Abstract
We have demonstrated that mannose-binding lectin (MBL) recognizes various slow-growing, pathogenic mycobacteria [Mycobacterium tuberculosis (MTB), M. bovis, M. kansasii, M. gordonae] as well as non-pathogenic M. smegmatis. Recognition resulted in activation of the lectin pathway (LP) of complement and an enhancement of phagocytosis (shown for M. tuberculosis). Although MBL may be considered the main factor activating the LP upon recognition of mycobacteria, involvement of ficolins has also to be considered. Interaction of ficolin-3 with M. tuberculosis, M. bovis and M. kansasii, and ficolin-1 with M. tuberculosis and M. bovis was shown for the first time. Binding of recombinant MBL or ficolin-3 to MTB H37Rv led to the agglutination of bacteria and promoted their phagocytosis, but little effect was apparent with ficolin-1 or ficolin-2. Data from Western blots suggest mannosylated lipoarabinomannan (ManLAM) to be one of the main cell components of slow-growing mycobacteria, involved in LP activation. However, the LP was also activated by other cell fractions. Results presented here supplement considerably the data concerning the ability of complement-activating lectins to interact with mycobacteria. Ficolins (especially ficolin-3) might influence host response to infection and thus have clinical significance, at least as disease modifiers.
Keywords: complement, host–pathogen interactions, phagocytosis
Introduction
Collectins and ficolins, families of pattern recognition molecules (PRMs), are considered to be important components of the innate immune system. These collagen-related factors participate in two crucial anti-microbial defence mechanisms, opsonophagocytosis and complement activation via the lectin pathway (LP). The latter property is uniquely possessed by all known ficolins (-1, -2, -3 or M-, L-, H-, respectively) and certain collectins [mannose-binding lectin (MBL), collectin (CL)-10, and CL-11], co-operating with MBL-associated serine proteases (MASPs) and related non-enzymatic proteins.
MBL binds with high affinity to microbial polysaccharides or glycoconjugates rich in d-mannose (Man), N-acetyl-d-glucosamine (GlcNAc) or l-fucose (Fuc), in a calcium-dependent manner. It is known to bind to numerous bacteria, fungi, protozoa and viruses (reviewed in [1]). Among bacterial species recognized by this lectin, Mycobacterium tuberculosis, M. leprae, M. avium, M. bovis and M. africanum have been reported [2–5].
Ficolins, generally, recognize N-acetylated compounds like GlcNAc, N-acetyl-d-galactosamine (GalNAc) and related structures and, like MBL, are synthesized mainly in the liver. Ficolin-1 (M-ficolin), however, is synthesized in monocytes and neutrophils; it is exposed on the surface or stored inside granules [6–8], but is also present in serum [9]. Ficolin-1 shares with ficolin-2 the capacity to bind GlcNAc, GalNAc, N-acetylated cysteine as well as an artificial ligand, acetylated human albumin [10]. Ficolin-1, but not ficolin-2 or ficolin-3, recognizes sialic acids [11] and it has been shown to bind to several Streptococcus agalactiae strains possessing capsular polysaccharides (CPS) containing sialic acid [12]. Recently, interaction of ficolin-1 with CPS of Str. pneumoniae and Str. mitis has been demonstrated, suggesting N-acetylmannosamine to be the target structure for the lectin [13]. Ficolin-1 also recognizes Gram-negative bacteria: Escherichia coli and Salmonella Typhimurium [6,7].
Ficolin-2 (L-ficolin or P35) uniquely possesses four binding sites in its fibrinogen (FBG) domain and interacts with a relatively broad range of ligands (GlcNAc, GalNAc, N-acetyl-d-mannosamine, galactose and non-sugar compounds such as N-acetylated cysteine or acetylcholine) [14]. It has been shown to bind to several strains of capsulated streptococci and staphylococci, Pseudomonas aeruginosa, Escherichia coli, S. Typhimurium (Ra chemotype) and M. bovis as well as pathogenic protozoa (Trypanosoma cruzi, Giardia lamblia) and fungi (Aspergillus fumigatus) (reviewed in [15,16]). Recently, its interaction with M. tuberculosis has been reported [17].
Ficolin-3 (also called H-ficolin or Hakata antigen) is synthesized in both liver and lungs, therefore it may take part in both systemic (bloodstream) and local (respiratory system) immune responses. Like other ficolins, it recognizes acetylated sugars, but also d- and l-fucose and galactose [14]. Among its microbial ligands, an extracellular polysaccharide of Aerococcus viridans and a few lipopolysaccharides (from S. Typhimurium and S. Minnesota E. coli O111 and Hafnia alvei strain 1200) have been reported [18–20]. Moreover, it binds to T. cruzi, G. lamblia and pandemic H1N1 influenza A virus [21–23].
The unique feature of the mycobacterial cell wall is the abundance of mannosylated molecules such as mannose-capped lipoarabinomannan (ManLAM), lipomannan (LM) and phosphatidyl-myo-inositol mannosides (PIMs), contributing to the pathogenicity of M. tuberculosis. In the case of slow-growing, pathogenic mycobacteria (e.g. M. tuberculosis, M. avium or M. bovis), ManLAM is built up from a carbohydrate core composed of d-mannose and d-arabinose residues, a mannosylphosphatidyl-myo-inositol (MPI)-anchor and mannose-capping motifs. Fast-growing mycobacteria (e.g. M. smegmatis) synthesize LAM with phospho-myo-inositol caps (PILAM) or are uncapped (AraLAM) [24]. Mannose-rich cell surface structures of M. avium – ManLAM, LM, PIM, AraLAM – as well as cord factor (dimycolated trehalose) were shown to be targets for MBL [3]. Furthermore, lipoarabinomannan was suggested to be recognized by ficolin-2 [17]. Here we present data demonstrating binding of MBL and human ficolins to various mycobacteria, the subsequent complement activation and the impact of those molecules on M. tuberculosis H37Rv phagocytosis.
Materials and methods
Bacterial strains and culture conditions
M. tuberculosis H37Rv [MTB H37Rv; American Type Culture Collection (ATCC), Manassas, VA, USA], M. bovis [bacillus Calmette–Guérin (BCG); ATCC] and M. smegmatis (MC2 155; ATCC) came from the collection of the Laboratory of Mycobacterium Genetics and Physiology, IMB PAS, whereas M. kansasii and M. gordonae strains were kindly gifted by Professor E. Augustynowicz-Kopec (Institute of Tuberculosis and Lung Diseases, Warsaw, Poland). Bacteria were cultivated at 37°C in Middlebrook 7H9 medium, enriched with oleic albumin dextrose catalase (OADC) (Becton Dickinson, San Jose, CA, USA), in roller bottles until the bacteria reached the exponential growth phase [optical density (OD)600 = 0·8–1·0] and then heat- (80°C, 20 min) or paraformaldehyde (PFA)-inactivated [5% PFA (BioShop, Burlington, ON, Canada), 4°C, 16 h].
Green fluorescent protein (GFP)-tagged bacteria
The GFP was amplified based on pJFR11 plasmid as a template [25] and cloned into pMV306KmR integrative vector previously digested by BamHI and HindIII. Next, the 400 base pairs (bp) fragment, encoding the putative ercc3 promoter, was released by BamHI digestion from pKSR 15 plasmid (Rajagopalan, unpublished) and cloned into integrative vector pMV306Kmr in front of GFP. The final construct (PerccGFPMVKm) was verified by sequencing. Subsequently, the PerccGFPMVKm plasmid was introduced by electroporation into M. tuberculosis H37Rv. The chromosomal DNA was isolated from kanamycin-resistant transformants and used as a template in polymerase chain reaction (PCR) with primers complementary to pMV306Km. The presence of GFP was confirmed by fluorescent microscopy.
Bacterial lipopolysaccharides
Lipopolysaccharide (LPS) from K. pneumoniae O3:K55–(KO3) came from the collection of the Laboratory of Immunobiology of Infections, IBM, PAS while LPS from H. alvei PCM1200 (H.a. 1200) was kindly provided by Professor J. Lukasiewicz (L. Hirszfeld Institute of Immunology and Experimental Therapy, PAS, Wroclaw, Poland).
Monocytic cell line
The monocytic U937 (ATCC) cell line came from the collection of the Laboratory of Immunobiology of Infections, IMB, PAS. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Sigma, St Louis, MO, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) and l-glutamine/penicillin/streptomycin (Gibco) (37°C, 5% CO2, 95% humidity). To induce differentiation into macrophages, U937 cells were cultured for 24 h in the presence of phorbol 12-myristate 13-acetate (PMA; Sigma) (20 ng/ml) and then for 24 h without PMA.
Reagents and antibodies
Recombinant human MBL and ficolin-1, -2 and -3 (M-, L-, H) came from R&D Systems (Minneapolis, MN, USA). Anti-MBL (clone 131-01) and anti-ficolin-1 (clone ABS031-01) monoclonal antibodies (mAbs) came from BioPorto (Gentofte, Denmark); and anti-ficolin-2 (clone 4F5) and anti-ficolin-3 (clone 4H5) mAbs were from Hycult Biotech (Uden, The Netherlands). Horseradish peroxidase (HRP)-conjugated rabbit anti-mouse, anti-human C4c and goat anti-rabbit immunoglobulin (Ig) antibodies were from Dako (Glostrup, Denmark). Fluorescein isothiocyanate (FITC)-labelled anti-mouse Ig antibodies came from AbD Serotec (Oxford, UK). Mannan-agarose was from Sigma and proteinase K from Thermo Scientific (Fremont, CA, USA). Western blot luminol reagent was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Texas Red-phalloidin and 4′,6-diamidino-2-phenylindole (DAPI) came from Life Technologies/Thermo Fisher Scientific (USA). Lipoarambinomannan (LAM) from M. tuberculosis Aoyama-B was obtained from Nacalai Tesque Inc. (Nakagyo-ku, Kyoto, Japan).
Human sera
Normal sera from healthy adult volunteers (all BCG-vaccinated) with known concentrations of MBL and ficolins were used as a source of pattern-recognition molecules (Table 1).
Table 1.
Normal human sera (NHS) used as a source of complement-activating pattern recognition molecules
| NHS1 | NHS2 | NHS3 | |
|---|---|---|---|
| MBL concentration (ng/ml) | 2000 | 345 | 120 |
| Ficolin-1 concentration (ng/ml) | 561 | 555 | 1100 |
| Ficolin-2 concentration (ng/ml) | 2600 | 3900 | 4900 |
| Ficolin-3 concentration (μg/ml) | 13·6 | 25 | 14·6 |
As a source of C4, serum from a healthy MBL-deficient individual (with normal C4 serum level), prediluted 1 : 3000, was used. Such a dilution prevents complement activation depending on MBL-MASP or ficolin-MASP complexes present in this serum and provides a sufficient amount of C4 factor in experimental conditions.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)/Western blot
Lysates of PFA-inactivated mycobacterial cells [digested with proteinase K (2 mg/ml, 90 min, 56°C [26]) or untreated], were separated in 12% polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membrane (Thermo Scientific). After blocking with 2% bovine serum albumin (BSA), the membrane was incubated overnight at 4°C with normal human serum (NHS)1 (Table 1) (or NHS1 pre-absorbed with mannan-agarose, 30 min on ice), prediluted 1 : 50 in MBL-binding buffer, containing 20 mM Tris, 1 M NaCl, 10 mM CaCl2, pH 7·4 (high ionic strength, preventing classical complement pathway activation) [27], supplemented with 0·1% BSA. After washing, the membrane was incubated with serum from an MBL-deficient individual, prediluted 1 : 3000 in TBS-Ca2+ buffer (10 mM Tris, 140 mM NaCl, 5 mM CaCl2, pH 7·4), supplemented with 0·1% BSA, for 2 h at 37°C, followed by rabbit anti-C4c and HRP-conjugated goat anti-rabbit Ig antibodies [for detection of MBL binding, specific mAbs (clone HYB-131-01; BioPorto) followed by HRP-conjugated anti-mouse Ig (Dako) were used]. Then, membrane was incubated with Western blot luminol reagent and exposed to an X-ray film. KO3 and H.a. 1200 lipopolysaccharides as well as LAM were used as controls. For the inhibition assay, NHS-1 (1:12·5) was pre-incubated (1 h, on ice) with an equal volume of 20 mM solution of d-mannose, N-acetyl-d-glucosamine, d-glucose or d-galactose (all from Sigma) in MBL-binding buffer and then used for Western blot. Membranes were incubated with serum pretreated with inhibitors for 2 h at 37°C (instead of 4°C, overnight).
Alternatively, PFA-fixed MTB H37Rv cells (1 × 107) were incubated with recombinant MBL or ficolins (0·3 μg) and, after washing, SDS-PAGE and transfer to PVDF, the bound proteins were detected with specific primary antibodies and HRP-conjugated anti-mouse Ig antibodies.
Enzyme-linked immunosorbent assay (ELISA)
U96 Maxisorp microtitre plates (Nunc, Roskilde, Denmark) were coated with heat- or PFA-inactivated mycobacterial cells (10 μg/well) and incubated at 4°C overnight. After blocking with 1% BSA, normal human serum was added (NHS1 for MBL; NHS2 for ficolin-2 and -3; NHS3 for ficolin-1), prediluted 1 : 10 in a buffer containing 40 mM imidazole, 1·25 M NaCl, 50 mM CaCl2 (pH 7·8), supplemented with 1% BSA. Next, plates were incubated 2 h at 37°C and overnight at 4°C and the bound proteins were detected with specific primary antibodies and HRP-labelled corresponding secondary antibodies. 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic)acid (ABTS) (Sigma) was used as substrate for HRP. Absorbance (at 405 nm) was read with the use of a Benchmark Plus microplate spectrophotometer (Bio-Rad, Hercules, CA, USA). For a negative control, the primary antibody was omitted. Absorbance values at least twofold higher [after standard deviation (s.d.) subtraction] compared with control were considered positive.
Flow cytometry assay
PFA-fixed bacteria (1 × 107) were washed with buffer containing 25 mM Hepes, 155 mM NaCl, 5 mM CaCl2 1 mM MgCl2 (pH 7·4) and incubated with recombinant MBL or ficolins (20, 5, 1, 0·5 μg/ml) for 2 h at 37°C. After washing, the bound proteins were detected with specific mAbs (1 μg/ ml) and FITC-labelled anti-mouse Ig antibodies. The reactivity was estimated using the LSR II flow cytometer (BD). For a negative control, mycobacterial cells were incubated with primary and secondary antibodies only, with no recombinant lectins.
Agglutination assay
GFP-tagged MTB H37Rv bacteria (1 × 107) were suspended in 100 μl of buffer containing 25 mM Hepes, 155 mM NaCl, 5 mM CaCl2 1 mM MgCl2 (pH 7·4) and incubated (4 h, 37°C) with 100 ng of recombinant proteins (or recombinant proteins and 10 nM ethylenediamine tetraacetic acid (EDTA), according to Ariki et al. [28]. Then, agglutination was observed by TE-200U fluorescent microscope (Nikon, Tokyo, Japan).
Phagocytosis assay
GFP-tagged MTB H37Rv cells (1 × 107) were opsonized with 2 μg of recombinant MBL or ficolins (15 min at 37°C). Next, bacteria were washed, resuspended in the DMEM and added to PMA-treated U937 (ratio: 30 : 1). After cultivation (90 min, 37°C) in DMEM (without FBS and antibiotics), the macrophages were washed to remove non-associated bacilli, fixed with BD Cytofix buffer and analysed using the BD LSR II flow cytometer. The data obtained were analysed by FlowJo software (Tree Star Inc., Ashland, OR, USA). Alternatively, U937 were cultivated and differentiated with PMA in eight-chamber slides and, after 90 min incubation at 37°C with GFP-tagged bacteria (ratio: 30 : 1), stained with Texas Red-phalloidin and DAPI. Phagocytosis was observed in C1 fluorescent confocal microscope (Nikon) and data were recorded photographically.
Statistical analysis
The Statistica (version 10; StatSoft, Kraków, Poland) software package was used for data management and statistical calculations. The analysis of variance (anova) test was used. P-values < 0·05 were considered statistically significant.
Results
Activation of the lectin pathway of complement by mycobacterial cell antigens
As shown in Fig. 1a, low molecular weight (approximately 10 kDa, 15–20 kDa and 25–35 kDa), proteinase K-resistant structures of slow-growing species (M. tuberculosis, M. kansasii, M. gordonae, M. bovis) were able to initiate the activation of the lectin pathway of complement, although that ability was relatively weak in the case of M. gordonae (Fig. 1a, lane 3). After treatment of normal serum with mannan-agarose (leading to the removal of MBL, and possibly other mannose-binding proteins), the reactivity appeared much weaker but was not abolished (Fig. 1b). This suggested an involvement of serum lectin(s) other than MBL in recognition of mycobacteria and subsequent complement activation. Indeed, the MBL binding pattern (proteins detected with specific antibodies) differed from that of C4c deposition (Fig. 1c). With non-pathogenic M. smegmatis, the LP was activated by the 10 kDa and 15–25 kDa cell fractions (Fig. 1a, lane 5). Heat inactivation (56°C, 30 min) of serum abolished LP activity (data not shown). Both lipopolysaccharides from K. pneumoniae O3 (a ligand for MBL) and H. alvei PCM1200 (a ligand for ficolin-3), used as controls, activated the lectin pathway of complement (Fig. 1a, lanes 7, and 8). For the former, that ability was inhibited by mannan-agarose (Fig. 1b, lane 7). Interestingly, removal of MBL weakened but did not abolish C4c deposition by LAM (Fig. 1a,b, lane 6).
Fig. 1.
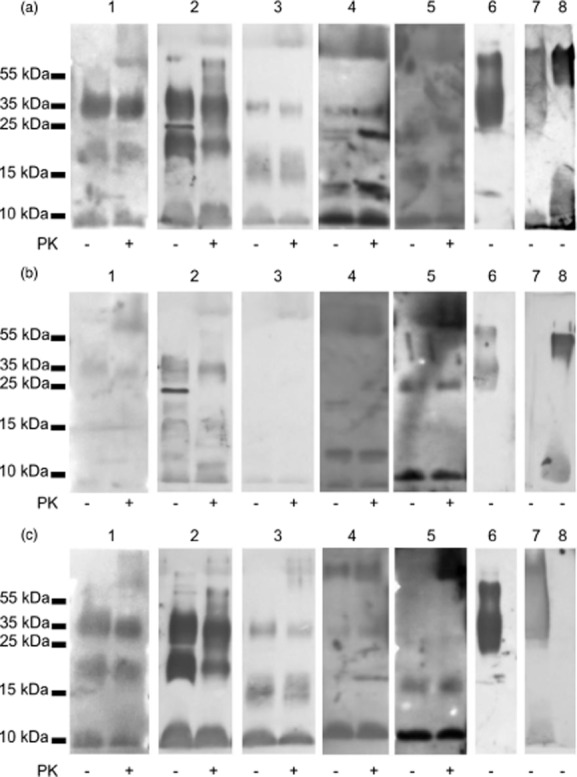
Complement lectin pathway activation (C4c deposition: a,b) and binding of mannose-binding lectin (MBL) (c) by mycobacterial cell fractions, demonstrated in Western blot. (a,c) normal human serum (NHS1); (b) mannan-agarose pretreated NHS1. 1: Mycobacterium tuberculosis; 2: M. kansasii; 3: M. gordonae; 4: M. bovis; 5: M. smegmatis; 6: LAM; 7: KO3 lipopolysaccharide (LPS); 8: H. a. 1200 LPS. PK: proteinase K.
Data from the competition assay seem to confirm the major role of MBL in the lectin pathway of complement activation by M. tuberculosis. Both Man and GlcNAc strongly inhibited C4c deposition (Fig. 2, lanes 2 and 3). A much weaker effect was observed in the case of Glc while Gal barely influenced C4 activation (Fig. 2, lanes 4 and 5).
Fig. 2.
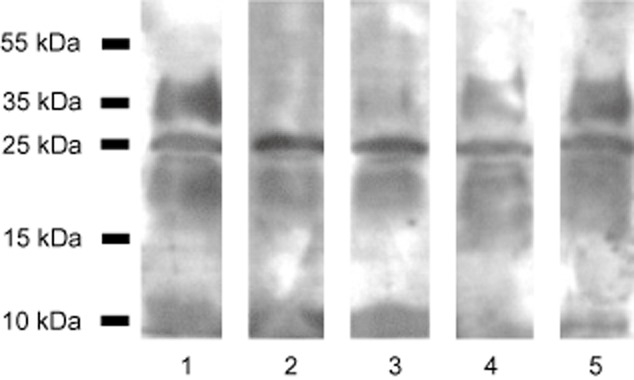
Inhibition of complement lectin pathway activation (C4c deposition) by monosaccharides (mannose, lane 2; N-acetyl-d-glucosamine, lane 3; d-glucose, lane 4 and d-galactose, lane 5). Positive control (no inhibitor added) is demonstrated in lane 1. PFA-inactivated M. tuberculosis H37Rv lysates (not pre-treated with proteinase K) were used.
Reactivity of lectin pathway factors with mycobacterial whole cells
Binding of serum MBL or ficolins to heat- or PFA-inactivated whole cells of various mycobacteria was investigated by ELISA (Fig. 3). The strongest reactivity was observed for MBL, recognizing heat-inactivated MTB H37Rv and M. kansasii bacteria (>25-fold difference compared with control), followed by M. bovis BCG, M. gordonae and M. smegmatis. MBL binding was much weaker for PFA-inactivated slow-growing species. An exception was M. bovis BCG: similarly to fast-growing M. smegmatis, practically no reaction was found (Fig. 3). Other serum factors tested were much less reactive (ficolin-1 and -3) or unreactive (ficolin-2) against mycobacteria (Fig. 3). Interestingly, ficolin-1 bound more strongly to PFA-treated M. kansasii and M. bovis BCG, while ficolin-3 recognized PFA-treated but not heat-inactivated M. tuberculosis H37RV (Fig. 3). Moreover, ficolin-1 was reactive against M. tuberculosis (both heat- and PFA-treated), whereas ficolin-3 was reactive against heat-inactivated BCG cells (Fig. 3).
Fig. 3.
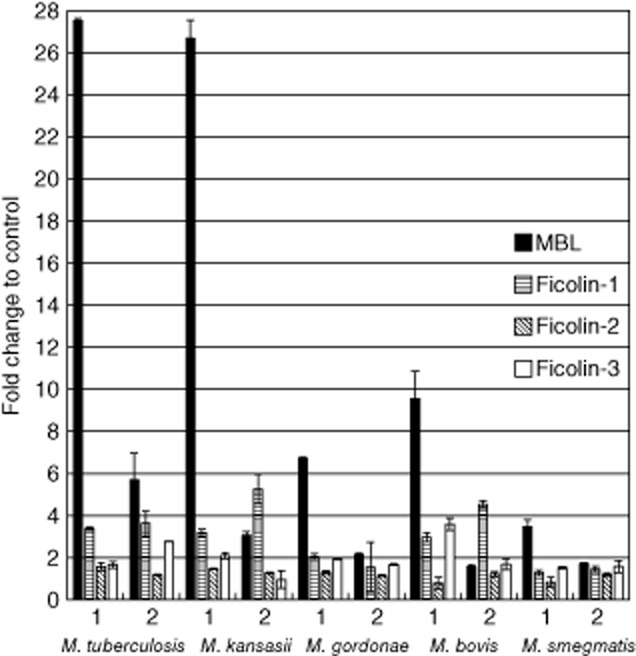
The reactivity of serum mannose-binding lectin (MBL), ficolin-1, -2 and -3 with heat- (1) or paraformaldehyde (PFA)- (2) inactivated mycobacteria. Data (mean ± standard deviation) from at least three independent experiments were used.
Reactivity of recombinant proteins with MTB H37Rv
The results obtained from normal sera were confirmed using recombinant lectin pathway factors and the M. tuberculosis H37Rv strain. In this experiment, PFA-inactivated bacteria were incubated with recombinant proteins, separated in SDS-PAGE and transferred to a PVDF membrane. MBL or individual ficolins were detected with specific antibodies. The strongest reactivity was evident for recombinant (r)MBL, followed by rficolin-3 and rficolin-1, while a weaker signal was observed in the case of rficolin-2 (Fig. 4).
Fig. 4.
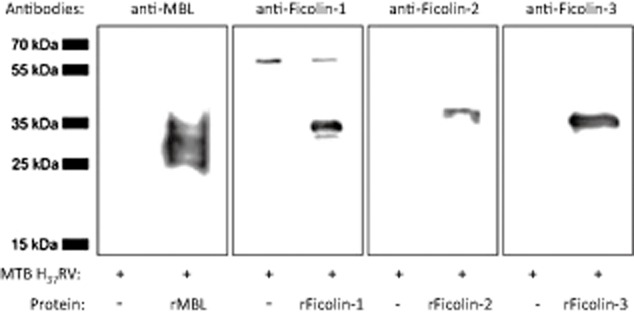
The reactivity of recombinant mannose-binding lectin (rMBL) and ficolins with Mycobacterium tuberculosis (MTB) H37Rv paraformaldehyde (PFA)-inactivated cells in Western blot. PFA-fixed MTB H37Rv cells were incubated with recombinant MBL or ficolins and, after washing, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer to polyvinylidene difluoride (PVDF) membrane, the bound proteins were detected with specific primary antibodies and horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin antibodies.
Flow cytometry revealed that rMBL recognized PFA-inactivated MTB H37Rv cells very efficiently at the highest (20 μg/ml) dose (>85% positive cells), but much less so (approximately 25%) throughout the physiological concentration range of MBL (Fig. 5a); rficolin-1 and rficolin-3 were bound in a dose-dependent manner at physiological concentrations (Fig. 5b,d). Again, the reactivity of rficolin-2 was weaker (<40% positive cells at the supraphysiological dose of 20 μg/ml, and <20% across the normal serum concentration range) (Fig. 5c).
Fig. 5.
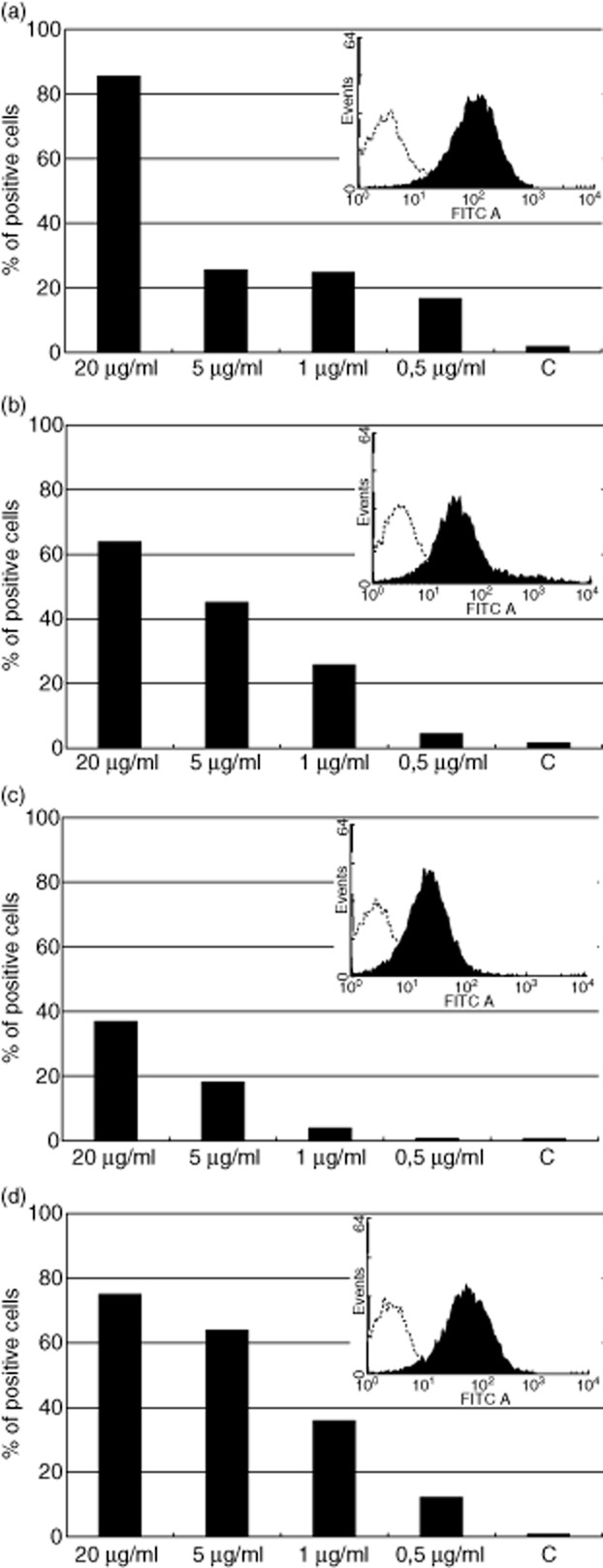
The reactivity of recombinant mannose-binding lectin (MBL) and ficolins (various doses) with Mycobacterium tuberculosis (MTB) H37Rv paraformaldehyde (PFA)-inactivated cells in flow cytometry. (a) MBL; (b) ficolin-1; (c) ficolin-2; (d) ficolin-3. C: controls (bacteria incubated in absence of MBL or ficolins). Histograms from the highest protein doses are presented in the right upper corners of each plot.
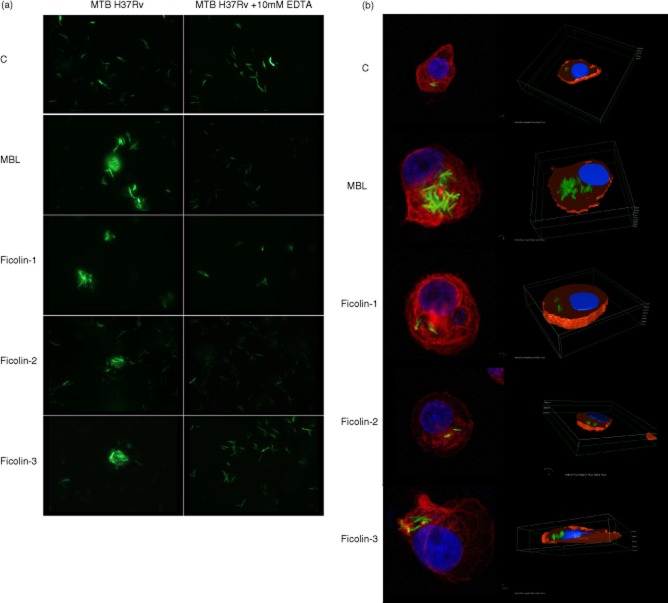
Agglutination of MTB H37Rv cells by lectin pathway factors and their contribution to phagocytosis
GFP-tagged MTB H37Rv bacteria were agglutinated by all recombinant proteins tested (Fig. 6a). Agglutination was inhibited by EDTA, suggesting a crucial role for calcium cations. Furthermore, opsonization with MBL or ficolin-3 led to increased uptake of GFP-tagged bacteria by phagocytes (Fig. 6b), although for ficolin-1 and -2 the effect appeared very modest. Phagocytosis was inhibited by pre-incubation of recombinant MBL with mannan or ficolin-3 with H. alvei PCM1200 LPS (not shown). These data were confirmed using flow cytometry (significantly more phagocytes containing engulfed bacteria opsonized with MBL or ficolin-3 but not ficolin-1 or -2, compared with control) (Fig. 7).
Fig. 6.
Influence of mannose-binding lectin (MBL) and ficolins on agglutination (a: data from fluorescent microscope) and phagocytosis (b: data from confocal fluorescent microscope) of green fluorescent protein (GFP)-tagged Mycobacterium tuberculosis (MTB) H37Rv bacteria. C: controls (bacteria non-opsonized with MBL or ficolins).
Fig. 7.
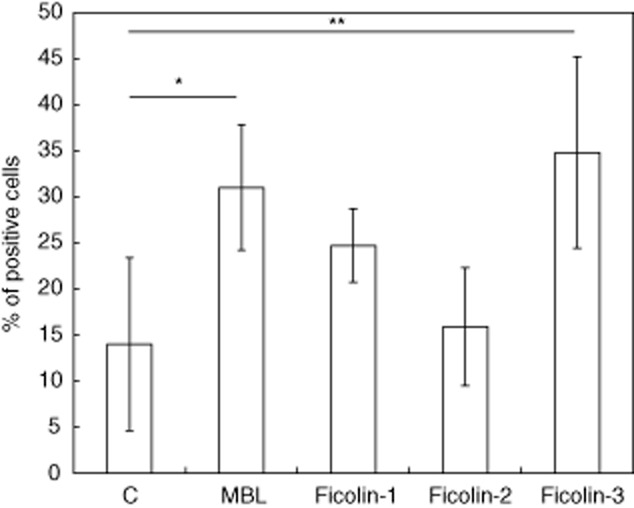
Contribution of mannose-binding lectin (MBL) and ficolins to phagocytosis of green fluorescent protein (GFP)-tagged Mycobacterium tuberculosis (MTB) H37Rv bacteria by U937 cells, differentiated to macrophages with phorbol myristate acetate (PMA). Data from flow cytometry (mean ± standard deviation from at least four experiments). C: controls (bacteria not opsonized with MBL or ficolins). *P < 0·05; **P < 0·01.
Discussion
Although the ability of MBL to interact with mycobacteria was first reported two decades ago [2,3], its role in mycobacterial infections is still controversial (analysed and reviewed in [29]). Fewer reports concerning ficolins have been published. Previously, only ficolin-2 had been shown to recognize M. bovis and M. tuberculosis [4,17]. Interestingly, its low serum concentration or corresponding FCN2 gene polymorphisms were suggested to influence the susceptibility not only to tuberculosis [17], but also to leprosy [30,31]. Moreover, the latter disease was postulated to be associated with ficolin-1 (FCN1) gene variation [32]. No data concerning ficolin-3 have emerged so far.
Our results confirmed the direct interaction of MBL with M. tuberculosis [2] and M. bovis [4]. We also found that MBL recognizes M. kansasii, M. gordonae and (more weakly) M. smegmatis (Figs 1 and 2), resulting in lectin pathway activation (Fig. 1), agglutination of bacteria (Fig. 6a) and enhancement of phagocytosis (Figs 6b and 7). Previously, Bonar et al. [33] have reported recombinant MBL to promote phagocytosis of the M. bovis BCG strain. Although MBL may be considered the principal factor activating the LP upon recognition of mycobacteria, involvement of ficolins in this process has to be taken into account, as pre-incubation of normal human serum with mannan-agarose weakened but did not abolish C4c deposition (Fig. 1b). This conclusion may be supported by the inhibition of lectin pathway activation by Man (the strongest effect) and GlcNAc (Fig. 2). Both are MBL ligands; however, acetylated compounds, including GlcNAc, are recognized by ficolins while mannose is recognized by another complement-activating collectin, CL-11 [34]. The pattern of positive control reaction (no inhibitor added, Fig. 2, lane 1) differs from that demonstrated in Fig. 1a. It probably reflects the twofold higher serum concentration used for the inhibition assay and different incubation conditions. Interestingly, a 25 kDa protein band (visible in Fig. 1 in the case of M. kansasii) is recognized neither by MBL nor any of the ficolins tested (not shown). As C4c deposition depending on this fraction is not influenced by any of the sugar inhibitors (in the case of M. kansasii by mannan-agarose as well, Fig. 1b), its specificity remains unclear.
Ficolin-2-dependent (as well as MBL-dependent) complement activation by M. bovis was concluded by Carroll et al. [4]; however, no direct evidence was provided. Recently, Luo et al. [17] demonstrated factor C4 activation due to interaction of recombinant ficolin-2 with M. tuberculosis H37Rv cells, and thus fully confirmed the conclusion of Carroll et al. Furthermore, they found that this molecule promoted phagocytosis of MTB by murine macrophages and was able to protect human lung cells as well as ficolin A-deficient mice (ficolin A is a murine homologue of ficolin-2) from infection with this strain. The ManLAM was shown to inhibit recognition of bacteria, thus it was suspected to be the crucial target for ficolin-2 [17]. In contrast to the cited papers, we could not demonstrate binding of ficolin-2 at a concentration (3·9 μg/ml) typical of normal serum [15] to various mycobacterial strains. Conversely, recombinant protein, at high doses, recognized MTB H37Rv cells (Fig. 4), consistent with data published recently (46·3% positive cells according to [17]; 37% in this investigation; both at a ficolin-2 dose of 20 μg/ml). There was, however, no significant effect on phagocytosis (Figs 6b and 7).
Ficolin-3 is the most abundant ficolin in human serum and the most potent activator of the LP in vitro, yet it fails to recognize most common pathogens and its function(s) is the least well-established of the LP activators. To our knowledge, we have shown for the first time that ficolin-3 recognizes the slow-growing mycobacteria M. tuberculosis, M. bovis and M. kansasii, as does ficolin-1 (Figs 5). Interaction of MTB H37Rv bacteria with recombinant ficolin-3 led to agglutination of bacteria and markedly promoted their phagocytosis. A similar but less marked effect was observed for rficolin-1 (Figs 6 and 7).
Either MBL or ficolin binding to mycobacteria, as well as subsequent complement activation, as expected, depended on glycosylation patterns: clear differences were shown when data from heat- and PFA-inactivated or proteinase K-treated and -untreated bacterial cells were compared. Thus, protein denaturation or enzymatic degradation modifies accessibility of pattern-recognition molecules by influencing their target structures. Generally, heat inactivation, leading to protein denaturation, seems to expose carbohydrate patterns interacting with the lectin domain of MBL. In contrast, PFA treatment might leave intact some non-sugar, acetylated compounds, accessible for ficolins' fibrinogen-like domains. This may explain the higher affinity of MBL to heat-inactivated and ficolins (with the exception of the ficolin-3–M. bovis interaction) to PFA-inactivated bacteria. Moreover, pretreatment of microbial cells with proteolytic enzymes might result in the loss of not only peptide but also sugar compounds, due to protein glycosylation.
Data from Western blotting (Fig. 1) suggest that ManLAM (30–35 kDa fractions) are one of the main cell components of slow-growing mycobacteria involved in lectin pathway activation. This seems to confirm data concerning interactions of MBL with M. avium [3] and ficolin-2 with M. tuberculosis [17]. However, other fractions able to activate LP were found (Fig. 1a).
Certain differences in the levels of reactivity of recombinant and natural (blood) lectins may reflect not only the variety of their concentrations in the normal sera used (Table 1) but also a possible modifying effect of other serum factors (including antibodies), interacting with mycobacteria (sera came from BCG-vaccinated individuals). On the other hand, findings from both are not contradictory.
The results presented here supplement considerably the previously published data concerning the ability of complement-activating lectins to interact with mycobacteria. Ficolin-3, in particular, might have an important influence on the host response (complement activation, opsonophagocytosis) to infection and thus have clinical significance as a disease modifier. As ficolin-1 is expressed in alveolar epithelial cells, monocytes and neutrophils, while ficolin-3 is synthesized in the lung and secreted into bronchi and alveoli, both might affect the local, pulmonary immune defence that is particularly important in tuberculosis.
Acknowledgments
This work was supported by the European Union within European Regional Development Fund, through grant Innovative Economy (POIG. 01·01·02–10-107/09) and by National Science Centre (Poland), grant 2011/01/M/NZ6/01764. We thank Dr K. Bednarska (Laboratory of Experimental Immunology, IMB, PAS) and Dr P. Przygodzka (Laboratory of Cellular Proteomics, IMB, PAS) for their excellent assistance in fluorescent/confocal microscopy, Professor J. Lukasiewicz (L. Hirszfeld Institute of Immunology and Experimental Therapy, PAS, Wroclaw, Poland) for providing H. alvei PCM1200 LPS and Dr D. C. Kilpatrick (Edinburgh, Scotland, UK) for critical reading of the manuscript and editorial suggestions.
Disclosure
Authors declare no conflict of interest.
References
- 1.Dinasarapu AR, Chandrasekhar A, Fujita T, Subramaniam S. Mannose/mannan-binding lectin. UCSD Mol Pages. 2013;2:8–18. [Google Scholar]
- 2.Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–131. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 3.Polotsky VY, Belisle JT, Mikusova K, Ezekowitz RA, Joiner KA. Interaction of human mannose-binding protein with Mycobacterium avium. J Infect Dis. 1997;175:1159–1168. doi: 10.1086/520354. [DOI] [PubMed] [Google Scholar]
- 4.Carroll MV, Lack N, Sim E, Krarup A, Sim RB. Multiple routes of complement activation by Mycobacterium bovis BCG. Mol Immunol. 2009;46:3367–3378. doi: 10.1016/j.molimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Thye T, Niemann S, Walter K, et al. Variant G57E of mannose binding lectin associated with protection against tuberculosis caused by Mycobacterium africanum but not by M. tuberculosis. PLOS ONE. 2011;6:e20908. doi: 10.1371/journal.pone.0020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teh C, Le Y, Lee SH, Lu J. M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-D-glucosamine and mediates monocyte adhesion and phagocytosis of E. coli. Immunology. 2000;101:225–232. doi: 10.1046/j.1365-2567.2000.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Endo Y, Iwaki D, et al. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J Immunol. 2005;175:3150–3156. doi: 10.4049/jimmunol.175.5.3150. [DOI] [PubMed] [Google Scholar]
- 8.Rorvig S, Honore C, Larsson LI, et al. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J Leukoc Biol. 2009;86:1439–1449. doi: 10.1189/jlb.1008606. [DOI] [PubMed] [Google Scholar]
- 9.Honore C, Rorvig S, Munthe-Fog L, et al. The innate pattern recognition molecule ficolin-1 is secreted by monocytes/macrophages and is circulating in human plasma. Mol Immunol. 2008;45:2782–2789. doi: 10.1016/j.molimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J Innate Immun. 2010;2:167–180. doi: 10.1159/000218324. [DOI] [PubMed] [Google Scholar]
- 11.Gout E, Garlatti V, Smith DF, et al. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J Biol Chem. 2010;285:6612–6622. doi: 10.1074/jbc.M109.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjaer TR, Hansen AG, Sorensen UB, Nielsen O, Thiel S, Jensenius JC. Investigations on the pattern recognition molecule M-ficolin: quantitative aspects of bacterial binding and leukocyte association. J Leukoc Biol. 2011;90:425–427. doi: 10.1189/jlb.0411201. [DOI] [PubMed] [Google Scholar]
- 13.Kjaer TR, Hansen AG, Sorensen UB, et al. M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain. Infect Immun. 2013;81:452–459. doi: 10.1128/IAI.01148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garlatti V, Belloy N, Martin L, et al. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–633. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick DC, Chalmers JD. Human L-ficolin (ficolin-2) and its clinical significance. J Biomed Biotechnol. 2012;2012:138797. doi: 10.1155/2012/138797. (doi: 10.1155/2012/138797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita M, Endo Y, Fujita T. Structural and functional overview of the lectin complement pathway: its molecular basis and physiological implication. Arch Immunol Ther Exp (Warsz) 2013;61:273–283. doi: 10.1007/s00005-013-0229-y. [DOI] [PubMed] [Google Scholar]
- 17.Luo F, Sun X, Wang Y, et al. Ficolin-2 defends against virulent Mycobacteria tuberculosis infection in vivo, and its insufficiency is associated with infection in humans. PLOS ONE. 2013;8:e73859. doi: 10.1371/journal.pone.0073859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugimoto R, Yae Y, Akaiwa M, et al. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J Biol Chem. 1998;273:20721–20727. doi: 10.1074/jbc.273.33.20721. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimura M, Ishida C, Sagara Y, et al. Detection of serum thermolabile β-2 macroglycoprotein (Hakata antigen) by enzyme-linked immunosorbent assay using polysaccharide produced by Aerococcus viridans. Clin Diagn Lab Immunol. 2001;8:454–459. doi: 10.1128/CDLI.8.2.454-459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swierzko A, Lukasiewicz J, Cedzynski M, et al. New functional ligands for ficolin-3 among lipopolysaccharides of Hafnia alvei. Glycobiology. 2012;22:267–280. doi: 10.1093/glycob/cwr119. [DOI] [PubMed] [Google Scholar]
- 21.Cestari Idos S, Krarup A, Sim RB, Inal JM, Ramirez MI. Role of early lectin pathway activation in the complement-mediated killing of Trypanosoma cruzi. Mol Immunol. 2009;47:426–437. doi: 10.1016/j.molimm.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Evans-Osses I, Ansa-Addo EA, Inal JM, Ramirez MI. Involvement of lectin pathway activation in the complement killing of Giardia intestinalis. Biochem Biophys Res Commun. 2010;395:382–386. doi: 10.1016/j.bbrc.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, White M, Vathipadiekal V, et al. Human H-ficolin inhibits replication of seasonal and pandemic influenza A viruses. J Immunol. 2012;189:2478–2487. doi: 10.4049/jimmunol.1103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dziadek J, Madiraju MV, Rutherford SA, Atkinson MA, Rajagopalan M. Physiological consequences associated with overproduction of Mycobacterium tuberculosis FtsZ in mycobacterial hosts. Microbiology. 2002;148:961–971. doi: 10.1099/00221287-148-4-961. [DOI] [PubMed] [Google Scholar]
- 26.Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–271. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 28.Ariki S, Kojima T, Gasa S, et al. Pulmonary collectins play distinct roles in host defense against Mycobacterium avium. J Immunol. 2011;187:2586–2594. doi: 10.4049/jimmunol.1100024. [DOI] [PubMed] [Google Scholar]
- 29.Denholm JT, McBryde ES, Eisen DP. Mannose-binding lectin and susceptibility to tuberculosis: a meta-analysis. Clin Exp Immunol. 2010;162:84–90. doi: 10.1111/j.1365-2249.2010.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Messias-Reason I, Kremsner PG, Kun JF. Functional haplotypes that produce normal ficolin-2 levels protect against clinical leprosy. J Infect Dis. 2009;199:801–804. doi: 10.1086/597070. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DF, Huang XQ, Wang D, Li YY, Yao YG. Genetic variants of complement genes ficolin-2, mannose-binding lectin and complement factor H are associated with leprosy in Han Chinese from Southwest China. Hum Genet. 2013;132:629–640. doi: 10.1007/s00439-013-1273-8. [DOI] [PubMed] [Google Scholar]
- 32.Boldt AB, Sanchez MI, Stahlke ER, et al. Susceptibility to leprosy is associated with M-ficolin polymorphisms. J Clin Immunol. 2013;33:209–210. doi: 10.1007/s10875-012-9770-4. [DOI] [PubMed] [Google Scholar]
- 33.Bonar A, Chmiela M, Rudnicka W, Rozalska B. Mannose-binding lectin enhances the attachment and phagocytosis of mycobacteria in vitro. Arch Immunol Ther Exp (Warsz) 2005;53:437–441. [PubMed] [Google Scholar]
- 34.Henriksen S, Brandt J, Andrieu JP, et al. Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J Immunol. 2013;191:6117–6127. doi: 10.4049/jimmunol.1302121. [DOI] [PubMed] [Google Scholar]