Abstract
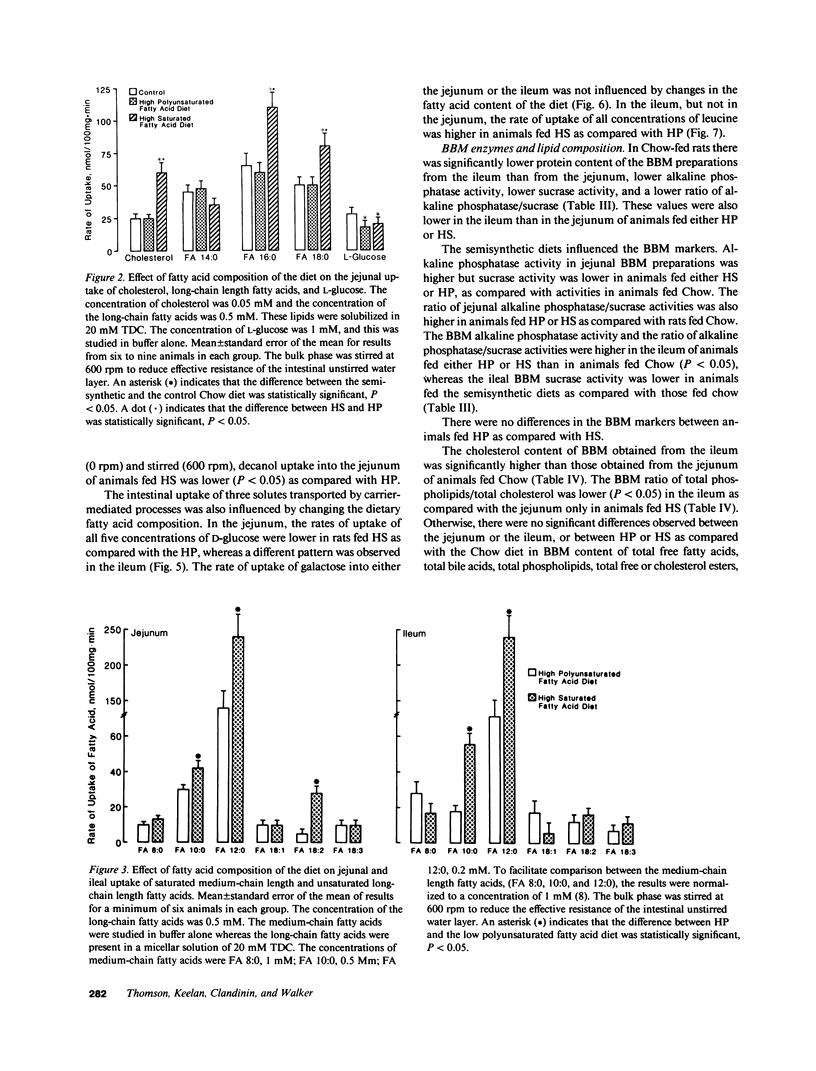
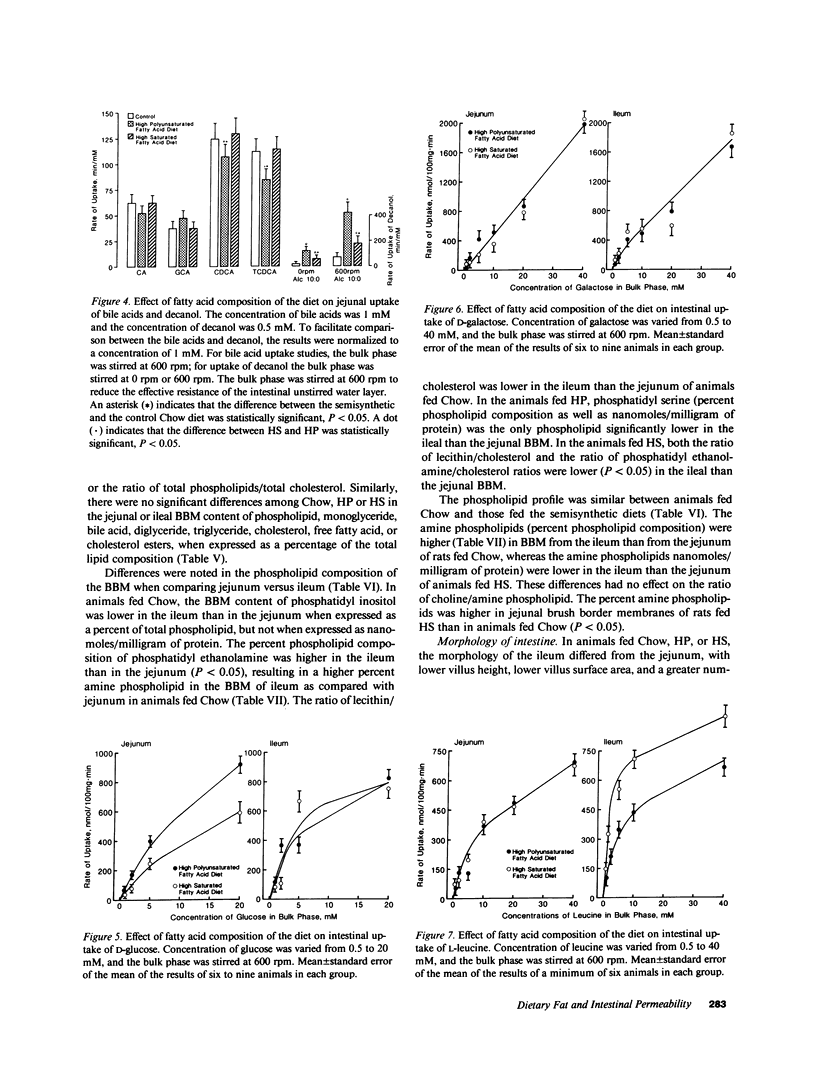
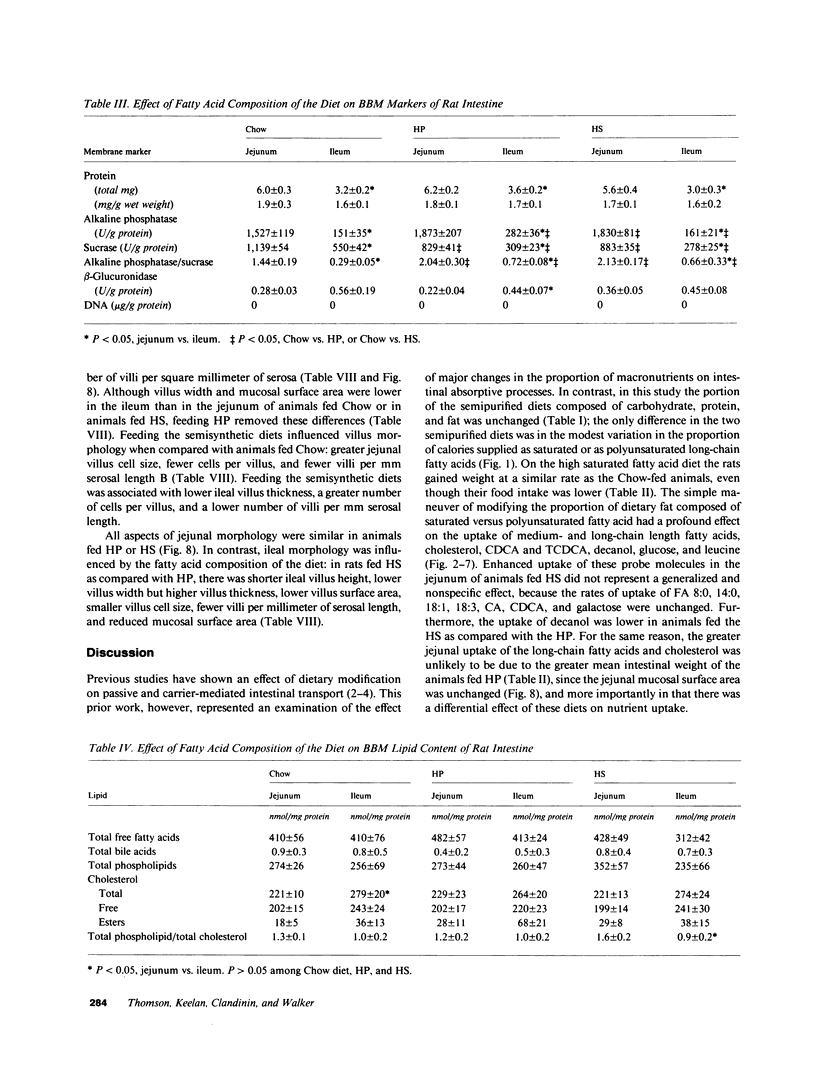
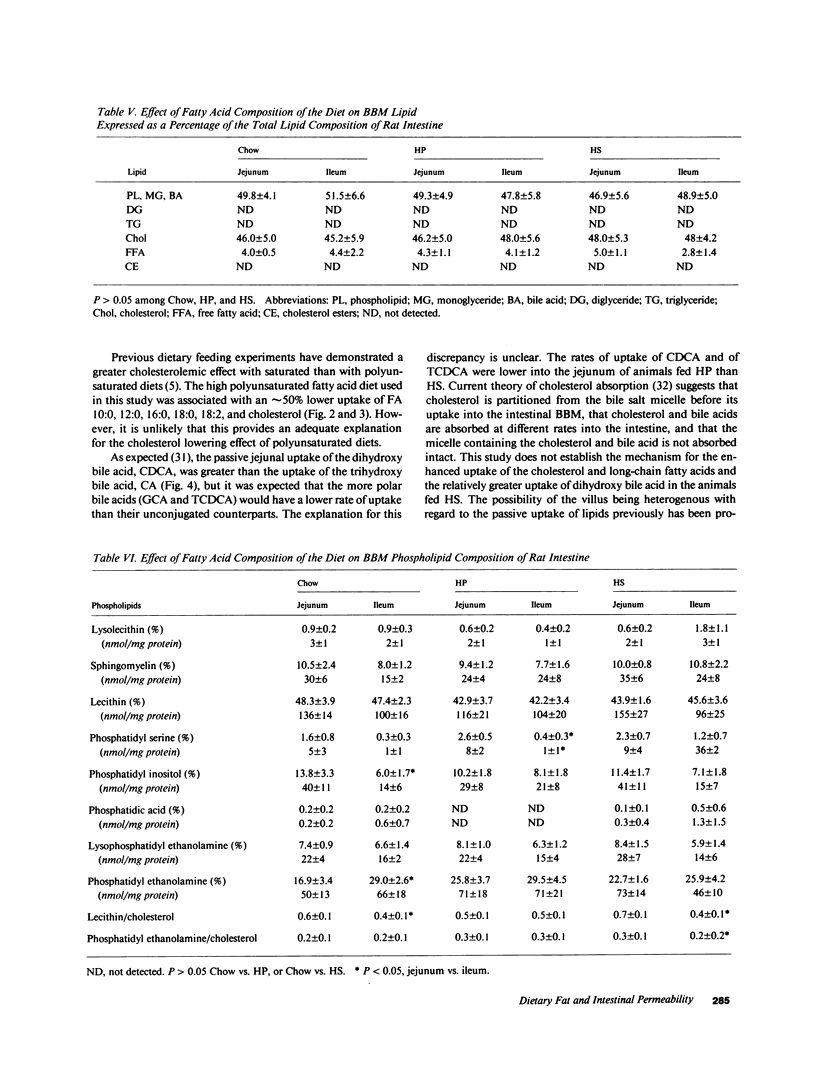
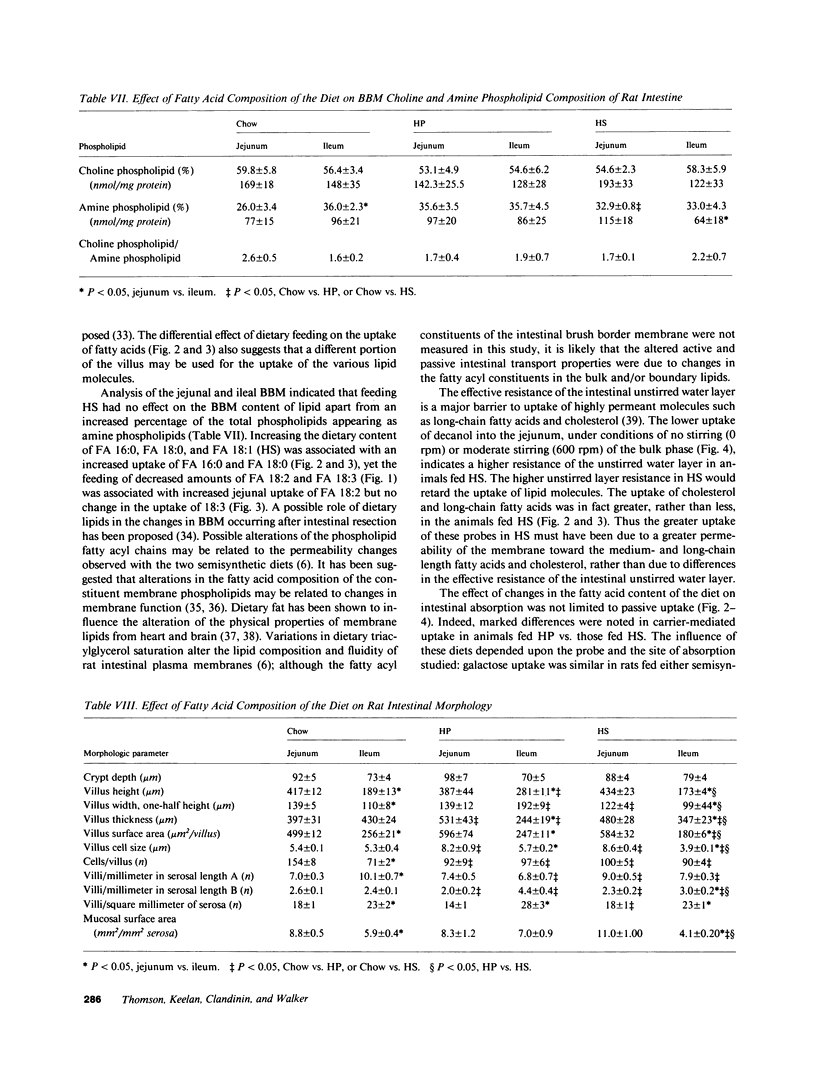
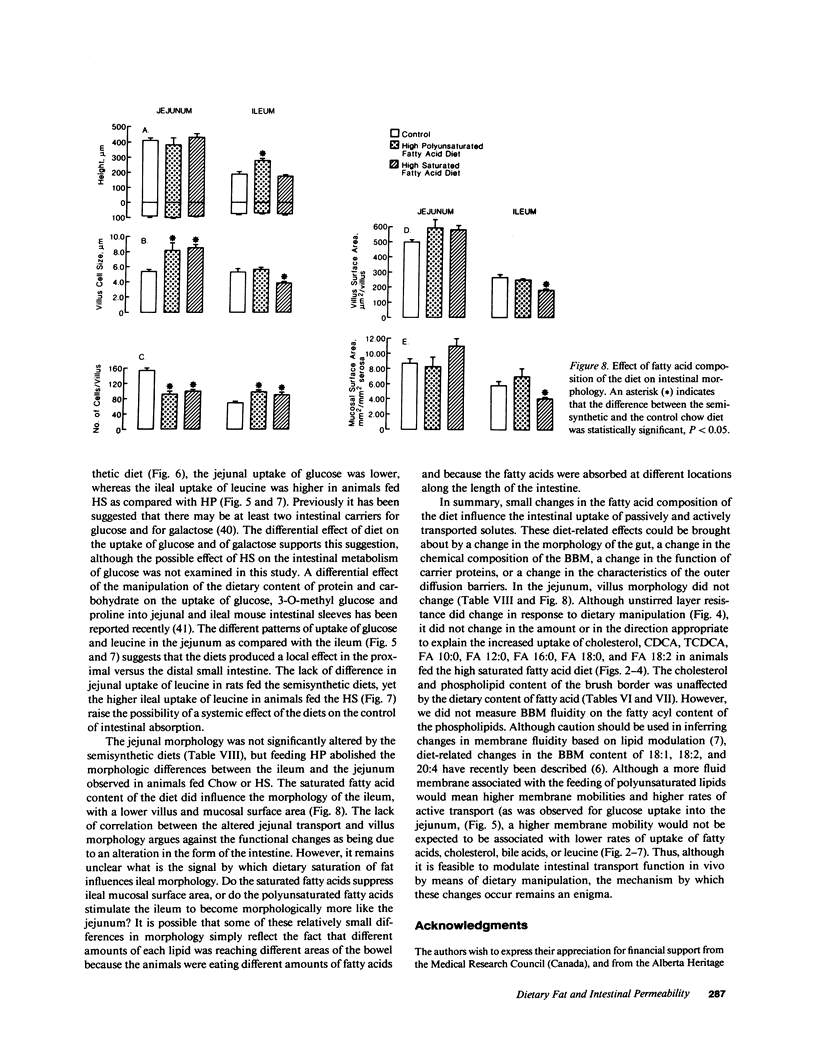
The influence of dietary fatty acid composition on intestinal active and passive transport function, brush border membrane composition, and morphology was examined in rats. Animals fed a semisynthetic diet high in saturated fatty acids demonstrated enhanced in vitro jejunal uptake of decanoic, dodecanoic, palmitic, stearic, and linoleic acid, as well as cholesterol and chenodeoxycholic and taurochenodeoxycholic acid, as compared with uptake in animals fed a semisynthetic diet high in polyunsaturated fatty acids but equivalent in total content of fat and other nutrients, or as compared with Purina chow. Feeding the saturated fatty acid diet was also associated with reduced jejunal uptake of a range of concentrations of glucose, enhanced ileal uptake of leucine, unchanged uptake of galactose, and lower uptake of decanol. The semisynthetic diets did not alter brush border membrane protein, sucrase or alkaline phosphatase activities, cholesterol, or total phospholipids, although the percentage of jejunal amine phospholipids was higher than in rats fed chow. The morphologic differences between the jejunum and ileum were abolished in animals fed the high polyunsaturated fatty acid diet; in rats fed the high saturated fatty acid diet, there was reduced mean ileal villus height, width, thickness, surface area, cell size, and villus density, as well as reduced mucosal surface area. The changes in jejunal transport were not correlated with the alterations in morphology, unstirred layer resistance, food intake, or body weight gain. It is proposed that small changes in the percentage of total dietary lipids composed of essential and nonessential fatty acids (without concurrent alterations in dietary total fat, carbohydrate, or protein) influence active and passive intestinal transport processes in the rat.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs J. M. Intermolecular hydrogen bonding between lipids: influence on organization and function of lipids in membranes. Can J Biochem. 1980 Oct;58(10):755–770. doi: 10.1139/o80-107. [DOI] [PubMed] [Google Scholar]
- Bowers G. N., Jr, Kelley M. L., McComb R. B. Continuous spectrophotometric measurement of serum alkaline phosphatase activity. Relative molecular activity and sensitivity of nine self-indicating phenolic substrates in 2-amino-2-methyl-1-propanol buffer. Clin Chem. 1967 Jul;13(7):608–610. [PubMed] [Google Scholar]
- Bowyer D. E., King J. P. Methods for the rapid separation and estimation of the major lipids of arteries and other tissues by thin-layer chromatography on small plates followed by microchemical assays. J Chromatogr. 1977 Sep 1;143(5):473–490. doi: 10.1016/s0378-4347(00)81794-6. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Davidson N. O., Schachter D. Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes. Biochim Biophys Acta. 1985 Jan 25;812(2):460–472. doi: 10.1016/0005-2736(85)90321-9. [DOI] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Clandinin M. T., Field C. J., Hargreaves K., Morson L., Zsigmond E. Role of diet fat in subcellular structure and function. Can J Physiol Pharmacol. 1985 May;63(5):546–556. doi: 10.1139/y85-094. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecknauer R., Vadakel T., Wepler R. Intestinal morphology and cell production rate in aging rats. J Gerontol. 1982 Mar;37(2):151–155. doi: 10.1093/geronj/37.2.151. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Foot M., Cruz T. F., Clandinin M. T. Influence of dietary fat on the lipid composition of rat brain synaptosomal and microsomal membranes. Biochem J. 1982 Dec 15;208(3):631–640. doi: 10.1042/bj2080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Innis S. M., Clandinin M. T. Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. Biochem J. 1981 Jan 1;193(1):155–167. doi: 10.1042/bj1930155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Davidson L. M., Shapiro I. L., Kim H. K., Kitagawa M., Malhotra S., Nair P. P., Clarkson T. B., Bersohn I., Winter P. A. Lipid metabolism and experimental atherosclerosis in baboons: influence of cholesterol-free, semi-synthetic diets. Am J Clin Nutr. 1974 Jan;27(1):29–50. doi: 10.1093/ajcn/27.1.29. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lukie B. E., Westergaard H., Dietschy J. M. Validation of a chamber that allows measurement of both tissue uptake rates and unstirred layer thicknesses in the intestine under conditions of controlled stirring. Gastroenterology. 1974 Oct;67(4):652–661. [PubMed] [Google Scholar]
- Mashige F., Imai K., Osuga T. A simple and sensitive assay of total serum bile acids. Clin Chim Acta. 1976 Jul 1;70(1):79–86. doi: 10.1016/0009-8981(76)90007-3. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Toyosato M., Yabumoto S., Tanimizu I., Hirakawa H. A new enzymatic method for colorimetric determination of free fatty acids. Anal Biochem. 1980 Oct;108(1):6–10. doi: 10.1016/0003-2697(80)90686-7. [DOI] [PubMed] [Google Scholar]
- Morin C. L., Ling V., Van Caillie M. Role of oral intake on intestinal adaptation after small bowel resection in growing rats. Pediatr Res. 1978 Apr;12(4 Pt 1):268–271. doi: 10.1203/00006450-197804000-00004. [DOI] [PubMed] [Google Scholar]
- Morin R. J. Rapid enzymatic determination of free and esterified cholesterol content of serum and tissues. Clin Chim Acta. 1976 Aug 16;71(1):75–80. doi: 10.1016/0009-8981(76)90277-1. [DOI] [PubMed] [Google Scholar]
- Okabe H., Uji Y., Nagashima K., Noma A. Enzymic determination of free fatty acids in serum. Clin Chem. 1980 Oct;26(11):1540–1543. [PubMed] [Google Scholar]
- Osuga T., Mitamura K., Mashige F., Imai D. Evaluation of fluorimetrically estimated serum bile acid in liver disease. Clin Chim Acta. 1977 Feb 15;75(1):81–90. doi: 10.1016/0009-8981(77)90502-2. [DOI] [PubMed] [Google Scholar]
- Schiff E. R., Small N. C., Dietschy J. M. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972 Jun;51(6):1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. B. Influence of dietary modifications on uptake of cholesterol, glucose, fatty acids, and alcohols into rabbit intestine. Am J Clin Nutr. 1982 Mar;35(3):556–565. doi: 10.1093/ajcn/35.3.556. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Rajotte R. Effect of dietary modification on the enhanced uptake of cholesterol in diabetic rats. Am J Clin Nutr. 1983 Feb;37(2):244–252. doi: 10.1093/ajcn/37.2.244. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Rajotte R. Effect of dietary modification on the uptake of glucose, fatty acids, and alcohols in diabetic rats. Am J Clin Nutr. 1983 Sep;38(3):394–403. doi: 10.1093/ajcn/38.3.394. [DOI] [PubMed] [Google Scholar]
- Thomson A. B. Unidirectional flux rate of cholesterol and fatty acids into the intestine of rats with drug-induced diabetes mellitus: effect of variations in the effective resistance of the unstirred water layer and the bile acid micelle. J Lipid Res. 1980 Aug;21(6):687–698. [PubMed] [Google Scholar]
- Vitiello F., Zanetta J. P. Thin-layer chromatography of phospholipids. J Chromatogr. 1978 Dec 11;166(2):637–640. doi: 10.1016/s0021-9673(00)95654-1. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976 Jul;58(1):97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winne D. The permeability coefficient of the wall of a villous membrane. J Math Biol. 1978 Jun 12;6(1):95–108. doi: 10.1007/BF02478521. [DOI] [PubMed] [Google Scholar]
- Yakymyshyn L. M., Walker K., Thomson A. B. Use of percollTM in the isolation and purification of rabbit small intestinal brush border membranes. Biochim Biophys Acta. 1982 Sep 9;690(2):269–281. doi: 10.1016/0005-2736(82)90331-5. [DOI] [PubMed] [Google Scholar]



