| Title: | LXR Modulators | ||
| Patent Application Number: | WO 2014/144037 A1 | Publication Date: | 18 September 2014 |
| Priority Application: | US 61/786,974 | Priority Date: | 15 March 2013 |
| Inventors: | Kick, E. K.; Bodas, M.; Mohan, R.; Valente, M.; Wurtz, N.; Patil, S. | ||
| Assignee Company: | Bristol-Myers Squibb Company; Route 206 and Province Line Road, Princeton, NJ 08543 (US) | ||
| Exelixis Patent Company LLC; 210 East Grand Avenue, South San Francisco, CA 94080 (US) | |||
| Disease Area: | Coronary heart disease, atherosclerosis, insulin resistance, aged and UV skin wrinkling, diabetes, cancer, and immunological disorders | Biological Target: | Liver X Receptors (LXRs) |
| Summary: | The invention in this patent application relates to pyrazole derivatives represented generally by formula (I) that modulate the activity of liver X receptors (LXRs). These compounds may be used for the treatment and/or prophylaxis of coronary heart disease, atherosclerosis, and other diseases or disorders related to LXR activities. | ||
| LXRs are adopted orphan members of the nuclear receptor superfamily. They are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXR agonists may be beneficial in the treatment of several diseases and disorders as highlighted below. | |||
| Blood cholesterol is a major risk factor for coronary heart disease (CHD). The cholesterol delivery is mediated by the low density lipoprotein (LDL). Macrophages in the subendothelial space accumulate cholesteryl esters and pro-inflammatory lipids to form foam cells. This causes a chronic inflammatory process leading ultimately to thrombosis and vessel occlusion and resulting in myocardial infarction (MI). The inverse process is known as reverse cholesterol transport (RCT), and it is mediated by high density lipoprotein (HDL). In RCT, excess cholesterol in peripheral tissues is transferred to the liver where it is secreted via the bile into the intestine and excreted in feces. Thus, stimulating RCT removes cholesterol from atherosclerotic lesions, and it is likely to be therapeutically beneficial in the treatment of CHD. The two LXR isoforms, LXRα and LXRβ, regulate cellular and whole-body reverse cholesterol transport (RCT) thus controlling the transport of peripheral cellular cholesterol to the liver and out of the body. LXRs are transactivated by specific oxysterol metabolites. Following their activation, they induce the expression of a variety of cholesterol efflux transporters, apolipoproteins, and lipoprotein modification pathways in multiple tissues that facilitate the removal of excess cellular and whole-body cholesterol as HDL-C. | |||
| LXRs inhibit the NF-KB-dependent induction in macrophages of a variety of inflammatory genes such as iNOS, COX-2, and IL-6, and LXR agonists inhibit inflammatory processes in vitro and in vivo. Recent studies suggest that LXR agonists could affect acquired immunity by limiting T-cell proliferative responses to activating signals. These effects on innate and acquired immunity are additional potential antiatherosclerotic mechanisms of LXR agonists. | |||
| LXRs have favorable effects on glucose homeostasis. Studies on diabetic mouse models have shown that LXR agonists inhibit the hepatic PGC-1, PEPCK, and glucose-6 phosphatase (G6 Pase) and stimulate hepatic glucokinase, thus affecting a marked inhibition of hepatic glucose output (HGO). Thus, LXR agonists lower plasma glucose in diabetic rodent models by both inhibiting hepatic glucose output and stimulating peripheral glucose disposal. | |||
| Studies have also shown LXRs to be key regulators of prostate cancer cell survival. Activation of LXR increases cholesterol efflux to cause disruption of lipid rafts. Decreasing membrane cholesterol results in a suppression of the AKT survival pathway and apoptosis. Therefore, stimulating the LXR-AKT pathway may be beneficial for the treatment of prostate cancer. Studies have also shown that activation of LXR may be beneficial in treating breast and pancreatic cancers. | |||
| LXR agonists may also be useful for the prevention and treatment of photo and chronological skin aging. | |||
| However, LXRs stimulate fatty acid and triglyceride (TG) synthesis in the liver. This is an undesirable side effect caused primarily by the specific activity of LXRα. Strategies to include identifying LXRβ selective compounds that are also partial agonists may minimize this undesirable lipid effects. Mouse study indicates that, while LXRα is the predominant mediator of LXR activity in the liver, LXRβ alone is sufficient to mediate the effects of LXR ligands on target gene expression in macrophages. Therefore, compounds with limited LXRα activity should have antiatherogenic activity while limiting unwanted hepatic effects. | |||
| Important Compound Classes: | 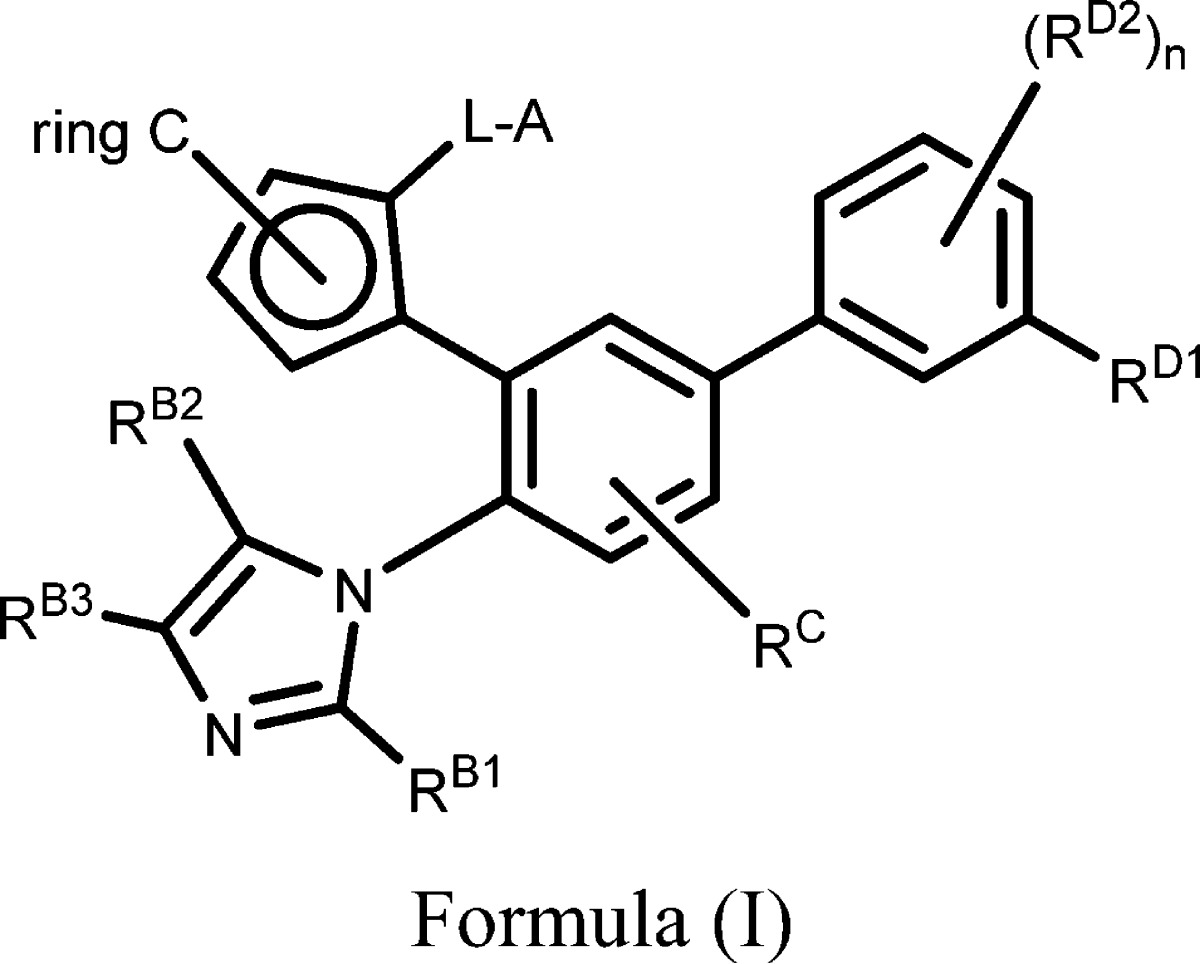 |
||
| Key Structures: | The inventors described a list of 647 examples of formula (I), including the following four compounds:
|
||
| Biological Assay: |
|
||
| Biological Data: | The biological data obtained from the co-transfection assay were listed for 647 examples. The values for the representative examples illustrated above are included in the following table:
|
||
| Recent Review Articles: | 1. Rai A. K.; Debetto P.; Sala F. D.. Indian J. Exp. Biol. 2013, 51 (11), 885–894. | ||
| 2. Xu P.; Li D.; Tang X.; Bao X.; Huang J.; Tang Y.; Yang Y.; Xu H.; Fan X.. Mol. Neurobiol. 2013, 48 (3), 715–728. | |||
| 3. Loren J.; Huang Z.; Laffitte B. A.; Molteni V.. Expert Opin. Ther. Pat. 2013, 23 (10), 1317–1335. | |||
The authors declare no competing financial interest.


