Abstract
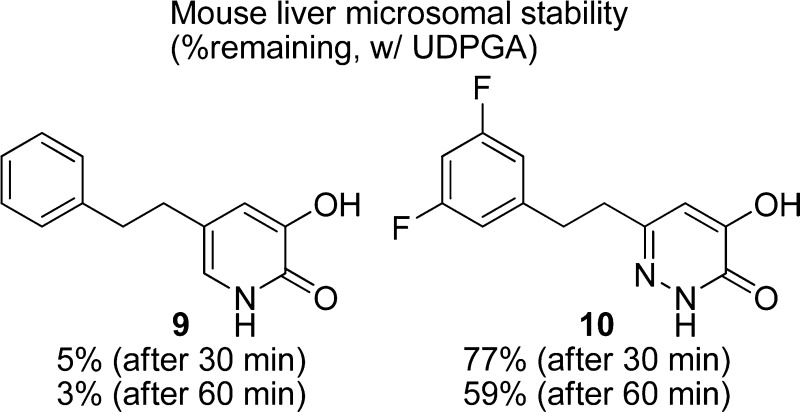
Representative d-amino acid oxidase (DAAO) inhibitors were subjected to in vitro liver microsomal stability tests in the absence or presence of uridine diphosphate glucuronic acid (UDPGA). While carboxylate-based DAAO inhibitors displayed little glucuronidation, most DAAO inhibitors containing α-hydroxycarbonyl moiety exhibited nearly complete glucuronidation within 30 min. The one exception was 6-[2-(3,5-difluorophenyl)ethyl]-4-hydroxypyridazin-3(2H)-one 10, which exhibited some degree of resistance to glucuronidation by liver microsomes from mice, rats, and humans.
Keywords: glucuronidation, d-amino acid oxidase (DAAO), drug metabolism
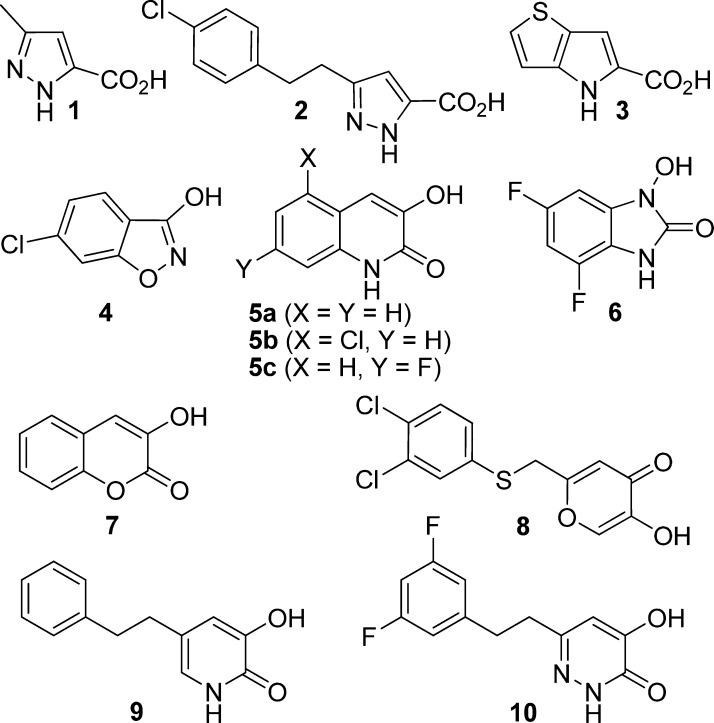
d-Amino acid oxidase (EC 1.4.3.3, DAAO) catalyzes the oxidation of d-amino acids including d-serine, an endogenous ligand for the glycine modulatory site of the N-methyl-d-aspartate (NMDA) receptors. Since hypofunction of NMDA receptors has been implicated in the pathophysiology of schizophrenia, enhancement of d-serine in the brain by inhibition of DAAO has gained substantial interest as a new therapeutic approach to schizophrenia. Indeed, a number of new DAAO inhibitors have appeared in the literature over the past decade.1,2 The common structural feature shared by these DAAO inhibitors is an aromatic ring system with a carboxylic acid or its bioisostere. As shown in Figure 1, representative carboxylate-based inhibitors include 5-methyl-1H-pyrazole-3-carboxylic acid (AS057278) 1,3 5-(4-chlorophenethyl)-1H-pyrazole-3-carboxylic acid 2,4 and 4H-thieno[3,2-b]pyrrole-5-carboxylic acid 3.5 6-Chloro-1,2-benzisoxazol-3(2H)-one (CBIO) 4(6) has an isoxazol-3-one as a carboxylic acid bioisostere. Compounds 5–107−11 contain an α-hydroxycarbonyl moiety, which appears particularly effective as a bioisosteric replacement for the carboxylate group. The successful replacement of the carboxylate group is primarily due to their ability to retain the critical interactions with the key residues of the active site of DAAO. For example, a crystal structure of human DAAO in complex with 5a (3G3E) shows the Tyr228 residue involved in hydrogen bonding to the 3-hydroxyl group of 5a as well as the Arg283 residue interacting with both the 3-hydroxyl group and the 2-carbonyl group of 5a.7 A very similar mode of binding has been reported for 7 (PDB 3ZNP),129 (PDB 3W4J),11 and 10 (PDB 3W4K)11 cocrystallized with human DAAO.
Figure 1.
Representative DAAO inhibitors.
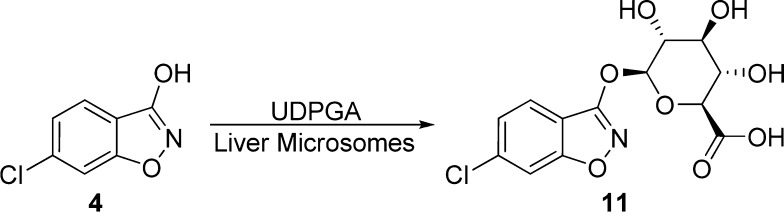
One of the advantages offered by the α-hydroxycarbonyl moiety is their considerably higher pKa values as compared to those of carboxylic acids. The 3-hydroxyl group of 5a has a pKa value of 8.77 and is only minimally ionized at the physiological pH. The low acidity of this bioisostere was expected to result in better membrane permeability and improved pharmacokinetics. Systematic pharmacokinetics studies, however, revealed that close analogues of 5a are not orally available in either mice or rats despite that the carboxylate-based inhibitors 1 and 3 show reasonable oral bioavailability (F = 33–97%) in both species.13 These findings suggest that there may be other factors potentially impacting the pharmacokinetics of DAAO inhibitors containing the α-hydroxycarbonyl bioisostere. Interestingly, we previously found that compound 4 is substantially metabolized by mouse and human microsomes in the presence of UDPGA, but not in the presence of NADPH, suggesting loss of the parent compound by glucuronidation (Figure 1).14 Indeed, the formation of the corresponding glucuronide 11 (Figure 2) was confirmed by LC/MS analysis in both mouse and human microsomes fortified with UDPGA.14 Subsequently, we found that compound 6 also undergoes substantial glucuronidation in microsomes.8 These in vitro metabolic studies imply that glucuronidation may represent a common metabolic pathway that potentially contributes to the poor oral pharmacokinetics of a broader range of DAAO inhibitors. In this letter, we report on the glucuronidation of representative DAAO inhibitors including those containing a carboxylic acid bioisostere to identify structural features that are subject to glucuronidation and examine their potential role in oral pharmacokinetics.
Figure 2.
Glucuronidation of 4 in liver microsomes.
An in vitro glucuronidation assay was conducted using mouse, rat, and/or human microsomes (0.5 mg protein/mL) in the absence or presence of UDPGA (2 mM). Mouse microsomes were used as the primary assay so that we could compare the in vitro stability data with the previously reported in vivo pharmacokinetics data obtained from mice. The remaining parent compounds were quantified after 30 and 60 min of incubation at 37 °C. The metabolic data are summarized in Table 1.
Table 1. Glucuronidation of DAAO Inhibitors.
| % remaining |
||||
|---|---|---|---|---|
| compd | hDAAO IC50 (nM)a | source of liver microsomes | 30 min | 60 min |
| 1 | 9103 | mouse | 92 ± 6 | 78 ± 4 |
| 2 | <10004 | mouse | 103 ± 4 | 102 ± 5 |
| 3 | 5.412 | mouse | 98 ± 1 | 100 ± 6 |
| 4 | 17.213 | mouse | 58 ± 2 | 33 ± 1 |
| 5a | 47 | mouse | <1 | <1 |
| 5b | 87 | mouse | <1 | <1 |
| 5b | rat | <1 | <1 | |
| 5c | 107 | mouse | <1 | <1 |
| 5c | rat | <1 | <1 | |
| 6 | 808 | mouse | <1 | <1 |
| 7 | 4409 | mouse | <1 | <1 |
| 8 | 10010 | mouse | 20 ± 3 | 5 ± 1 |
| 9 | 2011 | mouse | 5 ± 1 | 3 ± 1 |
| 10 | 7.011 | mouse | 77 ± 7 | 59 ± 4 |
| 10 | rat | 69 ± 1 | 51 ± 2 | |
| 10 | human | 81 ± 1 | 62 ± 0 | |
IC50 values are taken from the respective cited references.
In all cases, the parent compounds remained intact in the absence of UDPGA. In the presence of UDPGA, however, loss of the parent compounds took place to varying degrees, indicating that DAAO inhibitors of different chemotypes are subject to different degrees of glucuronidation. A clear distinction was observed between carboxylate-based DAAO inhibitors 1–3 and biosiostere-containing inhibitors 4–10 in degree of glucuronidation. Compounds 1–3 were found to be completely resistant to glucuronidation. The results are in good agreement with the reasonable oral bioavailability in mice previously reported for compounds 1 and 3 (F = 33% and 56%, respectively).13 Consistent with our previous data, compound 4 was glucuronidated in a time-dependent manner, and only 33% of the parent compound remained intact after 60 min of incubation. The rather gradual glucuronidation of 4, however, appears to result in the still reasonable oral bioavailability (F = 29%) in mice.13 In contrast, compounds 5–9 were glucuronidated much more rapidly than 4. Only 20% of compound 8 remained after 30 min of incubation. Moreover, compounds 5–7 and 9 were almost completely metabolized within 30 min. The possibility of N-glucuronidation cannot be ruled out for compounds 5, 6, and 9. However, the rapid glucuronidation of 7 lacking an amino group suggests that predominant metabolism of these compounds is O-glucuronidation.
The rapid glucuronidation by liver microsomes predicts significant loss of compounds through the first pass metabolism following oral administration. Indeed, compound 5b was reported to have negligible oral bioavailability in rats (F = 1.4%) and mice (F = 0.65%).13 Interestingly, compound 5c was reported to be orally available (F = 38%) in rats.7 To this end, we also examined glucuronidation of 5c in rat microsomes. Similar to mouse microsomes, however, compound 5c was completely consumed within 30 min by rat microsomes in the presence of UDPGA. A possible explanation for the reasonable oral bioavailability of 5c despite the substantial glucuronidation may be rapid deglucuronidation of the metabolite by intestinal β-glucuronidase followed by reabsorption of 5c, creating enterohepatic circulation.
In contrast to compounds 5–9, only partial glucuronidation was observed with a 4-hydroxypyridazin-3(2H)-one derivative 10. Nearly 60% of the parent compound remained intact even after 60 min of incubation. The reduced rate of glucuronidation of 10 was also seen in rat and human microsomes. A recent report exploring strategies for the modulation of phase II metabolism in protein kinase C epsilon (PKCε) inhibitors demonstrated that introduction of proximal polarity to the glucuronidation site is generally effective in attenuating O-glucuronidation while maintaining potency against PKCε.15 Although speculative, it is conceivable that increased polarity of the hydroxypyridazin-3(2H)-one moiety as compared to other α-hydroxycarbonyl-based pharmacophores is at least partially responsible for the slow glucuronidation of 10.
Nevertheless, the glucuronidation profiling certainly differentiates compound 10 from other DAAO inhibitors containing a bioisostere for a carboxylate group. Compound 10 was found to be stable in mouse liver microsomes (92% remaining after a 60 min incubation) in the presence of NADPH, a cofactor for CYP-dependent oxidation. Indeed, plasma pharmacokinetics studies in CD1 mice16 revealed that compound 10 is orally bioavailable (F = 31%) with a plasma clearance of 39 mL/min/kg. Although its plasma clearance is higher than compounds 1 (10 mL/min/kg) and 3 (21 mL/min/kg), the oral bioavailability (%F) of 10 appears to be comparable to that of 1 (33%) and 3 (56%).13 Additional reduction in the degree of glucuronidation through structural modification should lead to further improvement in oral bioavailability and plasma clearance.
The results demonstrate that the glucuronidation potential of DAAO inhibitors can be reduced without removing α-hydroxycarbonyl moiety crucial for DAAO inhibition. While our approach is purely empirical, our findings should have practical implication for the design of glucuronidation resistant molecules for other therapeutic targets. One notable example is catechol O-methyltransferase (COMT) inhibitors, many of which possess phenolic pharmacophores subject to glucuronidation.17
Glossary
Abbreviations
- DAAO
d-amino acid oxidase
- CBIO
6-chloro-1,2-benzisoxazol-3(2H)-one
- UDPGA
uridine diphosphate glucuronic acid
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by National Institutes of Health (R01MH091387 to T.T. and a postbaccalaureate fellowship to C.B.).
The authors declare no competing financial interest.
Dedication
Dedicated to Professor Iwao Ojima on the occasion of his 70th birthday.
Funding Statement
National Institutes of Health, United States
References
- Ferraris D. V.; Tsukamoto T. Recent advances in the discovery of d-amino acid oxidase inhibitors and their therapeutic utility in schizophrenia. Curr. Pharm. Des. 2011, 17, 103–11. [DOI] [PubMed] [Google Scholar]
- Sacchi S.; Rosini E.; Pollegioni L.; Molla G. d-Amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr. Pharm. Des 2013, 19, 2499–511. [DOI] [PubMed] [Google Scholar]
- Adage T.; Trillat A. C.; Quattropani A.; Perrin D.; Cavarec L.; Shaw J.; Guerassimenko O.; Giachetti C.; Greco B.; Chumakov I.; Halazy S.; Roach A.; Zaratin P. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur. Neuropsychopharmacol. 2008, 18, 200–14. [DOI] [PubMed] [Google Scholar]
- Fang Q. K.; Hopkins S.; Jones S.. Pyrrole and pyrazole DAAO inhibitors. U.S. Pat. Appl. 20050143443, 2005.
- Sparey T.; Abeywickrema P.; Almond S.; Brandon N.; Byrne N.; Campbell A.; Hutson P. H.; Jacobson M.; Jones B.; Munshi S.; Pascarella D.; Pike A.; Prasad G. S.; Sachs N.; Sakatis M.; Sardana V.; Venkatraman S.; Young M. B. The discovery of fused pyrrole carboxylic acids as novel, potent d-amino acid oxidase (DAO) inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3386–91. [DOI] [PubMed] [Google Scholar]
- Ferraris D.; Duvall B.; Ko Y. S.; Thomas A. G.; Rojas C.; Majer P.; Hashimoto K.; Tsukamoto T. Synthesis and biological evaluation of d-amino acid oxidase inhibitors. J. Med. Chem. 2008, 51, 3357–9. [DOI] [PubMed] [Google Scholar]
- Duplantier A. J.; Becker S. L.; Bohanon M. J.; Borzilleri K. A.; Chrunyk B. A.; Downs J. T.; Hu L. Y.; El-Kattan A.; James L. C.; Liu S.; Lu J.; Maklad N.; Mansour M. N.; Mente S.; Piotrowski M. A.; Sakya S. M.; Sheehan S.; Steyn S. J.; Strick C. A.; Williams V. A.; Zhang L. Discovery, SAR, and pharmacokinetics of a novel 3-hydroxyquinolin-2(1H)-one series of potent d-amino acid oxidase (DAAO) inhibitors. J. Med. Chem. 2009, 52, 3576–85. [DOI] [PubMed] [Google Scholar]
- Berry J. F.; Ferraris D. V.; Duvall B.; Hin N.; Rais R.; Alt J.; Thomas A. G.; Rojas C.; Hashimoto K.; Slusher B. S.; Tsukamoto T. Synthesis and SAR of 1-hydroxy-1H-benzo[d]imidazol-2(3H)-ones as inhibitors of d-amino acid oxidase. ACS Med. Chem. Lett. 2012, 3, 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katane M.; Osaka N.; Matsuda S.; Maeda K.; Kawata T.; Saitoh Y.; Sekine M.; Furuchi T.; Doi I.; Hirono S.; Homma H. Identification of novel d-amino acid oxidase inhibitors by in silico screening and their functional characterization in vitro. J. Med. Chem. 2013, 56, 1894–190. [DOI] [PubMed] [Google Scholar]
- Raje M.; Hin N.; Duvall B.; Ferraris D. V.; Berry J. F.; Thomas A. G.; Alt J.; Rojas C.; Slusher B. S.; Tsukamoto T. Synthesis of kojic acid derivatives as secondary binding site probes of d-amino acid oxidase. Bioorg. Med. Chem. Lett. 2013, 23, 3910–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondo T.; Warizaya M.; Niimi T.; Namatame I.; Yamaguchi T.; Nakanishi K.; Hamajima T.; Harada K.; Sakashita H.; Matsumoto Y.; Orita M.; Takeuchi M. 4-Hydroxypyridazin-3(2H)-one derivatives as novel d-amino acid oxidase inhibitors. J. Med. Chem. 2013, 56, 3582–92. [DOI] [PubMed] [Google Scholar]
- Hopkins S. C.; Heffernan M. L.; Saraswat L. D.; Bowen C. A.; Melnick L.; Hardy L. W.; Orsini M. A.; Allen M. S.; Koch P.; Spear K. L.; Foglesong R. J.; Soukri M.; Chytil M.; Fang Q. K.; Jones S. W.; Varney M. A.; Panatier A.; Oliet S. H.; Pollegioni L.; Piubelli L.; Molla G.; Nardini M.; Large T. H. Structural, kinetic, and pharmacodynamic mechanisms of d-amino acid oxidase inhibition by small molecules. J. Med. Chem. 2013, 56, 3710–24. [DOI] [PubMed] [Google Scholar]
- Lange J. H.; Venhorst J.; van Dongen M. J.; Frankena J.; Bassissi F.; de Bruin N. M.; Besten C.; de Beer S. B.; Oostenbrink C.; Markova N.; Kruse C. G. Biophysical and physicochemical methods differentiate highly ligand-efficient human d-amino acid oxidase inhibitors. Eur. J. Med. Chem. 2011, 46, 4808–19. [DOI] [PubMed] [Google Scholar]
- Rais R.; Thomas A. G.; Wozniak K.; Wu Y.; Jaaro-Peled H.; Sawa A.; Strick C. A.; Engle S. J.; Brandon N. J.; Rojas C.; Slusher B. S.; Tsukamoto T. Pharmacokinetics of oral d-serine in d-amino acid oxidase knockout mice. Drug Metab. Dispos. 2012, 40, 2067–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. J.; Coon T.; Busch B. B.; Asgian J. L.; Hudson S.; Termin A.; Flores T. B.; Tran D.; Chiang P.; Sperry S.; Gross R.; Abt J.; Heim R.; Lechner S.; Twin H.; Studley J.; Brenchley G.; Collier P. N.; Pierard F.; Miller A.; Mak C.; Dvornikovs V.; Jimenez J. M.; Stamos D. Strategies for the modulation of phase II metabolism in a series of PKCepsilon inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3398–402. [DOI] [PubMed] [Google Scholar]
- Compound 10 was dosed either intravenously (10 mg/kg) or orally (30 mg/kg) to male CD1 mice (6–8 weeks old), and blood samples were obtained via cardiac puncture at 0.08, 0.25, 0.5, 1, 2, 3 (p.o. only), 4 (i.v. only), and 6 h (p.o. only) post dose (n = 3 per time point). Plasma samples were analyzed for levels of compound 10 by LC/MS/MS. The pharmacokinetic parameters were determined using WinNonlin, version 5.0.1; Pharsight Inc.: Mountain View, CA. [Google Scholar]
- Lautala P.; Kivimaa M.; Salomies H.; Elovaara E.; Taskinen J. Glucuronidation of entacapone, nitecapone, tolcapone, and some other nitrocatechols by rat liver microsomes. Pharm. Res. 1997, 14, 1444–8. [DOI] [PubMed] [Google Scholar]