel along a passage that was 12 cm wide and 20 cm long with an opaque guillotine door at the middle. When raised, the door left an opening that was 10 cm wide, and 20 cm high. Four food wells, 3.5 cm in diameter, were located 10 cm from the rear wall half way between the side wall and the partition that created each test compartment. Twenty-four pairs of objects were used for the experiment (3 for training and 24 for the test session), with the two members of each pair being identical. The objects were large enough to cover a food well, and light enough to be displaced from the food well by a rat. Any object with an obvious scent was excluded. Typical objects were a soap dispenser, an ashtray, Lego blocks, and an electric plug.
Procedure
Surgery and Histology
All rats were anesthetized using an isoflurane-oxygen mix before placement in a stereotaxic frame (Kopf Instruments, Tujunga, CA). Bone above the region to be lesioned was removed, and rats in the perirhinal group were infused with N-methyl-D-aspartic acid (NMDA; Sigma, Poole, U.K. dissolved in phosphate-buffered saline [pH 7.4] to provide a solution with a concentration of 9mM). These neurotoxins were administered through a 2-μl Hamilton syringe held with a microinjector (Kopf Instruments, Model 5000). Table 1 shows the coordinates and volume of infusions for rats in the perirhinal group. The total number of infusions per hemisphere was three, with an infusion rate of 0.20 μl/min and diffusion time of 4 min. Rats in the sham group received identical treatment with the exception that dura was perforated with a 25-gauge Microlance 3 needle (Becton Dickinson, Drogheda, Ireland), but no fluid was infused into the brain. A minimum of 14 days postoperative recovery was allowed.
Table 1. Stereotaxic coordinates and volume of NMDA or ibotenic acid for lesions of the hippocampus or perirhinal cortex, respectively.
| AP | ML | DV | Volume(μl) | |
|---|---|---|---|---|
| Hippocampus: (from bregma) | −5.4 | ± 4.2 | −3.9 | 0.10 |
| ± 5.0 | −6.1 | 0.08 | ||
| −5.3 | 0.08 | |||
| −4.5 | 0.09 | |||
| −4.7 | ± 4.0 | −7.2 | 0.10 | |
| −3.5 | 0.05 | |||
| ± 4.5 | −6.5 | 0.05 | ||
| −3.9 | ± 2.2 | −3.0 | 0.10 | |
| −1.8 | 0.10 | |||
| ± 3.5 | −2.7 | 0.10 | ||
| −3.1 | ± 1.4 | −3.0 | 0.10 | |
| −2.1 | 0.10 | |||
| ± 3.0 | −2.7 | 0.10 | ||
| −2.4 | ± 1.0 | −3.0 | 0.05 | |
| Perirhinal cortex: (from bregma) | −1.8 | ± 5.9 | −9.3 | 0.25 |
| −3.4 | ± 6.1 | −9.6 | 0.25 | |
| −5.0 | ± 6.2 | −9.0 | 0.25 |
Following behavioral testing, all rats received a lethal overdose of sodium pentobarbitone (Euthatal) and they were then transcardially perfused, first with 0.9% saline and then with 10.0% formal-saline. The brains were extracted, postfixed for 24 hr and then transferred to phosphate-buffered (0.1M) 30.0% sucrose solution in which they remained for a further 24 hr. Subsequently the brains were removed from the sucrose solution and frozen in a −20 °C cryostat. The brains were then sliced coronally. The 40-μm sections were collected on gelatine-coated slides, left to dry in room temperature over 24 hr and then stained with cresyl violet. Histological borders and nomenclature for the perirhinal cortex were taken from Burwell (2001).
Behavior
For the training in the swimming pool, both groups were required to escape from a rectangular pool with a submerged platform located in one corner. For each session, rats were transported to the room adjacent to the test room five at a time in light-tight boxes, which were placed on a shelf. There were four trials in a session, and for each trial rats were required to escape from the pool by swimming to the submerged platform. Rats were released by being lowered gently into the pool facing the center of one of the walls. The sequence of walls from which they were released varied randomly from session to session, with the constraint that each wall was used once in every session. Throughout each trial, rats were observed on the monitor. If a rat failed to find the platform within 60 s, the experimenter placed a finger approximately 5 cm in front of the rat’s nose and guided it to the platform. Rats were allowed to remain on the platform for 20 s before they were removed from the pool. After a trial, the rats were dried gently and returned to the light-tight box where they waited until the other four rats had received a single trial in the pool. This cycle was repeated until all rats had received four trials. After the squad of rats in the light-tight box had each received a single trial, the rectangular arena was rotated either clockwise or anticlockwise. The rectangle was always oriented along a north-south or east-west axis, where north, for the sake of the experiment, was defined as the middle of the entrance to the room. The sequence of rotations was varied randomly from session to session and the rectangle could be moved through more than 90° in one rotation. A record was taken on every trial of how long it took the rat to reach and climb on to the platform after it was released from the edge of the pool.
For odd numbered sessions from 1 to 24, the four walls of the rectangle were white, and for the even numbered sessions all four walls were black. For half the rats in each group the platform was located in a corner where a short wall was to the left of a long wall in the white rectangle, and in a corner where a short wall was to the right of a long wall in the black rectangle. The opposite of these relationships were used for the remaining rats. From Sessions 25 to 37, two trials within each session were conducted in the white rectangle and two were conducted in the black rectangle. The sequence in which the rectangles were used varied randomly from session to session. Occasionally test trials were conducted in which rats were placed in the pool with the platform removed and allowed to swim for 60 s. Such test trials replaced the fourth training trial in Sessions 21, in which the test took place in a white pool, and in Session 22, in which the test took place in the black pool. There were also test trials in Sessions 35 and 37, for which half the rats in each group were tested in the black rectangle in Session 35, and the white rectangle in Session 37; the opposite was true for the remaining rats.
For each test trial the rats were released from the center of the pool and allowed to swim for 60 s. During this trial the amount of time spent in four circular search zones was recorded. Each zone was 30 cm in diameter with its center on a point that was 25 cm from a corner on a line that bisected the corner. The search zones in the two opposite corners where the platform had previously been located are referred to as the correct zones; the zones in the remaining two corners are referred to as the incorrect zones.
For the test of object recognition, rats were first trained over eight days to run from one compartment to the other in the test chamber and to move each of two standard objects situated above the food wells. On Session 1 of this pretraining, pairs of rats were placed in the apparatus for 20 min with no objects, with the guillotine door raised, and with 45 mg food pellets (Noyes, Purified Rodent Diet, Lancaster, NH, USA) scattered freely on the floor and in the food wells. The door was again raised for Sessions 2 and 3, during which rats were placed in the chamber individually for 10 min and required to shuttle from one side to the other in order to retrieve food pellets from the four wells. Additional pellets were placed in the wells after those previously placed in them were consumed by the rats. From Session 4 onwards the behavior of the rats was controlled by the guillotine door. A single food pellet was placed in each well, and the wells were consistently replenished throughout each session. Once the pellets in both wells of the side of the chamber currently occupied by the rat had been consumed the door was raised. The door was lowered after the rat had passed through it and it remained in this position until the rat had eaten the food in the two wells. Training continued in this manner for Sessions 6 through 8, except identical objects were located over each of the food wells. The rats were required to displace these objects in order to retrieve the food. The three different pairs of objects that were used for this training were not used for the test phase of the experiment. The duration of Sessions 4 to 8 was 10 min. By the end of Session 8, every rat would run through the door as soon as it was opened and would displace one object and then the other from above the two food wells.
The test phase of the experiment comprised a single session of 20 trials. A rat was placed in one side of the chamber, with the door closed, and with only one object, A, over one food well, which contained a single food pellet. The door was raised after 60 s to permit the rat access to the other side of the chamber which contained two different objects over the two food wells, each of which contained a single food pellet. One of the objects was identical to the one just seen, A (now regarded as familiar), and the other one was novel, B. The door was closed once the rat had passed through and remained shut for a further 60 s. During this period the rat ate the food, and explored the objects. Once the door was again opened, the rat passed through it to be confronted by a replica of the object that had just been introduced, B, and a novel object, C, both of which were above food wells containing food. Training continued in this manner until the rat had received 20 trials and, hence, had been exposed to 21 different objects. The left-right placement of the novel and familiar objects varied according to a pseudo-random schedule. For half the subjects in each group the sequence in which the objects were shown was the opposite to that used for the remaining subjects. A camera located above the center of the guillotine door was used to record on videotape the behavior of each rat throughout the 20 trials.
During presentation of every pair of objects a record was taken of the amount of time that was spent exploring each of them. Exploration was defined as having the nose nearer than 1 cm to the object, or touching it with the nose or paws.
A Type I error of p<.05 was adopted throughout this report.
Results
Histology
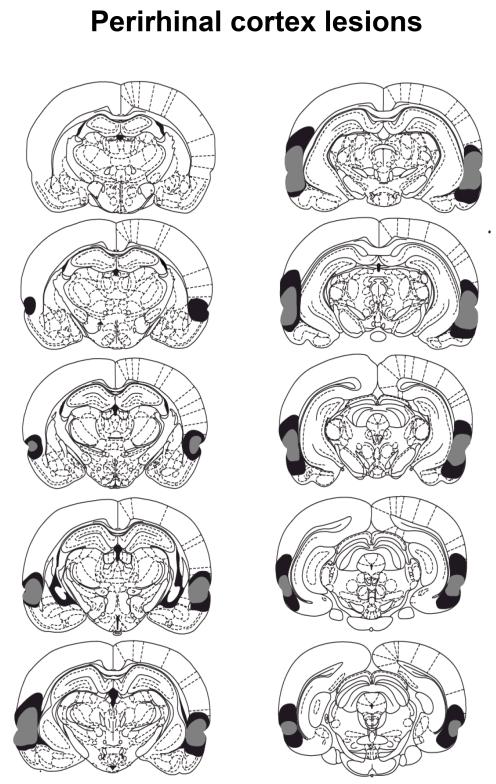
The cytoarchitectonic borders of the perirhinal cortex (areas 35 and 36) are taken from those described by Burwell (2001). Figure 3 depicts a series of coronal section (adapted form Paxinos and Watson, 1998) showing the extent of the maximum and minimum perirhinal cortex lesions. The lesions were centered on the rhinal sulcus and extended from the anterior parts of the amygdala to the posterior parts of the hippocampus. All rats sustained complete damage to the perirhinal cortex (areas 35 and 36) as well as damage to the lateral entorhinal cortices and the temporal association cortex immediately above area 36 (area Te2). Eight rats sustained damage to the deep layers of the auditory association cortices. In two of those eight rats there was extensive damage to the superficial areas of the association cortices in the left hemisphere. Damage to the dorsal piriform cortex was present in all rats and included the endopiriform nucleus. All rats had sparing of the external capsule, the subiculum and the hippocampus. Two rats were excluded from the behavioral analysis due to extensive dorsal damage including the auditory and visual cortices in both hemispheres as well as extensive ventral damage including the piriform cortex. In addition those rats sustained damage to the posterior parts of CA1. The final number of rats in Group Perirhinal was 10, with 12 in Group Sham.
Figure 3.
The coronal sections taken throughout the dorsoventral extent of the brain depict the extent of the damage for the perirhinal lesioned rats. The rat with the greatest extent of lesion is represented in gray whereas the rat with the smallest extent of lesion is represented in black shading. The sections are posterior to and at specific distances (in mm) from Bregma (top to bottom, left then right: 2.12, 2.80, 3.30, 3.80, 4.30, 4.80, 5.30, 5.80, 6.30, 6.80) taken from the Paxinos and Watson (1996) stereotaxic atlas.
Behavior
Spatial discrimination
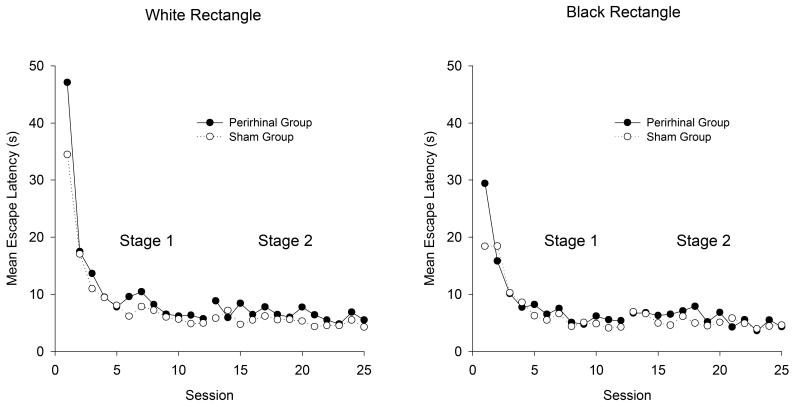
The left-hand panel of Figure 4 shows the mean escape latencies for the two groups for each of the sessions conducted in the white rectangle throughout Stages 1 and 2 of the experiment. Similar results for the training in the black rectangle can be seen in the right-hand panel. There is a marked reduction in latencies at the outset of the experiment, and the transition from Stage 1 to Stage 2 had relatively little influence on this measure. In both stages there is an indication that the escape latencies were longer for the perirhinal than the sham group. To compare the performance of the two groups, mean escape latencies were calculated for individual rats, for each session of training in each rectangle, for the two stages of the experiment. A two-way ANOVA of individual mean latencies for all the sessions of Stage 1 combined revealed a significant effect of group, F(1, 20) = 15.56, and color of rectangle (with latencies being shorter in the black than the white rectangle), F(1, 20) = 57.48, but the interaction was not significant, F(1, 20) = 1.58. A similar analysis for Stage 2 again revealed a significant effect of group, F(1, 20) = 7.51, but the effect of color of rectangle and the interaction were not significant, Fs(1, 20) < 2. 49.
Figure 4.
The mean escape latencies for the two groups during the training for the spatial discrimination in the white rectangle (left-hand panel) and in the black rectangle (right-hand panel).
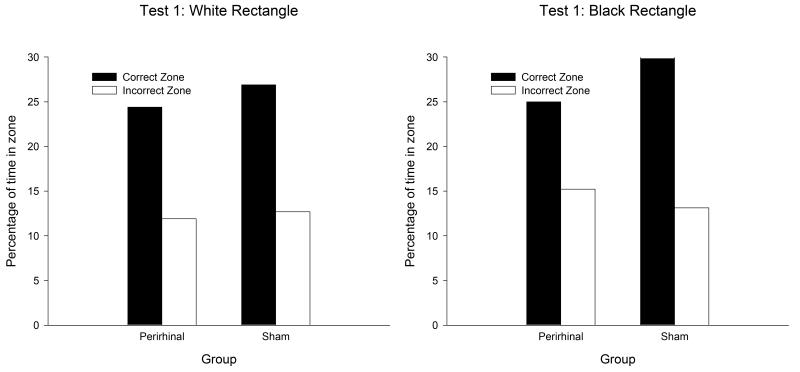
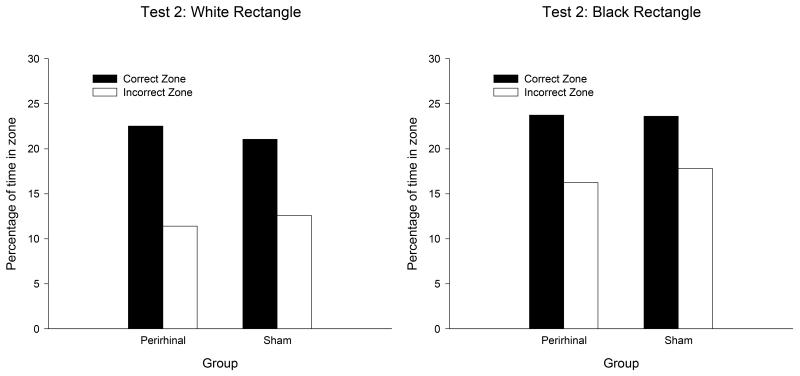
During the test trials at the end of Stage 1 and Stage 2, the two groups expressed a clear preference for searching in the correct rather than the incorrect search zones in both rectangles. Moreover, the extent of this preference was similar in both groups. The results from the first test, which was conducted on the twelfth session of training in the white rectangle, and the twelfth in the black rectangle, can be seen in Figure 5. A three-way ANOVA of individual durations of time spent in the correct and incorrect search zones revealed a significant preference for the correct over the incorrect zones, F(1, 20) = 78.25, and a significant effect of color, F(1, 20) = 4.46. Neither the effect of group nor any of the interactions was significant, Fs(1, 20) < 2.38. The equivalent results from the second test, which took place on Sessions 35 and 37, are displayed in Figure 6. Once again, there was a significant effect of search zone, F(1, 20) = 36.55, and of color, F(1, 20) = 23.66. The effect of group, and none of the interactions was significant, Fs(1, 20) < 3.2.
Figure 5.
The mean percentages of time spent by the two groups in the correct and incorrect search zones during the first test trial of the spatial discrimination in the white rectangle (left-hand panel) and the black rectangle (right-hand panel).
Figure 6.
The mean percentages of time spent by the two groups in the correct and incorrect search zones during the second test trial of the spatial discrimination in the white rectangle (left-hand panel) and the black rectangle (right-hand panel).
Object recognition test
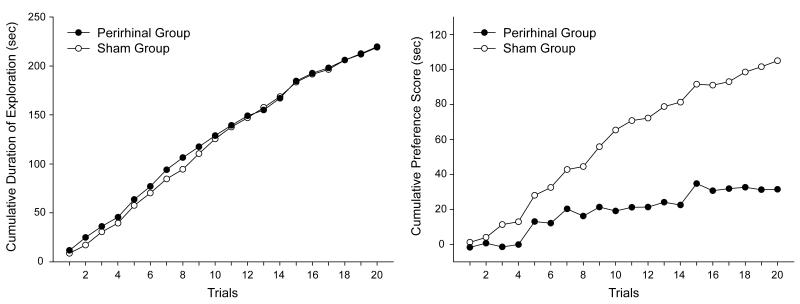
In order to determine whether the lesions to the perirhinal cortex affected the tendency to explore preferentially the novel objects, the total time spent exploring both objects was calculated for each pair of objects for every subject. The left-hand panel of Figure 7 shows the sum of these scores across successive presentations of the 20 different pairs of objects. The figure thus depicts the total time spent exploring both objects as training progressed. It is apparent that the two groups show a similar increase in the cumulative time spent exploring both objects (novel and familiar). Moreover, the trial by trial increase in the total amount of time spent exploring both objects was reasonably constant, which indicates that both groups spent approximately the same amount of time exploring each pair of objects throughout training. In keeping with the observation that the performance of both groups was very similar, statistical analysis revealed that by Trial 20 the cumulative time spent exploring both objects was not significantly different between the two groups, t(20) = 0.021.
Figure 7.
Left-hand panel: The cumulative time spent by the two groups of rats exploring both familiar and novel objects across successive trials of the test for spontaneous object recognition. Right-hand panel: Cumulative preference scores (time spent exploring novel object less the time spent exploring the familiar object) across successive trials of the same test for spontaneous object.
In order to compare the amount of time spent exploring the novel and the familiar object on each test trial, a preference score was calculated for each subject. This score was obtained by subtracting the time spent exploring the familiar object from the time spent exploring the novel object. The right-hand panel of Figure 7 shows for both groups the cumulative total of these preference scores for each of the 20 trials. It is apparent that the cumulative totals increased more rapidly for the sham than the perirhinal group, thus demonstrating that the preference for exploring the novel rather than the familiar object was greater in the control than the lesioned group. In support of this observation it was found that by Trial 20 the cumulative total of the difference scores was significantly higher for the control than the lesioned group, t(20) = 6.14.
Discussion
Despite the complexity of the design of the experiment that took place in the rectangular pool, the results were quite straightforward. With the platform removed from the pool, rats with lesions of the perirhinal cortex were able to identify the correct corners in the rectangular pool, even though one type of corner was correct in the white pool, and the other type was correct in the black pool. These test trial results thus confirm that despite their lesion, rats in the perirhinal group were able to solve a complex discrimination in which it was necessary to combine information about the color and the length of the walls of the rectangular arena.
The results from the other measure of behavior are not, however, in keeping with the results from the test trials. The escape latencies were significantly longer by the perirhinal-lesioned rats than the control rats but this difference, it is worth noting, was not large numerically. Although these results indicate an influence of the lesions on the acquisition of the discrimination, the results from the test trials indicate this influence was not a consequence of making it difficult to tell the difference between the correct and incorrect corners. Instead, this effect of the lesions to the perirhinal cortex may have occurred for more indirect reasons. Aggleton et al., (2004), for instance, suggested that damage to the perirhinal cortex may encourage rats to keep close to the wall of the apparatus when they are released into a swimming pool. If this occurred in the present experiment, then it would explain why it took the perirhinal group longer than the sham group to find the platform during the training sessions. On being released into the pool, if the rat was heading for a correct corner on the opposite side of the pool, any tendency to remain close to the wall would increase the length of the journey and result in the escape latency being longer than if the rat headed directly for the corner.
Turning to the second part of the experiment, the perirhinal group showed a much weaker preference for exploring novel than familiar objects than the control group. In keeping with the results from previous studies (Ennaceur et al., 1998; Norman & Eacott, 2004), the present experiment again demonstrates that the perirhinal cortex is important for allowing rats to distinguish between novel and familiar objects. It thus appears that the perirhinal cortex is important for some discriminations – judging whether an object is novel or familiar – but it is not so important for discriminations between combinations of stimuli that signal the presence or absence of reward. We shall explore in some detail in the General Discussion why the lesions were more effective in the spontaneous exploration of novel objects task than in the spatial navigation task.
General Discussion
The results from the phase of the experiment that took place in the swimming pool demonstrate that rats with bilateral lesions of the perirhinal cortex were capable of integrating geometric and non-geometric visual information. The results from the subsequent phase of the experiment revealed that the lesions adversely affected the spontaneous exploration of novel objects. In the following discussion we consider the implications of our findings for several different theories of the function of the perirhinal cortex.
One reason for conducting the experiments was to evaluate the perceptual mnemonic / feature conjunction (PMFC) model of perirhinal function put forward by Bussey and Saksida (2002; see also Bussey et al., 2005). They regard the perirhinal cortex as the final station in the ventral visual stream (Ungerleider & Mishkin, 1982), and that as one ascends this stream increasingly more complex visual representations are constructed. In terms of the connectionist network they developed, such complex representations are regarded as units that fire fully whenever a particular combination of features is presented, and lesions of the perirhinal cortex are assumed to eliminate these so-called, conjunctive units. Bussey and Saksida (2002) argued from these proposals that the perirhinal cortex is responsible for solving discriminations involving feature ambiguity where individual stimuli, by themselves, are of ambiguous significance about the trial outcome. In the case of the spatial task in the swimming pool, the performance on the test trials in the black and white rectangles indicates that rats with perirhinal lesions were able to integrate the color of the walls with geometric information in order to identify where the hidden goal was located. This finding appears to pose a problem for the PMFC theory because both types of cue alone provided ambiguous information about the location of the goal. It is possible, however, that the stimuli created by the rectangular pools were not of sufficient complexity to require an intact perirhinal cortex for the discriminations to be solved. Bussey et al. (2005) acknowledge that the perirhinal cortex is responsible for integrating information about complex stimuli, and that more caudal regions of the ventral visual stream are responsible for representing configural information based on relatively simple stimuli. On the basis of this proposal, it could then be inferred that the cues on which the spatial discriminations depended were not of sufficient complexity to require an intact perirhinal cortex for the necessary conjunctive representations to be formed.
An implication of the foregoing proposal is that the objects used for the object recognition test were of greater complexity than the spatial cues and thus depended upon an intact perirhinal cortex for one to be distinguished from the other (Cowell, Busey, & Saksida, 2006). The problem with this analysis is that it is hard to predict with the PMFC model, as it is currently formulated, whether or not lesions of the perirhinal cortex will disrupt a discrimination. Indeed, given the emphasis placed by this model on feature ambiguity, with discriminations involving considerable feature ambiguity being more prone to disruption by perirhinal lesions than discriminations where such ambiguity is slight, it might be thought that the present results should have revealed the opposite outcome. All of the stimuli that were involved for the spatial discrimination can be said to have been of ambiguous significance, and yet the lesions did not prevent it from being solved. In contrast, given the diversity of the objects used for the recognition task, there were many distinctive features that allowed one object to be differentiated from another, yet the lesions disrupted the discrimination between them. The PMFC model would thus seem to be in need of further elaboration if it is to prove satisfactory for predicting how novel discriminations will be affected by damage to the perirhinal cortex. It is possible that lesions of the perirhinal cortex disrupt discriminations involving only complex stimuli – such as the objects used for the spontaneous recognition test, but why this should be, and what constitutes a complex stimulus, is not fully explained by the PMFC model. Another possibility is that not all discriminations are solved by the same neural system. McDonald, Murphy, Guarrqaci, Gortler, White and Baker (1997) have argued that conditional contextual discriminations are not processed in the same way by the brain as discriminations involving feature ambiguity such as negative patterning. It is thus conceivable that the PMFC model does not apply to the swimming pool task adopted for the present study.
An alternative characterisation of the function of the perirhinal cortex is that it is responsible for processing knowledge about objects, whereas information about scenes is believed to be processed in the hippocampus (Buckley and Gaffan, 1998). This distinction fits reasonably well with the present results. Although it may not always be easy to specify when a combination of cues should be regarded as an object (but see Cassaday & Rawlins, 1995, 1997), it seems entirely reasonable to assume that the stimuli used for the test of spontaneous recognition were individual objects. If the capacity to represent objects depends upon an intact perirhinal cortex, then the outcome of the object recognition test should not be surprising. Morever, a white rectangular arena might reasonably be regarded as a scene, in which case the capacity to find a hidden goal with reference to this environment would not be expected to be affected by lesions of the perirhinal cortex, but it would be affected by lesions of the hippocampus. The results from the spatial discrimination confirmed the first of these predictions, and support for the latter can be found in Pearce, Good, Jones, & McGregor (2004) who demonstrated that rats with lesions of the hippocampus find it difficult to locate a hidden goal by reference to the geometric cues provided by a rectangular environment (see also, Jones, Pearce, Davies, Good & McGregor, 2007; McGregor, Hayward, Pearce & Good, 2004).
A problem with the suggestion that the perirhinal cortex is important for processing information about objects is posed by the findings that rats with lesions of the perirhinal cortex are able to solve a biconditional discrimination based on pairs of adjacent patterns (Davies, Machin, Sanderson, Pearce & Aggleton, 2007, but see Machin & Gaffan, 2001). It seems reasonable to suppose the stimuli would be regarded as objects by the rats and thus, according to the proposals of Buckley and Gaffan (1998), the lesions should have impaired the acquisition of the discrimination. To return to the discussion of Bussey et al. (2002), it is conceivable that each pattern was too simple, and that an object must be more complex if a discrimination in which it is involved is to be disrupted by damage to the perirhinal cortex. However, this suggestion raises the questions of how complexity can be measured, and why is it so important? Be that as it may, it is pertinent to note that the demonstrations with rats of a failure of find an effect of perirhinal lesions on the acquisition of a discrimination involving feature ambiguity have all involved relatively simple stimuli. In contrast, successful demonstrations with monkeys of an effect of perirhinal lesions on similar discriminations have used complex stimuli, such as photographs (Buckley & Gaffan, 1998; Bussey, Saksida, & Murray, 2002, 2003).
A rather different approach to understanding the present results is to assume that one dedicated function of the rat perirhinal cortex is to support recognition memory by differentiating between familiar and novel items (Aggleton & Brown, 1999). More specifically, it is proposed that the perirhinal cortex is required for item but not contextual information (Aggleton & Brown, 2006; Diana et al., 2007). On the basis of these proposals lesions of the perirhinal cortex would not be expected to influence the outcome of the spatial discrimination that we conducted, but they would be expected to have a profound impact on the object recognition test. While the present results are entirely in keeping with these predictions, these proposals do not provide a comprehensive account of perirhinal cortex function. For example, it is not clear why perirhinal cortex damage should disrupt the acquisition of some but not all discrimination tasks by monkeys and rats (Bartko et al., 2007; Buckley & Gaffan, 2003; Bussey et al., 2002; Davies et al., 2007; Hampton, 2005; Machin & Eacott, 1999). It remains for future work to identify an additional function of the perirhinal cortex.
We noted in the Introduction that lesions to the perirhinal cortex disrupt the capacity of animals to integrate information about objects and where they occur (e.g. Norman and Eacott, 2005). A related effect was reported by Iordanova et al. (2009) who found that such lesions make it difficult to identify in which environment an auditory cue has been presented. An implication of these results is that rats with lesions of the perirhinal cortex should be unable to learn about the location of a hidden goal, such as a submerged platform, in a distinctively shaped environment with walls of a distinctive color. The results from the test in the rectangular swimming pool failed to confirm this prediction. An important difference between these studies is that it was only in the present one that rats gained reward – through escape from the pool – by learning about the position of a goal relative to the context. Perhaps, if learning where an object (or auditory cue) was presented increased the likelihood of reward, then the impact of the lesions of the perirhinal cortex would also have been minimal in the studies by Norman and Eacott (2005) and Iordanova et al. (2009).
The results from test in the rectangular pool have important implications for the suggestion that animals possess a geometric module which is important for navigating in environments with a distinctive shape (Cheng, 1986; Gallistel, 1990). According to this point of view, when an animal is placed in an environment with a distinctive shape, then information about the geometric properties of the shape is processed in a specialised module which allows the position of the goal to be identified with reference to just geometric cues. Gallistel (1990) further proposed that this module is impervious to non-geometric information, which means that it should not have been possible for information about the shape of the rectangular pool to be integrated with the color of its walls in order for rats to solve the spatial discrimination. The success of the rats on this problem thus joins several other studies (Graham, Good, McGregor & Pearce, 2006; Pearce, Graham, Good, Jones & McGregor, 2006) in showing that if animals possess a geometric module then it is not impervious to all non-geometric information (see also Cheng & Newcombe, 2005).
The results from the spatial discrimination pose a problem for the most general form of the proposal by Bussey and Saksida (2002) that the perirhinal cortex is responsible for creating configural representations that are essential if discriminations involving ambiguous cues are to be solved. It is possible that the stimuli in Experiments 1 and 2 were too simple to provide a suitable test of their proposals. If this is correct, then the present experiments may help to identify the boundary conditions under which lesions of the perirhinal cortex will, and will not disrupt the acquisition of complex, conditional discriminations.
Acknowledgments
This work was supported by grants from the Medical Research Council and the Biotechnology and Biological Sciences Research Council of the United Kingdom, and an Overseas Research Studentship awarded to Murray Horne. We are grateful to Lloyd Uncles for his assistance with the experiments.
References
- Albasser MM, Poirier GL, Aggleton JP. Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel versus familiar objects as revealed by c-Fos imaging. European Journal of Neuroscience. 2009 doi: 10.1111/j.1460-9568.2009.07042.x. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Interleaving brain systems for episodic recognition memory. Trends in Cognitive Science. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Kyd R, Bilkey DK. When is the perirhinal cortex necessary for the performance of spatial memory tasks? Neuroscience and Biobehavioral Reviews. 2004;28:611–624. doi: 10.1016/j.neubiorev.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. Journal of Neuroscience. 2008;28:2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learning and Memory. 2007;14:821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices. Journal of Comparative Neurology. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhnal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience. 2002;15:365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Impairments in visual discriminations after perirhinal cortex lesions: testing ‘declarative’ vs ‘perceptual-mnemonic’ views of of perirhinal cortex function. European Journal of Neuroscience. 2003;17:649–660. doi: 10.1046/j.1460-9568.2003.02475.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic / feature conjunction model of perirhinal cortex function. Quarterly Journal of Experimental Psychology. 2005;58B:269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Rawlins JNP. Fornix-fimbria section and working memory deficits in rats: Stimulus complexity and stimulus size. Behavioral Neuroscience. 1995;109:594–606. doi: 10.1037//0735-7044.109.4.594. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Rawlins JNP. The hippocampus, objects, and their context. Behavioral Neuroscience. 1997;111:1228–1244. doi: 10.1037//0735-7044.111.6.1228. [DOI] [PubMed] [Google Scholar]
- Cheng K. A purely geometric module in the rat’s spatial representation. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Cheng K, Newcombe NS. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychonomic Bulletin and Review. 2005;12:1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. The Journal of Neuroscience. 2006;26:12186–12197. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Machin PE, Sanderson DJ, Pearce JM, Aggleton JP. Neurotoxic lesions of the rat perirhinal and postrhinal cortices and their impact on biconditional visual discrimination tasks. Behavioural Brain Research. 2007;176:274–283. doi: 10.1016/j.bbr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three component model. Trends in Cognitive Science. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous test of object recognition: evidence of object-location and object-context recognition. Behavioural Brain Research. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. Behavioral and Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave NJ, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behavioural Brain Research. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A. Interaction of the perirhinal cortex with the fornix-fimbria: Memory for objects and “object in place” memory. Journal of Neuroscience. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. MIT press; Cambridge, MA: 1990. [Google Scholar]
- Graham M, Good MA, McGregor A, Pearce JM. Spatial learning based on the shape of the environment is influenced by properties of the objects forming the shape. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:44–59. doi: 10.1037/0097-7403.32.1.44. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Monkey perirhinal cortex is critical for visual memory, but not for visual perception: Re-examination of the behavioural evidence from monkeys. Quarterly Journal of Experimental Psychology. 2005;58B:283–299. doi: 10.1080/02724990444000195. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Burnett DJ, Aggleton JP, Good MA, Honey RC. The role of the hippocampus in mnemonic integration and retrieval: Complementary evidence from lesion and inactivation studies. European Journal of Neuroscience. 2009 doi: 10.1111/j.1460-9568.2009.07010.x. in press. [DOI] [PubMed] [Google Scholar]
- Jones PM, Pearce JM, Davies VJ, Good MA, McGregor A. Impaired processing of local geometric features during navigation in a water maze following hippocampal lesions in rats. Behavioral Neuroscience. 2007;121:1258–1271. doi: 10.1037/0735-7044.121.6.1258. [DOI] [PubMed] [Google Scholar]
- Machin PE, Eacott MJ. Perirhinal cortex and visual discrimination learning in the rat. Psychobiology. 1999;27:470–479. [Google Scholar]
- McGregor A, Hayward AJ, Pearce JM, Good MA. Hippocampal lesions disrupt navigation based on the shape of the environment. Behavioral Neuroscience. 2004;118:1011–1021. doi: 10.1037/0735-7044.118.5.1011. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Spooner RIW. Watermaze software (computer software) Watermaze Software; Edinburgh, UK: 1990. [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural Brain Research. 2004;148:79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesion of the perirhinal cortex and postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. Academic; Sydney: 1998. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Good MA, Jones PM, McGregor A. Transfer of spatial behavior between different environments: Implications for theories of spatial learning and for the role of the hippocampus in spatial learning. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:135–147. doi: 10.1037/0097-7403.30.2.135. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Graham M, Good MA, Jones PM, McGregor A. Potentiation, overshadowing, and blocking of spatial learning based on the shape of the environment. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:201–214. doi: 10.1037/0097-7403.32.3.201. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]