Abstract
T helper type 2 (Th2)-characterized inflammatory responses are highly dynamic processes initiated by epithelial cell damage resulting in remodelling of the tissue architecture to prevent further harm caused by a dysfunctional epithelial barrier or migrating parasites. This process is a temporal and spatial response which requires communication between immobile cells such as epithelial, endothelial, fibroblast and muscle cells and the highly mobile cells of the innate and adaptive immunity. It is further characterized by a high cellular plasticity that enables the cells to adapt to a specific inflammatory milieu. Incipiently, this milieu is shaped by cytokines released from epithelial cells, which stimulate Th2, innate lymphoid and invariant natural killer (NK) T cells to secrete Th2 cytokines and to activate dendritic cells which results in the further differentiation of Th2 cells. This milieu promotes wound-healing processes which are beneficial in parasitic infections or toxin exposure but account for increasingly dysfunctional vital organs, such as the lung in the case of asthma and the colon in ulcerative colitis. A better understanding of the dynamics underlying relapses and remissions might lead ultimately to improved therapeutics for chronic inflammatory diseases adapted to individual needs and to different phases of the inflammation.
Keywords: cytokine receptors, cytokines, dendritic cells, inflammation, Th1/Th2 cells
T helper type 2 (Th2)-characterized immunological responses
Parasitic worms such as helminths elicit strong immunological responses by engagement of both the innate (macrophages and eosinophils) and adaptive (antigen-presenting and Th2 cells) immune system [1]. These immunological reactions have most probably co-evolved with these parasites and specifically aim to expel and kill the parasites, protect the hosts from the toxicity of certain egg proteins and initiate wound healing and remodelling of the colon and liver architecture where migration and colonization had caused tissue damage. When infections become chronic parasites, in turn, have the capacity to dampen the immune response to prevent the killing of the host by excessive activation, thereby allowing for co-existence with the host. Unlike bacteria, viruses and fungi, which elicit immunological responses via recognition by pathogen-associated molecular patterns (PAMPs), the mechanism by which helminths and worms instruct Th2 immune responses has not yet been elucidated. Surprisingly, the research of allergic reactions has provided a novel insight into how these highly specific reactions occur [2]. Allergic reactions have long been thought to be a manifestation of an uncontrolled immune system reacting to a false alarm, which somehow mimicked a parasitic infection. Recent observations, however, suggest that allergen-driven reactions might not only be pathological and a nuisance, but also provide protection to tissue damage induced by venoms, and might be instrumental in detoxification; these reactions might even protect against future venom exposure [3]. For example, venoms such as bee venom are a mixture of enzymes, cell lytic peptides, proteases and bioactive amines [4]. One of the major components is phospholipase A2 (PLA2), an enzyme that leads to damage of cellular membranes and is at the same time considered the major allergen in bee venom [5]. In addition, Amb a from ragweed pollen [6], Bla g from cockroach [7], Asp f 5, f 6 and f 11 from Aspergillus [8] and Der p 1, p 3 and p 9 from house dust mites [8], are proteases which damage the tight junctions of epithelial cells and lead to barrier dysfunction. It is the thought that this dual role of allergens is not accidental but of biological importance which might also change the perspective of Th2-characterized chronic inflammatory diseases such as asthma, atopic dermatitis (AD) and ulcerative colitis (UC).
Cells of the inflammatory milieu
Asthma, UC and AD are chronic diseases in which relapses are followed by periods of remission; as in helminthic infections, the immunological responses are highly dynamic and involve epithelial cells, cells of the adaptive and innate immune system, muscle cells and fibroblasts. The keywords are communication, integration of signalling, cellular mobility and plasticity. Cellular plasticity and ‘multi-tasking’ of cells in particular challenges our view of cellular development as a rigid and one-dimensional process, in which cells execute a function to which they seem assigned by their phenotypical features and location; for example, epithelial cells evolved from being passive bystanders to important immune regulators.
Epithelial cells as initiators of the immunological response
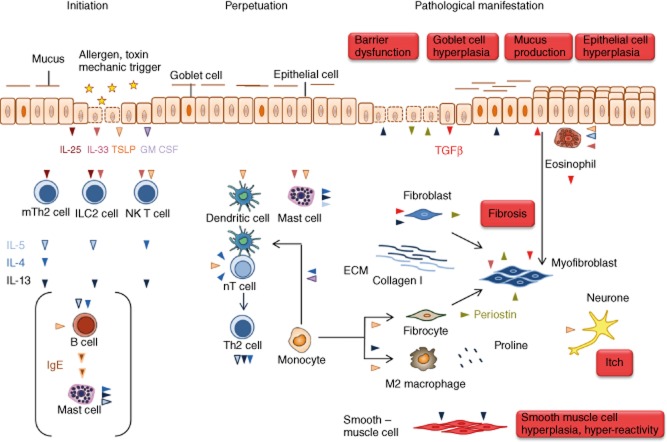
The foremost function of an epithelial cell has been described as a physical barrier to seal the body from the environment and to protect it from pathogens, aiming to feast on the body's rich nutritional sources and from environmental toxins that damage the barrier. However, we now know that epithelial cells lining the skin, colon and airways take an active part in the immunological response. They relay the signals triggered by epithelial cell damage to dendritic cells (DC), innate lymphoid cells (ILC), invariant natural killer (iNK) T cells and Th2 cells and thereby pivotally initiate and direct the immunological response (Fig. 1). Chemokines released by epithelial cells are responsible for the recruitment and influx of immune cells to sites of action [9], while the secreted cytokines direct and shape the adaptive and innate immune cell responses. The central mediators between the innate and adaptive immunity are the cytokines thymic stromal lymphopoietin (TSLP), interleukin (IL)-25 and IL-33.
Fig. 1.
The route to pathologies in T helper type 2 (Th2)-characterized chronic inflammatory diseases. The inflammatory cascade is initiated by the cytokines interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP) released from epithelial cells experiencing damage by allergens with proteolytic activity or toxins. The first addresses of these signals are MutT homologue 2 (mTh2) cells, innate lymphoid cells (ILCs) and invariant natural killer T2 (iNK T2) cells. These cells secrete the typical Th2 cytokines IL-4, IL-5 and IL-13 responsible for perpetuation of the inflammation and for pathological changes. TSLP also activates dendritic cells to enforce the differentiation of naive T cells to Th2 cells and thus further promotes the Th2 inflammatory process. In addition, TSLP activates mast cells which, in turn, release IL-4, IL5 and IL-13. This axis might be responsible for mast cell activation in the absence of immunoglobulin (Ig)E as it is sometimes observed in atopic dermatitis and is common in ulcerative colitis (UC). IL-4 and granulocye–macrophage colony-stimulating factor (GM-CSF) induce the differentiation of monocytes to dendritic cells which instruct more naive T cells to become Th2 cells. In concert with periostin, IL-13 induces the differentiation of monocytes to alternatively activated macrophages which release components of the extracellular matrix. Periostin also promotes the differentiation of monocytes to fibrocytes which are considered as crucial for the manifestation of fibrosis. Fibrosis is the pathological phenotype shared by all three diseases and the outcome of the activity of various cell types. IL-5 activates eosionohils and TSLP stimulate eosinophils to secrete transforming growth factor (TGF)-β, an important cytokine to induce fibrosis by stimulating the secretion of extracellular matrix proteins from myofibroblasts. Upon interaction of eosionophils with epithelial cells, epithelial cells undergo epithelial mesenchymal transition and turn into myofibroblasts. In addition, resident fibroblasts and monocyte-derived fibrocytes become myofibroblasts. Although the pathologies in all three diseases differ according to the affected organ, it is obvious that epithelial cells are simultaneously activator and target of the inflammatory response. They secrete periostin in response to exposure to IL-13, which induces barrier dysfunction and respond to to periostin by secreting TGF-β. There are some differences in all three diseases which might reflect different molecular mechanisms underlying the disease. In asthma, IL-4 and IL-5 in concert with periostin promote the clonal expansion and maturation of B cells and induces the IgM/IgE switch. Released IgE activate mast cells to secrete IL-4, IL-5 and IL-13 and histamines and chemokines which promote the influx of inflammatory cells into the tissue. IL-13 induces goblet cell hyperplasia resulting in increased mucus production and smooth muscle cell hyperplasia and hyperactivity which are prominent pathological features of asthma. In atopic dermatitis, IgE release signifies the disease in many cases; however, it does not seem to be a prerequisite for the manifestation of the disease. In atopic dermatitis, IL-13 induces epithelial hyperplasia resulting in the thickening of the skin, and TSLP is most probably the cytokine responsible for itch. Colitis is characterized by severe epithelial damage responsible for diarrhoea and extensive fibrosis, which accounts for the loss of function of the colon. IL-13 is considered the key effector cytokine responsible for barrier dysfunction of the gut epithelia and fibrosis. Another specificity of UC is that the colon hosts the microflora, which has no contact with the apical side of the gut epithelia in a healthy condition, but which can invade the tissue when the barrier is defective.
TSLP, IL-33 and IL-25 relay signals received by epithelial cells
TSLP is a novel IL-7-like cytokine that was identified originally as a growth factor in the supernatant of thymic stromal cells which supported the development of B cells [10]. TSLP is expressed primarily in epithelial cells of the skin, lung and intestine, and the expression is induced by proteases which are, for example, secreted by helminths or are components of allergens such as Amb a from ragweed pollen [6], Bla g from cockroach [7], Asp f 5, f 6 and f 11 from Aspergillus [8] and Der p 1, p 3 and p 9 from house dust mites [11]. These serine proteases activate the protease activated receptor (PAR)-2 resulting in increased expression of TSLP [12].
Additionally, epithelial cells can be triggered to produce TSLP upon sensing microbial products over Toll-like receptors (TLR). Several studies described increased TSLP production upon TLR ligation [13–18] in epithelial cells and introduced the idea that the TLR–TSLP relationship may promote the Th2 response and chronic infection beneficial to the pathogen. In rhinoviral infections dsRNA activates TLR-3, which supports viral clearance by promoting enhanced mucus production and tips the balance towards a Th2 response to keep the viral-induced Th1 response in check [19]. In asthma, however, this might be the beginning of a vicious cycle in which rhinoviral infections induce asthma exacerbations, creating a milieu that facilitates further viral infections. Similarly, diacetylated lipoproteins from Staphylococcus aureus membrane activate TLR-2–TLR-6 and induce TSLP production, extending the vicious cycle between the S. aureus colonization and atopic dermatitis [13]. Moreover, TLR-mediated TSLP production has been proposed to play a role in gut tolerance towards commensal microbiota [20].
Monocytes and DC exhibit the highest co-expression of the TSLP receptor and IL-7Rα, and are therefore the preferred targets of TSLP [21]. TSLP promotes the Th2 response by activating CD11c+ (myeloid) DC that, in turn, attract and stimulate naive CD4+ cells towards the Th2 phenotype characterized by the expression of IL-4, IL-5 and IL-13 [22]. Besides usual DC priming and activation, TSLP induces OX40 ligand expression in DC, therefore promoting inflammatory [tumour necrosis factor (TNF)-α-characterized)] instead of the conventional (IL-10-characterized) Th2 response. Furthermore, TSLP-activated DC provide signals essential for maintenance of Th2 memory cells [23].
In mouse CD4+ cells TSLP has been shown to induce IL-2-independent IL-4 secretion [24–26] and to directly induce the secretion of IL-13 from NK T cells [27]. In addition, TSLP acts on eosinophils and mast cells and is thereby able to induce AD-like pathologies in the absence of increased immunoglobulin (Ig)E levels [28,29]. It directly activates mast cells to secrete IL-4, IL-5 and IL-13. Finally, TSLP seems to be the missing link between AD and itch, as it has been shown that it activates primary sensory afferents to evoke itch [30,31].
The signalling of TSLP follows a common pathway observed in the heterodimeric interleukin receptor family. The initial binding to the specific receptor, also termed ‘driver receptor’, occurs with low affinity. This binary complex then recruits the so-called trigger receptor which, by itself, is not capable of binding to the respective cytokine but which turns the assembled ternary complex into a high-affinity complex. In the case of the TSLP complex, IL-7αR is the driver receptor, whereas the TSPL receptor (TSPLR) is required for high-affinity binding and is thus considered the trigger receptor [32–34]. The complex activates the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway by inducing phosphorylation of the Janus kinase family, JAK1 and JAK2, and six members of the STAT transcription factor family, STAT-1, -3, -4, -5a, -5b and -6, STAT-5 and/or STAT-6, depending on the targeted cell [35,36]. In DC, STAT-5 binds to the promoter of the chemokine gene CCL17 and thus might be responsible for secretion of the Th2-attracting cytokine CCL17 [35].
IL-25 is a cytokine with high homology to IL-1 which is, like TSLP, secreted by epithelial cells upon damage, exposure to protease allergens and activation of PAR2 [37]. IL-25 binds to the IL-17-RA and IL-17RB receptor complex [38], expressed on type 2 innate lymphoid cells (ILC2), which respond immediately to secrete the Th2 cytokines. In addition, IL-25 promotes the development of multi-potent progenitor cells from circulating haematopoietic stem cells that accumulate in the mucosa and give rise to mast cells, basophils and macrophages [39]. Thus, the release of Th2 cytokines and the promotion of antigen-presenting cells and granulocytes ensure an immediate response to epithelial cell damage prior to T lymphocyte activation.
Furthermore, a recent study by Petersen et al. has reported a type 2 myeloid population induced by IL-25, playing an important role in steroid-resistant airway disease [40]. IL-25 exerts its activity in T cells by inducing the phosphorylation of STAT-6 [41].
IL-33 belongs to the IL-1 family, which is expressed in the epithelial lining of the skin, gut mucosa and lung, in fibroblasts, endothelial cells, adipocytes, astrocytes, DCs and macrophages [41]. Unlike the other members of the family, which are activated by the inflammasome by proteolytic cleavage, IL-33 is active in its full-length form and released from epithelial cells upon cellular damage. Therefore, it is thought to act like an ‘alarmin’ to induce responses which lead ultimately to wound healing. The prominent target cells of IL-33 are ILC2, which reside in the skin and sense the necrotic skin cells [42]. Upon exposure to IL-33, ILC2 release type 2 cytokines and thus direct the inflammation towards a type 2 phenotype. IL-33 binds to the heterodimer ST2/IL-1RAP receptor complex on mast cells, basophils, eosinophils, Th2 cells and ILC2 and activates these cells to release their specific cytokines [43]. Of note, in contrast to the activation of mast cells by TSLP, the presence of IgE is required for mast cell degranulation by IL-33 [44]. Upon activation, myeloid differentiation primary response gene 88 (MyD88) and IL-1 receptor-associated kinase (IRAK)-1/4 are recruited, leading to activation of the transcription factor, nuclear factor-κB (NF-κB), and the mitogen-activated protein kinase (MAPK) pathway, which is mediated by the activation of the MAPKs extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK), and ultimately to the production of Th2 cytokines and chemokines [45].
Granulocyte–macrophage colony-stimulating factor (GM-CSF)
Another important actor during inflammation is GM-CSF, which is released by epithelial cells and stimulates the proliferation, maturation and function of antigen-presenting cells. GM-CSF promotes the secretion of IL-33 from epithelial cells and might be responsible for the increased susceptibility to allergens during infections [46]. In concert with IL-4, GM-CSF induces the differentiation of monocytes to DC which, in turn, drive more naive T cells to differentiate to Th2 cells [47]. GM-CSF shares its driver receptor with IL-3 and IL-5 and signals towards Akt, Erk and JNK activation [48].
Innate lymphoid cells as primary target cells of IL-25 and IL-33
Recently, a novel type of lymphocyte in epithelial locations has been identified, pushing T cells from the centre of immune orchestration [49,50]. To date, these so-called innate lymphoid cells (ILC) comprise three groups (ILC1, 2 and 3) that provide an important source of inflammatory cytokines. As they do not express a T cell receptor, they react in an antigen-independent manner. ILC2 are a novel, lineage-negative cell population residing in the skin, liver, respiratory and gastrointestinal tissues characterized by production of Th2-cytokines IL-4, IL-5, IL-10 and IL-13. Several studies discovered this innate Th2-like population independently, all of them describing them as cells responding independently of and prior to any adoptive Th2 immune response [51–53]. ILC2 are stimulated by IL-25 and IL-33, and thus respond directly to the damage signals released by epithelial cells and prime the system for a type 2 response before T lymphocyte activation [40].
Epithelial cells as effector cells of pathological changes
In addition to their role in barrier formation, epithelial cells are actively involved in tissue remodelling and fibrosis. First, they exhibit an enormous plasticity upon injury, termed epithelial to mesenchymal transition (EMT), and turn into myofibroblasts to take an active role in the wound-healing process [54]. The EMT process is promoted by diverse cell types of innate immunity, such as eosinophils and macrophages, in direct interaction with epithelial cells [54]. Eosinophils also have the capacity to sense danger signals from stressed and necrotic epithelial cells [55] and promote EMT, thereby supporting fibrosis [54]. This process is enhanced further by the release of transforming growth factor (TGF)-β by eosinophils upon exposure to IL-5 [56]. Thus, eosinophils are instrumental in tissue repair and remodelling and it is therefore not surprising that elevated levels of eosinophils are also found in phases of remission on colonic tissue of UC patients [57]. In contrast to the eosinophils detected in the acute phase, they are not signified by the degranulation marker CD66b, and thus may contribute ‘silently’ to tissue remodelling [58]. They also seem to be the gatekeepers of plasma cells in the niches of the bone marrow by providing the proliferation-inducing ligand (APRIL) and IL-6 [59].
TSLP is also involved in fibrosis, as it induces the secretion of collagen 1 by fibrocytes [60]. Together with IL-13, TGF-β, IL-4 and various growth factors, it also induces the differentiation of monocytes into alternatively activated macrophages, which seem to have an ambiguous role in fibrosis. On one hand, they promote fibrosis by recruiting fibroblasts and promote their proliferation [61]; on the other hand, M2 macrophages seem to be required for suppression and resolution of fibrosis. In response to IL-4 and IL-13, M2 macrophages express elevated levels of arginase I, which is essential for converting L-arginine into ornithine, which is metabolized further to proline. Thus, it was thought initially that M2 macrophages promote fibrosis by providing an additional source of proline, the major component of collagen. However, depleting arginase I in alternatively M2 macrophages exacerbated liver fibrosis following infection with Schistosoma mansoni in mice, suggesting an alternative role for M2 macrophages [62]. Depleting the pool of arginine M2 macrophages might limit the capacity of myofibroblasts to produce collagen and thus keep the wound-healing process in check.
The cytokine milieu promoting fibrosis is influenced by the production of TGF-β by injured epithelial cells. TGF-β secretion is initiated by periostin and induces extracellular matrix protein secretion by myofibroblasts [63]. Periostin is a matricellular protein which interacts with several integrin molecules such as αvβ1, αvβ3 and αvβ5, inducing remodelling and enhancing fibrosis by binding to other proteins of the extracellular matrix [64–66]. Periostin is not only secreted by epithelial cells but acts on them, and enhances proliferation and differentiation in keratinocytes and induces TSLP secretion in epithelial keratinocytes, thus perpetuating the inflammatory process [67]. In addition to epithelial cells, periostin is expressed by fibroblasts and fibrocytes [68]. Periostin gene expression is under the control of IL-13, and periostin serum levels have been identified as a biomarker for patients susceptible to treatment with lebrikizumab, a monoclonal antibody trapping IL-13 [69].
T cells as effector cells to perpetuate the immunological response and pathological changes
T cells have long been assigned the key position to orchestrate immunological responses. Indeed, the plasticity of T cells results in a repertoire of functions covering the entire process of acute and chronic inflammation. The cytokine signature directs the type of inflammation, regulates the response and develops memory subsets for future immunity. This also certainly applies to Th2-driven inflammatory responses. Upon receiving the damage signal from the epithelial cells, DC drive naive T cells to differentiate into a Th2 phenotype and to secrete the crucial cytokines IL-4, IL-5 and IL-13 that are responsible for development and perpetuation of the inflammation, tissue repair and pathological manifestations of chronic inflammatory diseases. These include abnormal mucus production, fibrosis, tissue remodelling, smooth muscle cell hyperplasia and hyperreactivity.
Invariant NK T cells
Another important source of Th2 cytokines in inflammatory diseases are iNK T cells, which are also considered part of the innate immunity. They express an invariant T cell receptor (Vα14-Jα281 in mice and Vα24-Jα18 in humans) linked to a restricted repertoire of Vβ chains, which enables them to recognize glycolipid antigens presented by highly conserved major histocompatibility complex (MHC) class I molecules (CD1 in mice and CD1d in humans) presented to them by CD4+/CD8+-positive thymocytes in the thymus [70,71]. While some NK T cells mature in the thymus, most of them mature in the periphery. CD1d is expressed on the apical side of epithelial cells [72], and could directly present glycolipid antigens to iNK T cells. Activation of the iNK T cells is dependent upon the inflammatory milieu, and in the case of Th2-instructed immunological responses a subgroup of iNK T cells (CD4+ IL-17RB+), also referred to as NK T2 cells, produce IL-4, IL-13, IL-9, IL-10, IL-17A and IL-22 upon exposure to IL-25 [73]. Thus, like ILC, iNK T cells respond directly to damage signals released by epithelial cells.
IL-4 and IL-13 are key cytokines secreted by T cells, innate lymphoid cells and NK T cells
Although IL-4 and IL-13 are structurally analogous and share functional receptors, their function is quite different. It is now assumed that IL-4 acts mainly as a regulatory cytokine and IL-13 as an effector cytokine [74]. The allocation to these specific roles is facilitated by the IL-4/IL-13 signalling pathways, which are fine-tuned by spatial, temporal and cell-specific expression of receptors and ligands [75]. Signalling by IL-4 is mediated by type I and type II receptor complexes [76]. Expression of the type I complex is restricted to haematopoietic cells, whereas type II is expressed ubiquitously but not in haematopoietic cells, with the exception of human B cells which also express the type II receptor [77]. According to this effector function, IL-13 is responsible for epithelial barrier dysfunction [78,79], goblet cell metaplasia in asthma [79,80], epithelial hyperplasia [81,82], fibrosis [60,83] and smooth muscle cell hyperplasia [84].
Signalling through IL-13 is modulated further by the expression of IL-13Rα2 in macrophages. IL-4 and IL-13, in concert with TNF-α, induce the expression of IL-13Rα2 which is then activated by IL-13 to promote the secretion of TGF-β [85,86].
IL-5
IL-5 plays a key role in eosinophil proliferation, differentiation, maturation, migration to tissue sites and survival, as well as prevention of eosinophil apoptosis [87,88]. The driver receptor in this signalling complex is IL-5Rα, the trigger receptor of the common βc receptor [89].
Animal models: oxazolone-induced inflammatory diseases
Oxazolone-induced colitis and dermatitis in mice have become recognized models in the study of the immunological mechanisms underlying the diseases [90,91]. Oxazolone is considered a haptenizing agent which penetrates the mucosal or epithelial barrier and triggers an immunological response, mimicking the described diseases [92]. When applied to the skin of hairless mice (hr/hr) in low doses for a period of 3 weeks, mice develop symptoms characteristic for AD to include barrier dysfunction, secretion of IgE, epithelial cell hyperplasia, fibrosis and influx of inflammatory cells into the dermis and epidermis and secretion of Th2-characterized interleukins [90]. Similarly, when oxazolone is applied rectally, C57BI/10 or BALB/c mice develop colitis-like symptoms. The clinical activity score increases and the histological inspection of the colon reveals an influx of inflammatory cells into the lamina propria, destruction of the mucosa, fibrosis and secretion of IL-4 and IL-13 by CD4+ cells and NK T cells [91]. In our mouse models for UC [93], which rely upon immune-incompetent non-obese diabetic (NOD)-severe combined immunodeficiency (SCID) IL-2Rγ null mice engrafted with human peripheral blood mononuclear cells (hPBMC) derived from patients suffering from UC or AD, we observed some unexpected results which were not consistent with the idea that the capacity of oxazolone to modify proteins and thus turn them into allergens is the trigger for the immunological reactions. First, oxazolone applied to non-engrafted animals proved to be highly toxic in our UC model, and the presence of PBMCs seemed to mitigate the effect. This result contradicted the assumption that the observed pathology in immunocompetent mice resulted solely from an inflammatory cascade initiated by an allergen-like trigger. Secondly, we observed the same pathological manifestations when we applied 50% ethanol to the engrafted mice. This was similarly surprising, as this experiment was also considered as a control experiment. In light of the hypothesis that one biological important function of Th2-driven inflammatory responses is to protect the organism from toxic agents and to support detoxification, however, both results become consistent with the hypothesis. In the presence of lymphocytes, as in engrafted animals or immune-competent mice, oxazolone and ethanol damage the epithelial cells, which initiate a detoxification and healing process which in many aspects mimics colitis.
The Th2 inflammatory milieu and cancer
Tumours are characterized by infiltrates of cells of the adaptive and innate immune system; understanding of the mechanisms of how cancer cells can escape from immune recognition and destruction is crucial for cancer therapy in general and specifically for immunotherapy. It has been shown that Th2 inflammatory markers such as TSLP or IL-33 are associated with poor prognosis in pancreatic, hepatocellular, non-small cell lung carcinoma, gastric and breast cancer [94–97], and it is now assumed that the inflammatory cascade which ultimately favours immune protection follows the scheme observed in the inflammatory milieu in Th2-characterized inflammatory diseases. Tumour cells are stressed due to a variety of reasons to include metabolic stress caused by malnutrition and hypoxia, leading ultimately to the release of the epithelial damage signals IL-33 and TSLP. As in the Th2-driven inflammatory milieu, both cytokines induce the release of IL-13 by ILC2s patrolling the tumour and the activation of DC to further instruct Th2 cells, respectively. As in chronic inflammatory diseases, IL-13 and TSLP seem to be key effector cytokines which promote the proliferation of tumour cells and the deposition of collagen, which creates a physical barrier impairing the infiltration of lymphocytes. IL-13 is also responsible for the proliferation of myeloid-derived suppressor cells (MDSC), which strongly suppress immune recognition [98]. In addition, TGF-β released by epithelial cells promotes the generation and infiltration of regulatory T cells, which further dampens the inflammatory response.
A further fatal trait of cancer cells is the capacity to disseminate to different organs. Dissemination requires EMT or stemness features of cancer cells, both of which are induced or maintained by periostin. Periostin induces a mesenchymal phenotype in epithelial cells and breast cancer cells resulting in increased invasion and multi-lineage differentiation [99]. It also contributes to creating a microenvironment favouring cancer stem cell maintenance. This is achieved by invasion of bone marrow-derived mesenchymal stem cells (BM MCS) to become part of the tumour microenvironment. These cells highly express periostin and thus might contribute to tumour progression and metastasis. MCS also migrate to sites of inflammation and their immunomodulatory activities have been the basis for using them as therapeutics in UC [100]. In light of their ability to promote cancer progression, however, they might be one of the reasons why the incidence of the development of cancer in UC patients is elevated. A milieu which promotes EMT and simultaneously provides niches for the maintenance of cells with potential stemness-like features might favour the development of cancer. Thus, as in chronic inflammatory diseases, tumour cells shape and are shaped by the inflammatory milieu. Therapeutically targeting this milieu might provide a double-edged sword to render the tumour cell vulnerable to cytotoxic T cells and simultaneously to preventing the development of cancer cells with stem-like features.
Therapeutics in development
Therapeutic programmes increasingly reflect the dynamics of the immunological response and acknowledge the specificity of Th2-characterized inflammatory diseases. Intervention points of novel therapeutics do not aim to generally dampen the immunological response, but to block the initiation, perpetuation and the pathological changes which specifically characterize Th2 inflammatory diseases. TSLP and its receptor have become targets of interest to interrupt the danger signals released by epithelial cells. Most programmes are in preclinical development, except for AMG 157, which is currently being tested for asthma and atopic dermatitis. Another important intervention point is the influx of T cells into the site of inflammation. This is being studied using inhibitors developed against the chemokine receptor ThCR2, also referred to as prostaglandin receptor D2, which is expressed on Th2 cells and responds to chemokines released by epithelial cells. In order to specifically abolish the influx of inflammatory cells to the colon, antibodies against the integrin a4β7 have been developed. IL-4, and especially IL-13, as important cytokines responsible for perpetuation and pathological manifestation, are addressed by therapeutics targeting the cytokines themselves by trapping them with an antibody or by inhibiting the IL-4Ra or IL-13Ra1 receptors. As IgE is instrumental in initiating and perpetuating the disease, the IgE signalling is blocked by antibody traps. Finally, as IL-5R and IL-5 are instrumental in the activation and proliferation of eosinophils, both are addressed by antibodies to trap the ligand or inhibit signalling. Table 1 depicts the therapeutics in clinical development.
Table 1.
Novel therapeutics for asthma, atopic dermatitis (AD) and ulcerative colitis (UC)
| Intervention points | Target | Compound | Characteristics | Disease | Phase | Company |
|---|---|---|---|---|---|---|
| Activation of Th2 immune response | TSLPR/IL-7R | AMG 157 | mAb; human | Asthma, AD | II, I | Amgen |
| Influx of neutrophils | CXCR2:IL-8 R | SB-656933 | Small molecule | UC | II | Glaxo SmithKline |
| AZD5069 | Small molecule | Asthma | II | AstraZeneca | ||
| Influx of monocytes, NK and T cells, dendritic cells, basophils | CCR3 | IPI ASM8 | Anti-sense | Asthma | II | Pharmaxis |
| Influx of Th2 cells | Prostaglandin-receptor D2 | ADC 3680 | Small molecule | Asthma | II | Pulmagen |
| OC000459 | Small molecule | Asthma | IIb | Oxagen | ||
| QAW039 | Small molecule | Asthma | II | Novartis | ||
| ARRY-502 | Small molecule | Asthma | II | Array BioPharma | ||
| Migration of leucocytes into intestinal tissue | Integrin a4ß7 | AMG 181 | mAb; humanized | UC | I | Amgen |
| Etrolizumab; RG7413 | mAb; humanized | UC | III | Roche | ||
| MLN 0002; Vedolizumab | mAb; humanized | UC | III | Millennium | ||
| MED 17183 | mAb; humanized | UC | II | AstraZeneca | ||
| Migration of leucocytes | S1P1-5 receptor | FTY720; Gilenea; Fingolimod | Small molecule | Asthma | II | Novartis |
| Activation of mast cells | IgE | Xolair; Omalizumab | mAb | Asthma, AD | Approved, II | Novartis |
| Activation and proliferation of lymphocytes | SYK | Bay 61–3606 | Small molecule | Asthma | Preclinical | Bayer |
| JAK3 | Tofacitinib; Xeljanz; CP 690550 | Small molecule | UC | III | Pfizer | |
| STAT-1 transcription factor | AVT 01 | Decoy DNA | Asthma | IIa | Avontec | |
| Proliferation of T cells | IL-2 R; CD 26 | Basiliximab Simulect | mAb | UC | II | Cermon Pharmaceuticals |
| Survival of T cells | Ox 40 R | RG 4930 | mAb; human | Asthma | I | Genmab |
| anti Ox40L | mAb; human | Asthma | I | Genentech; Roche | ||
| Differentiation and proliferation of CD4+ T cells, IgM /IgE switch | IL-4 | Pascolizumab | mAb; humanized | Asthma | II | Protein Design Lab |
| IL-4Ra | AEROVANT™;AER 001; Pitrakinra | Protein | Asthma, AD | II | AEROVANCE | |
| AMG 317 | mAb; human | UC | I | Amgen | ||
| Differentiation and proliferation of CD4+ T cells, IgM /IgE switch, fibrosis, barrier dysfunction, mucus production, smooth muscle cell hyperplasia and hyperactivity | IL-4Ra/IL-13 Rα1 | nd | Peptide | Asthma | Preclinical | Allostera |
| Dupilumab | mAb | Asthma, AD | II | Regeneron/Sanofi | ||
| Activation, proliferation of eosinophils | IL-5 | Mepolizumab; Bosatria | mAb; humanized | Asthma | II | Glaxo SmithKline |
| IL-5 R | Medi 563 | mAb; human | Asthma | II | MedImmune | |
| Benralizumab | mAb; human | Asthma | III | AstraZeneca | ||
| TPI ASM8 | Anti-sense | Asthma | II | Pharmaxis | ||
| Fibrosis, barrier dysfunction, mucus production, smooth muscle cell hyperplasia and hyperactivity | IL-13 | Lebrikizumab; MILR 1444A | mAb; humanized | Asthma | III | Genentech; Roche |
| QAX 576 | mAb; humanized | Allergic Asthma | qa | Novartis | ||
| QAX 576 | mAb; humanized | Allergic Asthma | I/II | Novartis | ||
| CNTO 607 | mAb | Asthma | Preclinical | Centocor | ||
| CDP 7766 | antibody fragment | Asthma | Preclinical | UCB | ||
| Anrukinzumab; IMA 638 | mAb; humanized | UC | II | Wyeth | ||
| Anrukinzumab; IMA 638 | mAb; humanized | Asthma | II | Wyeth | ||
| Tralokinumab | mAb; humanized | Asthma | I | MedImmune | ||
| IMA 026 | mAb; humanized | Asthma | I | Wyeth | ||
| IL-13 Rα1 | inhibitor | IL-13 peptide fragment 17-mer | Asthma | Preclinical | Synairgen | |
| MK 6105 | mAb; human | Asthma | Preclinical | CSL; Merck |
JAK3 = Janus kinase; STAT-1 = signal transducer and activator of transcription-1; IL = interleukin; mAb = monoclonal antibody.
Concluding remarks
Most of our knowledge relies upon in-vitro analysis of cellular responses in the presence or absence of respective signalling molecules, followed by verification of assumptions in vivo by respective mouse models or by analysis of distinct proteins in a disease setting. Although each single piece of information leads to the composition of the inflammatory puzzle and to a better understanding of diseases, we might fail to understand the dynamics of the inflammatory milieu in individual disease manifestations and the dynamics underlying relapse and remission if methods are not developed to analyse multi-cellular interactions. As in Vexierbildern (picture puzzles), scientists seem to focus upon single important aspects, and the angle they are looking from determines the focus of the scientific approaches. This might be misleading, as every single cell involved is an activator and responder subjected to the temporal milieu by which it is shaped and which it shapes. The presence and concentration of cytokines and their respective receptors might be influenced by the immunological challenge that each patient is currently experiencing and/or by the individual genetic setting. That specific molecular signatures are now attributed to different phases of each disease is an important improvement, and a better understanding of the inflammatory milieu will lead ultimately to dynamic therapies that constantly adapt to the demands of the respective phase that the patient is currently experiencing.
Acknowledgments
This work was funded by the Bundesministerium für Bildung und Forschung (grant number VIP0410). We thank Eric Whalley for critically reading the manuscript and for his involvement in the project.
Disclosure
None of the authors has a financial interest related to the work presented in the manuscript.
References
- 1.MacDonald AS, Araujo MI, Pearce EJ. Immunology of parasitic helminth infections. Infect Immun. 2002;70:427–433. doi: 10.1128/iai.70.2.427-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 5.King TP, Sobotka AK, Kochoumian L, Lichtenstein LM. Allergens of honey bee venom. Arch Biochem Biophys. 1976;172:661–671. doi: 10.1016/0003-9861(76)90121-1. [DOI] [PubMed] [Google Scholar]
- 6.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 7.Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol. 2002;110:589–595. doi: 10.1067/mai.2002.127798. [DOI] [PubMed] [Google Scholar]
- 8.Tai HY, Tam MF, Chou H, et al. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 9.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 11.Asokananthan N, Graham PT, Stewart DJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 12.Hideaki T, Gyohei E, Shuh N, Yoshiki M, Kenji K. Essential roles of actin cytoskeleton formation through mDia1 in skin dendritic cell functions. J Invest Dermatol. 2009;129:S1–S. [Google Scholar]
- 13.Vu AT, Baba T, Chen X, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol. 2010;126:985–993. doi: 10.1016/j.jaci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Le TA, Takai T, Vu AT, et al. Flagellin induces the expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Int Arch Allergy Immunol. 2011;155:31–37. doi: 10.1159/000318679. [DOI] [PubMed] [Google Scholar]
- 15.Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–1325. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Takai T, Chen X, Okumura K, Ogawa H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. 2012;66:233–237. doi: 10.1016/j.jdermsci.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Saito H, Kimura Y, et al. G-DNA suppresses poly(I:C)-induced TSLP production in human laryngeal arytenoid fibroblasts. Cytokine. 2012;57:245–250. doi: 10.1016/j.cyto.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Takai T, Chen X, Xie Y, et al. TSLP expression induced via Toll-like receptor pathways in human keratinocytes. Methods Enzymol. 2014;535:371–387. doi: 10.1016/B978-0-12-397925-4.00021-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3–EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009;40:610–619. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 22.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Liu YJ, Arima K. Cellular and molecular mechanisms of TSLP function in human allergic disorders – TSLP programs the ‘Th2 code’ in dendritic cells. Allergol Int. 2012;61:35–43. doi: 10.2332/allergolint.11-RAI-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 25.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 28.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmariah SB, Lerner EA. The missing link between itch and inflammation in atopic dermatitis. Cell. 2013;155:267–269. doi: 10.1016/j.cell.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong J, Kim MS, Chaerkady R, et al. TSLP signaling network revealed by SILAC-based phosphoproteomics. Mol Cell Proteomics. 2012;11:M112–017764. doi: 10.1074/mcp.M112.017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 34.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wohlmann A, Sebastian K, Borowski A, Krause S, Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol Chem. 2010;391:181–186. doi: 10.1515/bc.2010.029. [DOI] [PubMed] [Google Scholar]
- 37.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25:829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012;18:751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 42.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y, Yasuda K, Sakaguchi Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pushparaj PN, Tay HK, H'ng SC, et al. The cytokine interleukin-33 mediates anaphylactic shock [Retracted article; see vol. 109, pp. 15527, 2012] Proc Natl Acad Sci USA. 2009;106:9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Liew FY. IL-33: a Janus cytokine. Ann Rheum Dis. 2012;71(Suppl. 2):i101–104. doi: 10.1136/annrheumdis-2011-200589. [DOI] [PubMed] [Google Scholar]
- 46.Llop-Guevara A, Chu DK, Walker TD, et al. A GM-CSF/IL-33 pathway facilitates allergic airway responses to sub-threshold house dust mite exposure. PLOS ONE. 2014;9:e88714. doi: 10.1371/journal.pone.0088714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obermaier B, Dauer M, Herten J, Schad K, Endres S, Eigler A. Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol Proced Online. 2003;5:197–203. doi: 10.1251/bpo62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22:1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- 49.Walker JA, Barlow JL, McKenzie ANJ. Innate lymphoid cells – how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 50.Walker JA, McKenzie A. Innate lymphoid cells in the airways. Eur J Immunol. 2012;42:1368–1374. doi: 10.1002/eji.201242425. [DOI] [PubMed] [Google Scholar]
- 51.Liang Y, Jie Z, Hou L, et al. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Barlow JL, Bellosi A, Hardman CS, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Yasukawa A, Hosoki K, Toda M, et al. Eosinophils promote epithelial to mesenchymal transition of bronchial epithelial cells. PLOS ONE. 2013;8:e64281. doi: 10.1371/journal.pone.0064281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenfeldt AL, Wenneras C. Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils. Immunology. 2004;112:605–614. doi: 10.1111/j.1365-2567.2004.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Liu B, Wang L, et al. IL-5 up-regulates the expression of TGF-beta1 in human blood eosinophils in vitro. J Huazhong Univ Sci Technolog Med Sci. 2005;25:665–667. doi: 10.1007/BF02896165. [DOI] [PubMed] [Google Scholar]
- 57.Lampinen M, Ronnblom A, Amin K, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714–1720. doi: 10.1136/gut.2005.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lampinen M, Waddell A, Ahrens R, Carlson M, Hogan SP. CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J Leukoc Biol. 2013;94:1061–1070. doi: 10.1189/jlb.1212640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 60.Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–7242. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLOS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 65.Kii I, Nishiyama T, Li M, et al. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masuoka M, Shiraishi H, Ohta S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naik PK, Bozyk PD, Bentley JK, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–1056. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsumoto H. Serum periostin: a novel biomarker for asthma management. Allergol Int. 2014;63:153–160. doi: 10.2332/allergolint.13-RAI-0678. [DOI] [PubMed] [Google Scholar]
- 70.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 71.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 72.Somnay-Wadgaonkar K, Nusrat A, Kim HS, et al. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a beta2-microglobulin-associated form. Int Immunol. 1999;11:383–392. doi: 10.1093/intimm/11.3.383. [DOI] [PubMed] [Google Scholar]
- 73.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Junttila IS, Mizukami K, Dickensheets H, et al. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LaPorte SL, Juo ZS, Vaclavikova J, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mueller TD, Zhang JL, Sebald W, Duschl A. Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim Biophys Acta. 2002;1592:237–250. doi: 10.1016/s0167-4889(02)00318-x. [DOI] [PubMed] [Google Scholar]
- 77.Andrews RP, Rosa LR, Daines MO, Hershey GKK. Reconstitution of a functional human type IIIL-4/IL-13 receptor in mouse B cells: demonstration of species specificity. J Immunol. 2001;166:1716–1722. doi: 10.4049/jimmunol.166.3.1716. [DOI] [PubMed] [Google Scholar]
- 78.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–2038. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 80.Park KS, Korfhagen TR, Bruno MD, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steenwinckel V, Louahed J, Lemaire MM, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 82.Taniguchi K, Yamamoto S, Aoki S, Toda S, Izuhara K, Hamasaki Y. Epigen is induced during the interleukin-13-stimulated cell proliferation in murine primary airway epithelial cells. Exp Lung Res. 2011;37:461–470. doi: 10.3109/01902148.2011.596894. [DOI] [PubMed] [Google Scholar]
- 83.Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-beta1 in airway fibroblasts in asthma. Eur Respir J. 2014;43:464–473. doi: 10.1183/09031936.00068712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–368. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- 85.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 86.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 87.Shahabuddin S, Ponath P, Schleimer RP. Migration of eosinophils across endothelial cell monolayers: interactions among IL-5, endothelial-activating cytokines, and C-C chemokines. J Immunol. 2000;164:3847–3854. doi: 10.4049/jimmunol.164.7.3847. [DOI] [PubMed] [Google Scholar]
- 88.Sitkauskiene B, Johansson AK, Sergejeva S, Lundin S, Sjostrand M, Lotvall J. Regulation of bone marrow and airway CD34+ eosinophils by interleukin-5. Am J Respir Cell Mol Biol. 2004;30:367–378. doi: 10.1165/rcmb.2002-0311OC. [DOI] [PubMed] [Google Scholar]
- 89.Rossjohn J, McKinstry WJ, Woodcock JM, et al. Structure of the activation domain of the GM-CSF/IL-3/IL-5 receptor common beta-chain bound to an antagonist. Blood. 2000;95:2491–2498. [PubMed] [Google Scholar]
- 90.Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 92.Mullink H, Boorsma DM, Klein JC, Oostendorp R, Henzen-Logmans SC, Scheper RJ. A rapid and simple hapten conjugation method for monoclonal antibodies to be used in immunoenzyme single and double staining procedures. J Immunol Methods. 1987;99:199–204. doi: 10.1016/0022-1759(87)90128-1. [DOI] [PubMed] [Google Scholar]
- 93.Nolte T, Zadeh-Khorasani M, Safarov O, et al. Oxazolone and ethanol induce colitis in non-obese diabetic-severe combined immunodeficiency interleukin-2Rgamma(null) mice engrafted with human peripheral blood mononuclear cells. Clin Exp Immunol. 2013;172:349–362. doi: 10.1111/cei.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bergis D, Kassis V, Ranglack A, et al. High serum levels of the interleukin-33 receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma. Transl Oncol. 2013;6:311–318. doi: 10.1593/tlo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu LA, Fu Y, Zhang DN, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:2563–2566. doi: 10.7314/apjcp.2013.14.4.2563. [DOI] [PubMed] [Google Scholar]
- 96.Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci. 2011;56:3596–3601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 97.De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jovanovic IP, Pejnovic NN, Radosavljevic GD, et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134:1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Liu J, Wang Z, et al. Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLOS ONE. 2013;8:e72962. doi: 10.1371/journal.pone.0072962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martinez-Montiel Mdel P, Gomez-Gomez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol. 2014;20:1211–1227. doi: 10.3748/wjg.v20.i5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]