Abstract
Pre-eclampsia is one of the most serious disorders of human pregnancy and T helper type 1 (Th1)/Th2 imbalance plays a major role in its aetiology. The Th2 cytokine, interleukin (IL)-10, plays a significant role in the maintenance of pregnancy. The present study is aimed at understanding the role of IL-10 promoter polymorphisms (−1082 G/A; −592 A/C and −819 C/T) and their haplotypes in early-onset pre-eclampsia. A total of 120 patients and an equal number of women with normal pregnancy, from Government Maternity Hospital, Petlaburz, Hyderabad, India, were considered for the present study. A standard amplification refractory mutation system–polymerase chain reaction (ARMS–PCR) was carried out for genotyping followed by agarose gel electrophoresis. Appropriate statistical methods were applied to test for the significance of the results. It was found that the IL-10 −819 C allele (P = 0·003) and −592 A (P = 0·005) allele frequencies increased significantly in patients compared to controls. No significant difference was found with regard to −1082 promoter polymorphism. Haplotype analysis of the IL-10 single nucleotide polymorphisms (SNPs) revealed a significant association with ACC haplotype with a twofold increased risk in patients compared to controls. The frequencies of two common IL-10 haplotypes (GCC and ATA) did not show any significant difference. Further, the diplotype analysis revealed five genotypes: −1082A with −819C (P = 0·0016); −1082G with −819C (P = 0·0018); −819C with −592C (P = 0·001); −1082A with −592C (P = 0·032); and −1082G with −592C (P = 0·005) associated with the disease. These findings support the concept of contribution of IL-10 gene polymorphisms in the pathogenesis of early-onset pre-eclampsia.
Keywords: haplotype, interleukin-10, pre-eclampsia, reproductive immunology, Th1/Th2
Introduction
Pre-eclampsia is a common but complex disease which affects 2·5–3% of pregnancies occurring after 20 weeks of gestation, and leads to maternal and fetal mortality and morbidity, also causing perinatal death, preterm birth, intrauterine growth restriction (IUGR) and, in a few cases, intrauterine death (IUD) of the fetus. Pre-eclampsia symptoms might be revealed from 20 weeks of gestation up to 6 weeks postpartum and is considered early-onset before 34 weeks of gestation [1]. The maternal syndrome is driven by a dysfunctional uteroplacental circulation caused by shallow implantation of placenta leading to hypoxia and the release of proinflammatory trophoblast-derived factors [2], causing an excessive maternal inflammatory response with associated endothelial dysfunction resulting in hypertension and proteinuria [3]. Placental ischaemia caused by inadequate endometrial invasion of trophoblasts and endothelial damage is considered to play a crucial role in the pathogenesis [4,5]. Normal pregnancy is also associated with a vascular inflammatory response secondary to the presence of the placenta. It is not only of lower intensity but is characterized by a type 2 immune bias, in contrast to the type 1 bias of pre-eclampsia. Altered concentrations of various cytokines may also be involved in defective placental invasion and endothelial damage in pre-eclampsia. Despite intensive research, the aetiology and pathogenesis of pre-eclampsia were not completely understood. Wegmann et al. [6] proposed that successful pregnancy is T helper type 2 (Th2)-dependent, where the Th1 responses are inhibited for the survival of the fetus. Therefore, a Th1/Th2 balance is required for proper placentation. Th1 cells produce proinflammatory cytokines such as interleukin (IL)-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α and are involved in cell-mediated responses and delayed-type hypersensitivity reactions, whereas Th2 cells produce anti-inflammatory cytokines such as IL-4, IL-5, IL-10 and IL-13 and evoke humoral immunity [6]. Th1 and Th2 regulate each other's function reciprocally [7,8]. Among cytokines, IL-10 plays a key role in Th2 immunity, which is located on human chromosome 1 (1q31–1q32) [9,10]. Several single nucleotide polymorphisms (SNPs) were reported in the proximal (−1082A/G, −819T/C and −592A/C) and distal regions of the promoter region of the IL-10 gene [11] and were found to regulate the transcriptional rate of IL-10 [12,13]. It is a key regulator of the inflammatory process and has a pleiotropic activity, and also acts as an immunosuppressive cytokine, expressed throughout the pregnancy by epithelial cells and leucocytes in the endometrium and placenta [14]. Studies on circulating IL-10 levels report that higher IL-10 production is required for successful pregnancy and its maintenance [15]. Reduced IL-10 production in spontaneous abortions [16] and in pre-eclampsia (both circulating and placental) [17,18] has been reported recently, thereby highlighting the important role of IL-10 in successful normal pregnancy. Based on the potential importance of IL-10 in pregnancy, the purpose of this study was to investigate whether functional polymorphisms in the promoter region of IL-10 could predispose pregnant women to early-onset pre-eclampsia.
Materials and methods
Selection of cases and controls
A total of 120 pregnant women with early-onset pre-eclampsia and an equal number of age-matched women with normal pregnancy attending the gynaecological unit of Government Maternity Hospital, Hyderabad were considered for the present study during 2011–12. Women with no complications throughout their gestational period, such as infections, fetal anomalies, hypertension and diabetes, were considered as the control subjects. Information regarding the demographic features such as age, parity, gestational age, family history, consanguinity, etc. were obtained from all the subjects with the help of a standard structured questionnaire. The study was approved by the Institutional Ethical committee.
Criteria for patients
Inclusion criteria
A case was defined as follows: pre-eclampsia was diagnosed with minimum criteria of blood pressure >130/90 mm Hg on two occasions, 6 h apart, and onset of proteinuria >2 + by dipstick test in urine samples, and those who showed blood pressure >150/100 mm Hg and proteinuria >3 + by dipstick test in urine samples were considered to be patients with severe pre-eclampsia.
Exclusion criteria
Patients with a previous history of intrauterine fetal deaths and other complications were not considered for the study.
Criteria for controls
Inclusion criteria
The inclusion criteria were pregnant women with a gestational age of more than 20 weeks, normal blood pressure, normal fetal growth and with no other physiological abnormalities. Controls were selected randomly at the same time as the case selection. The controls were administered the same questionnaire.
Exclusion criteria
Pregnant women with heart problems, with previous history of eclampsia or blood pressure were not included, as per the normal standard, in the study.
Sample collection
Five ml of venous blood was collected from all subjects for biochemical and molecular analysis and aliquoted in plain and ethylenediamine tetraacetic acid (EDTA) vacutainers. Serum and plasma was separated after centrifugation at 189 g for 10 min. All samples were stored at −20°C for further analysis.
Determination of IL-10 polymorphisms
Genomic DNA was extracted from the samples using the salting-out method [19]. The isolated DNA was subjected to a standard amplification refractory mutation system–polymerase chain reaction (ARMS–PCR) [20]. The primer sequences for determining the polymorphism are given in Table 1. The optimized reaction conditions for the amplification were performed in 10 μl with a 25–50 ηg DNA sample, 20 mM Tris-HCl (pH 8·4), 50 mM KCl, 2 mM MgCl2, 0·2 mM each deoxynucleotide triphosphates (dNTPs), 2 μM each specific/common primers and 0·25 units of Taq DNA polymerase. The cycling conditions were as follows: an initial denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, 64·2°C for 50 s and 72°C for 1·5 min. The final extension step was at 72°C for 5 min. The PCR products were separated by electrophoresis on an agarose gel (2%) stained with ethidium bromide. The gel was visualized under ultraviolet light with a 100 base pairs (bp) ladder. All the collected samples were genotyped successfully. Results were cross-checked with internal positive [796 bp of human leucocyte antigen (HLA) gene] and negative controls (Millipore water). Ten per cent of the samples were taken randomly, and the assay was repeated. The findings were similar on replicative study, with 100% concordant results.
Table 1.
Interleukin-10 promoter primer sequences
| IL-10 | Primer sequence (5′– 3′) | Band length | |
|---|---|---|---|
| −1082 | Common reverse | GTA AGC TTC TGT GGC TGG AGT C | 161 base pairs (bp) |
| G- forward | AAC ACT ACT AAG GCT TCT TTG GGT G | ||
| A- forward | AAC ACT ACT AAG GCT TCT TTG GGT A | ||
| −819 | Common reverse | AGG ATG TGT TCC AGG CTC CT | 223 bp |
| C- forward | CCC TTG TACAGG TGA TGT AAC | ||
| T- forward | ACC CTT GTA CAG GTG ATG TAA T | ||
| −592 | Common reverse | CAA GCC CCT GAT GTG TAG A | 600 bp |
| C- forward | CTG TGA CCC CGC CTG TC | ||
| A- forward | CTG TGA CCC CGC CTG TA |
Statistical analysis
Allele and genotype frequencies were calculated on patient and control subjects. Findings were considered statistically significant at a P-value < 0·05. Analysis for deviations from Hardy–Weinberg equilibrium was performed based on the χ2 test. Statistical analysis for the differences between groups was determined by χ2 test using SNPstat [21]. The coefficient (D') of pairwise linkage disequilibrium (LD) between the SNPs was calculated using the software haploview version 4·2 [22].
Results
The demographic characteristics of patients and controls revealed a significant difference with respect to fetal growth (P = 0·02). However, there was no variation with regard to age, number of children, mode of delivery and oedema (Table 2). Fifty-nine per cent of the patients and 14% of the controls showed consanguinity. Such an observation might suggest the involvement of genetic factors in the aetiology of early-onset pre-eclampsia.
Table 2.
Demographic features of patients and controls
| PE cases (n = 120) | Controls (n = 120) | P-value | |
|---|---|---|---|
| Age (years) | 26·54 ± 4·603 | 24·73 ± 4·2 | 0·0017* |
| Gestational age (weeks) | 25·26 ± 3·84 | 24·38 ± 4·14 | 0·0891 |
| Primiparity n (%) | 63 (52·5) | 68 (56·6) | 0·6041 |
| SBP | 137·16 ± 9·63 | 116 ± 4·919 | <0·0001** |
| DBP | 91·16 ± 6·88 | 73 ± 4·78 | <0·0001** |
| Mode of delivery | |||
| Normal n (%) | 58 (48·3) | 69 (56·6) | |
| Caesarean n (%) | 62 (51·6) | 51 (42·5) | 0·1962 |
| Fetal growth | |||
| IUGR n (%) | 17 (14·1) | 7 (5·8) | |
| IUD n (%) | 37 (30·8) | 2 (1·6) | 0·02278* |
| Birth weight (kg) | 2·609 ± 0·426 | 3·23 ± 0·297 | <0·0001** |
P-value < 0·05;
P < 0·0001.
SBP = systolic blood pressure; DBP = diastolic blood pressure; IUGR = intrauterine growth restriction; IUD = intrauterine death; PE = pre-eclampsia.
Further, we analysed three bi-allelic IL-10 promoter polymorphisms at positions −1082 G/A, −819 T/C and −592 A/C. The data reported in Table 3 show allele and genotype distributions of different promoter polymorphisms of IL-10. The results indicate that the distribution of genotype and allele frequencies of IL-10 −1082 were not statistically different between the two groups, but the other two polymorphisms (−819 and −592) showed a significant difference between the two groups. The IL-10 −819 C [P-value = 0·0003, odds ratio (OR) = 1·96, 95% confidence interval (CI) = 1·37–2·829] allele is associated with pre-eclampsia patients. Analysis using different models also showed a significant distribution – co-dominant model: CC versus TT (P ≤ 0·0001, OR = 0·34, 95% CI = 0·174–0·67), dominant model: CC versus CT + TT (P ≤ 0·0001, OR = 0·29, 95% CI = 0·16–0·51), over-dominant model: CT versus CC + TT (P = 0·0014, OR = 0·42, 95% CI = 0·25–0·72) and recessive model: TT versus CT + CC (P = 0·25, OR = 0·71, 95% CI = 0·40–1·26). Further, the frequency of the IL-10 −592 A (P-value = 0·0058, OR = 1·693, 95% CI = 1·177–2·435) allele is increased significantly in patients compared to controls. There is a statistical difference in the distribution of genotypic frequencies when compared with different models – co-dominant model: AA versus CC (P = 0·0026; OR = 2·31, 95% CI = 1·15–4·97), dominant model: AA versus CA + CC (P = 6e–04, OR = 2·57, 95% CI = 1·48–4·46), over-dominant model: CA versus AA + CC (P = 0·13, OR = 1·93, 95% CI = 1·15–3·24) and recessive model: CC versus CA + AA (P = 0·36, OR = 1·32, 95% CI = 0·73–2·14).
Table 3.
Frequencies of interleukin (IL)-10 (−1082,−819,−592) genotypes and alleles in patients and control subjects
| Genotypes and alleles | Control group n (%) | Patient group n (%) | OR (95% CI) | P-value | P′ |
|---|---|---|---|---|---|
| IL-10 −1082 | |||||
| Co-dominant | |||||
| A/A | 53 (44·2%) | 41 (34·2%) | |||
| G/A | 43 (35·8%) | 49 (40·8%) | 1·47 (0·83–2·63) | 0·2430 | |
| G/G | 24 (20%) | 30 (25%) | 1·62 (0·82–3·17) | 0·27 | 0·44 |
| Dominant | |||||
| A/A | 53 (44·2%) | 41 (34·2%) | |||
| G/A + G/G | 67 (55·8%) | 79 (65·8%) | 1·52 (0·90–2·57) | 0·11 | |
| Recessive | |||||
| A/A + G/A | 96 (80%) | 90 (75%) | |||
| G/G | 24 (20%) | 30 (25%) | 1·33 (0·73–2·45) | 0·35 | |
| Over-dominant | |||||
| A/A + G/G | 77 (64·2%) | 71 (59·2%) | |||
| G/A | 43 (35·8%) | 49 (40·8%) | 1·24 (0·73–2·08) | 0·43 | |
| Alleles | |||||
| G | 149 | 131 | |||
| A | 93 | 109 | 0·734 (0·51–1·056) | 0·11 | |
| IL-10 −819 | |||||
| Co-dominant | |||||
| C/C | 25 (20·8%) | 57 (47·5%) | |||
| C/T | 58 (48·3%) | 34 (28·3%) | 0·26 (0·14–0·48) | 0·00003476** | |
| T/T | 37 (30·8%) | 29 (24·2%) | 0·34 (0·17–0·68) | <0·0001** | 0·00014** |
| Dominant | |||||
| C/C | 25 (20·8%) | 57 (47·5%) | |||
| C/T + T/T | 95 (79·2%) | 63 (52·5%) | 0·29 (0·16–0·51) | <0·0001** | |
| Recessive | |||||
| C/C + C/T | 83 (69·2%) | 91 (75·8%) | |||
| T/T | 37 (30·8%) | 29 (24·2%) | 0·71 (0·40–1·26) | 0·25 | |
| Over-dominant | |||||
| C/C + T/T | 62 (51·7%) | 86 (71·7%) | |||
| C/T | 58 (48·3%) | 34 (28·3%) | 0·42 (0·25–0·72) | 0·0014** | |
| Alleles | |||||
| C | 148 | 108 | |||
| T | 92 | 132 | 1·96 (1·37–2·829) | 0·0003** | |
| IL-10 −592 | |||||
| Co-dominant | |||||
| A/A | 54 (45%) | 29 (24·2%) | |||
| C/A | 41 (34·2%) | 60 (50%) | 2·72 (1·49–4·97) | 0·001598** | |
| C/C | 25 (20·8%) | 31 (25·8%) | 2·31 (1·15–4·62) | 0·0026** | 0·057* |
| Dominant | |||||
| A/A | 66 (55%) | 91 (75·8%) | |||
| C/A + C/C | 95 (79·2%) | 89 (74·2%) | 2·57 (1·48–4·46) | 0·00006** | |
| Recessive | |||||
| A/A + C/A | 95 (79·2%) | 89 (74·2%) | |||
| C/C | 25 (20·8%) | 31 (25·8%) | 1·32 (0·73–2·41) | 0·36 | |
| Over-dominant | |||||
| A/A + C/C | 79 (65·8%) | 60 (50%) | |||
| C/A | 41 (34·2%) | 60 (50%) | 1·93 (1·15–3·24) | 0·013* | |
| Alleles | |||||
| A | 149 | 118 | |||
| C | 91 | 122 | 1·693 (1·177–2·435) | 0·0058** |
P value < 0·05;
P value < 0·01; P′ after Bonferroni correction.
OR = odds ratio; CI = confidence interval.
Combinations of the three polymorphisms in the population allowed the observation of eight haplotypes (Table 4). Haplotype ACC is more prevalent in patients than in controls (P = 0·012, OR = 2·87, 95% CI = 1·27–6·49) and was associated significantly with pre-eclampsia. There was no significant change in OR value in any other haplotype when the two study groups were compared. Further, the bi-allelic analysis (Table 5) revealed a significant association of −1082A with −819C (OR = 2·33, P = 0·0016); −1082G with −819C (OR = 2·12, P = 0·0018); −819C with −592C (OR = 2·96, P = 0·001); −1082A with −592C (OR = 1·7, P = 0·032); and −1082G with −592C (OR = 2·25, P = 0·005) with pre-eclampsia. As three outcome measures were tested against eight hypothesized predictors, a Bonferroni-adjusted significance level was calculated to account for the increased possibility of a type I error, obtaining a value of P′ = 0·0119* for the haplotype.
Table 4.
Interleukin-10 haplotype distributions in patients with pre-eclampsia and controls
| S.No | −1082 | −819 | −592 | Controls | Patients | OR (95% CI) | P-value | P′ |
|---|---|---|---|---|---|---|---|---|
| 1 | G | C | A | 0·1714 | 0·18 | 1·00 | – | |
| 2 | A | T | A | 0·2063 | 0·1209 | 0·66 (0·35–1·27) | 0·22 | 0·204 |
| 3 | A | C | C | 0·0776 | 0·2136 | 2·87 (1·27–6·49) | 0·012* | 0·0119* |
| 4 | A | C | A | 0·1648 | 0·1194 | 0·67 (0·30–1·48) | 0·32 | 0·287 |
| 5 | A | T | C | 0·1779 | 0·092 | 0·53 (0·25–1·13) | 0·1 | 0·096 |
| 6 | G | T | C | 0·0875 | 0·0991 | 1·31 (0·57–3·05) | 0·53 | 0·441 |
| 7 | G | T | A | 0·0783 | 0·0714 | 0·94 (0·30–2·90) | 0·91 | 0·662 |
| 8 | G | C | C | 0·0362 | 0·1037 | 2·24 (0·64–7·81) | – |
P-value < 0·05; P′ after Bonferroni correction. OR = odds ratio; CI = confidence interval.
Table 5.
Diplotype analysis in patients and control subjects
| Locus1 | Locus 2 | OR | CI (95%) | P-value | P′ |
|---|---|---|---|---|---|
| −1082A | −819C | 2·33 | (1·39–3·90) | 0·0016** | 0·016* |
| −1082G | −819C | 2·12 | (1·33–3·37) | 0·0018** | 0·031* |
| −1082A | −592C | 1·70 | (1·05–2·77) | 0·032* | 0·032** |
| −1082G | −592C | 2·25 | (1·28–3·95) | 0·005** | 0·005** |
| −819C | −592C | 2·96 | (1·56–5·63) | 0·001** | 0·001** |
| −1082G | −592A | 1.44 | (0·87–2·39) | 0·16 | 0·153 |
| −819T | −592A | 0·87 | (0·50–1·51) | 0·63 | 0·531 |
| −819T | −592C | 0·90 | (0·56–1·44) | 0·66 | 0·552 |
P-value < 0·05;
P-value < 0·01; P′ after Bonferroni correction.
OR = odds ratio; CI = confidence interval.
Linkage disequilibrium analysis
LD analysis, defined by the delta coefficient (D′), was determined for both patients and controls for the three SNPs, IL-10 C−592A, C −819T and G −1082A. No linkage disequilibrium was observed between the three SNP polymorphisms (Fig. 1).
Fig. 1.
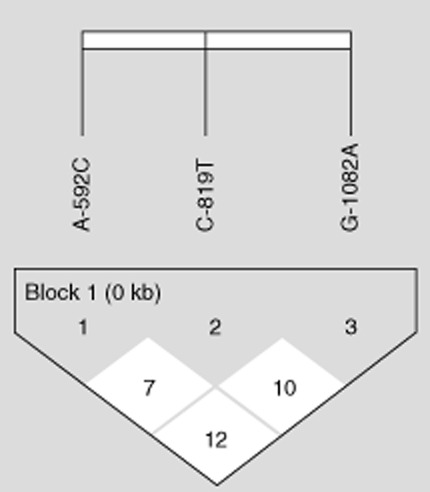
Linkage disequilibrium pattern of the genomic region in chromosome 1 located between single nucleotide polymorphisms (SNP) C−592A, C−819T and G−1082A.
Discussion
It is a widely accepted theory that deviation towards a Th2 response is important for a successful and normal pregnancy [23], and pre-eclampsia is believed to be caused by a Th1 shift. IL-10, an important cytokine for successful pregnancy, has been identified to have an inhibitory effect on Th1-type immune responses [14]; therefore, many researchers believe that low IL-10 is associated with pre-eclampsia. The transcriptional, phenotypic and functional characteristics of a gene are under the influence of promoter polymorphisms [24,25]. In the IL-10 gene promoter, polymorphic changes at three well-characterized sites, −1082, −819, and −592, are thought to contribute to dysregulated IL-10 production and to the onset and severity of pre-eclampsia. In addition, experiments on the level of IL-10 secretion showed that it is directly proportional to the level of IL-10 mRNA synthesized, and also that the IL-10 mRNA half-life from low IL-10 secretors was equal to that from high IL-10 secretors. Thus, differential secretion was likely to have its origins in differing rates of IL-10 mRNA synthesis which, in turn, might reasonably be ascribed to differences in the structure of the IL-10 promoter [26]. In the studies conducted by Crilly et al. [23] and Yilmaz et al. [28], in view of the differential effects of the IL-10 SNPs in regulating IL-10 mRNA expression and its protein secretion, considering ethnic variations, the effect of polymorphism on expression suggests an indirect role of IL-10 in down-regulating the expression of proinflammatory Th1 cytokines [27–29]. Therefore, this gene might conceivably be a candidate susceptibility gene in pre-eclampsia. In this respect, de Groot et al. [30] reported no significant difference between the polymorphisms in the IL-10 promoter region at positions −1082, −819 and −592 between pre-eclampsia patients and controls. Studies on individual promoter polymorphisms of IL-10 have demonstrated that there is a considerable change in the level of production [31]. Our study suggests no significance with respect to −1082 allelic and genotypic distributions between the two groups. Our observation is in concordance with the study by Stonek et al. [32] in an Austrian population, which revealed no association with the gene. A study by Rees et al. [29] on patients with pre-eclampsia, considering the influence of the promoter polymorphism on the level of production of IL-10, indicated that the −1082A allele confers a twofold increase in transcriptional activity of the IL-10 promoter compared to the G allele. Further, the other two polymorphisms, −819C (P = 0·0003) (Sowmya et al. [33]) and −592A (P = 0·0101) alleles showed a significant association with the disease. This observation is in concordance with the study by Zhang et al. [34], who reported that the C allele of −819 is associated with high production of IL-10. Studies by Lokossou et al. [35] and Temple et al. [35,36] suggested that the IL-10 −592 promoter carrying allele A is associated with low production of IL-10. In contrast, studies correlating promoter polymorphisms with the circulating levels and placental levels of IL-10 have shown no association. Makris et al. [37] has reported in an Australian population that the genotype of IL-10 promoter may not play a significant role in the circulating IL-10 levels, but has an effect on the placental levels of IL-10, suggesting that IL-10 plays an important role in proper placentation.
Our study showed no LD between the three promoter polymorphisms of IL-10 in both disease and controls. In contrast, previous studies documented strong LD between the −1082A/G, −819T/C and −592A/C SNPs [11] with only three haplotypes (ACC, ATA and GCC). Observations by Wilson et al. [38] in GCC, ACC and ATA individuals produced high, intermediate and low circulating IL-10 levels, respectively. In the present study, IL-10 haplotype distribution demonstrated an increased prevalence of the ACC haplotype (OR = 2·87, P = 0·012) among the patients. In contrast, a study by Kamali-Sarvestani et al. [39] showed an association with the GCC haplotype. Diplotype analysis also revealed an association with the allelic variants of IL-10, which are associated with high production of IL-10 (−1082A/−819C; −1082A/−592C and −819C/−592C). IL-10 not only plays an important role in maintaining the pregnancy but is necessary to protect the allogeneic fetal cells from rejection [40]. Proteinase activity is required for cytotrophoblast invasion of the uterine wall during placentation, and studies suggest that IL-10 has a role in regulating the expression of the serine proteinases [41] and matrix metalloproteinases [42] in a variety of cells. Roth and Fisher [43] showed that IL-10 is down-regulated as the invasion proceeds, thus permitting the production of matrix metallopeptidase 9 (MMP-9). However, if invading cytotrophoblasts encounter an immunologically hostile environment IL-10 production could be up-regulated, serving two important functions: high cytokine levels will suppress a harmful immune reaction, and invasion will be restricted to minimize the number of cytotrophoblasts in contact with maternal leucocytes. Therefore, observations suggest that IL-10 levels are elevated markedly in severe pre-eclampsia.
Finally, cytotrophoblast invasion of the uterine wall is one of the critical first steps for successful pregnancy. In addition to MMP-9 for normal pregnancy, cytotrophoblast expression of adhesion molecules, including integrins, cadherins and immunoglobulin (Ig) superfamily members, is modulated [44,45]. In pre-eclampsia, adhesion molecule expression is deregulated, leading to a shallow invasion of cytotrophoblasts [46]. Thus, the increased levels of IL-10 lead to improper implantation, suggesting that IL-10 is a major regulator in the maintenance of pregnancy.
Conclusion
In conclusion, our study revealed a significant association of the ACC haplotype in early-onset pre-eclampsia, which is associated with high production of IL-10. However, there is no strong LD between the three promoter polymorphisms in both patients and controls. Thus, the interesting possibility exists that elevated IL-10 may affect the production of these important molecules, thereby resulting in pre-eclampsia.
Disclosure
There are no conflicts of interest.
References
- 1.Salimi S, Farajian-Mashhadi F, Naghavi A, et al. Different profile of serum leptin between early onset and late onset preeclampsia. Dis Markers. 2014;2014:1–7. doi: 10.1155/2014/628476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:592–594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Borzychowski AM, Sargent IL, Redman CWG. Inflammation and preeclampsia. Semin Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Gammill HS. Preeclampsia. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 5.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Sad S. The expanding universe of T-cell subsets. Immunol Today. 1996;7:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 7.Liberman AC, Refojo D, Arzt E. Cytokine signaling/transcription factor cross-talk in T cell activation and Th1–Th2 differentiation. Arch Immunol Ther Exp (Warsz) 2003;51:351–365. [PubMed] [Google Scholar]
- 8.Matsuzaki J, Tsuji T, Imazeki I, Ikeda H, Nishimura T. Immunosteroid as a regulator for Th1/Th2 balance: its possible role in autoimmune diseases. Autoimmunity. 2005;38:369–375. doi: 10.1080/08916930500124122. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Brannan CI, Copeland NG. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- 10.Eskdale J, Kube D, Tesch H. Mapping of the human IL10 gene and further characterization of the 5′ flanking sequence. Immunogenetics. 1997;46:120–128. doi: 10.1007/s002510050250. [DOI] [PubMed] [Google Scholar]
- 11.D'Alfonso S, Rampi M, Rolando V, Giordano M, Momigliano-Richiardi P. New polymorphisms in the IL-10 promoter region. Genes Immun. 2000;1:231–233. doi: 10.1038/sj.gene.6363666. [DOI] [PubMed] [Google Scholar]
- 12.Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, Huizinga TW. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA. 1998;95:9465–9470. doi: 10.1073/pnas.95.16.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mormann M, Rieth H, Hua TD, et al. Mosaics of gene variations in the interleukin-10 gene promoter affect interleukin-10 production depending on the stimulation used. Genes Immun. 2004;5:246–255. doi: 10.1038/sj.gene.6364073. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 15.Marzi M, Vigano D, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makhseed M, Raghupathy R, Azizieh F, Farhat R, Hassan N, Bandar A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Hum Reprod. 2000;15:2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- 17.Wu MY, Chen HF, Chen SU, Chao KH, Yang YS, Ho HN. Increase in production of interleukin-10 early after implantation is related to the success of pregnancy. Am J Reprod Immunol. 2001;46:386–392. doi: 10.1034/j.1600-0897.2001.d01-29.x. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 19.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrey C, Turner SJ, Pravica V, Howell WM, Hutchinson IV. ARMS–PCR methodologies to determine IL-10, TNF-alpha, TNF-beta and TGF-beta 1 gene polymorphisms. Transpl Immunol. 1999;7:127–128. doi: 10.1016/s0966-3274(99)80030-6. [DOI] [PubMed] [Google Scholar]
- 21.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 24.Howell WM, Rose-Zerilli MJ. Cytokine gene polymorphisms, cancer susceptibility, and prognosis. J Nutr. 2007;137:194–199. doi: 10.1093/jn/137.1.194S. [DOI] [PubMed] [Google Scholar]
- 25.Schneider BG, Camargo MC, Ryckman KK, et al. Cytokine polymorphisms and gastric cancer risk: an evolving view. Cancer Biol Ther. 2008;7:157–162. doi: 10.4161/cbt.7.2.5270. [DOI] [PubMed] [Google Scholar]
- 26.Westendorp RGJ, Langermans JAM, Huizinga TWJ, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 27.Crilly A, Hamilton J, Clark CJ, Jardine A, Madhok R. Analysis of the 5′ flanking region of the interleukin 10 gene in patients with systemic sclerosis. Rheumatology (Oxf) 2003;42:1295–1298. doi: 10.1093/rheumatology/keg420. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz V, Yentur SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30:188–194. doi: 10.1016/j.cyto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Rees LE, Wood NA, Gillespie KM, Lai KN, Gaston K, Mathieson PW. The interleukin-10-1082 G/A polymorphism: allele frequency in different populations and functional significance. Cell Mol Life Sci. 2002;59:560–569. doi: 10.1007/s00018-002-8448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Groot CJ, Jansen MW, Bertina RM, Schonkeren JJ, Helmerhorst FM, Huizinga TW. Interleukin 10-2849 AA genotype protects against pre-eclampsia. Genes Immun. 2004;5:313–314. doi: 10.1038/sj.gene.6364092. [DOI] [PubMed] [Google Scholar]
- 31.Suarez A, Castro P, Alonso R, Mozo L, Gutierrez C. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation. 2003;75:711–717. doi: 10.1097/01.TP.0000055216.19866.9A. [DOI] [PubMed] [Google Scholar]
- 32.Stonek F, Hafner E, Metzenbauer M, et al. Absence of an association of tumor necrosis factor (TNF)-alpha G308A, interleukin-6 (IL-6) G174C and interleukin- 10 (IL-10) G1082A polymorphism in women with preeclampsia. J Reprod Immunol. 2008;77:85–90. doi: 10.1016/j.jri.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Sowmya S, Ramaiah A, Sunitha T, Nallari P, Jyothy A, Venkateshwari A. Role of IL-10 -819(t/c) promoter polymorphism in preeclampsia. Inflammation. 2014;37:1022–1027. doi: 10.1007/s10753-014-9824-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Hei P, Deng L, Lin J. Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol Hum Reprod. 2007;13:135–140. doi: 10.1093/molehr/gal106. [DOI] [PubMed] [Google Scholar]
- 35.Lokossou AG, Dechavanne C, Bouraïma A, et al. Association of IL-4 and IL-10 maternal haplotypes with immune responses to P. falciparum in mothers and newborns. BMC Infect Dis. 2013;13:215. doi: 10.1186/1471-2334-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Temple SE, Lim E, Cheong KY, et al. Alleles carried at positions –819 and –592 of the IL10 promoter affect transcription following stimulation of peripheral blood cells with Streptococcus pneumoniae. Immunogenetics. 2003;55:629–632. doi: 10.1007/s00251-003-0621-6. [DOI] [PubMed] [Google Scholar]
- 37.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta. 2006;27:445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JN, Rockett K, Jallow M, et al. Analysis of IL-10 haplotypic associations with severe malaria. Genes Immun. 2005;6:462–466. doi: 10.1038/sj.gene.6364227. [DOI] [PubMed] [Google Scholar]
- 39.Kamali-Sarvestani E, Kiany S, Gharesi-Fard B, Robati M. Association study of IL-10 and IFNgamma gene polymorphisms in Iranian women with preeclampsia. J Reprod Immunol. 2006;72:118–126. doi: 10.1016/j.jri.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem. 1992;267:8473–8477. [PubMed] [Google Scholar]
- 42.Mertz PM, DeWitt DL, Stetler SW, Wahl LM. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production. J Biol Chem. 1994;269:21322–21329. [PubMed] [Google Scholar]
- 43.Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol. 1999;205:194–204. doi: 10.1006/dbio.1998.9122. [DOI] [PubMed] [Google Scholar]
- 44.Damsky C, Sutherland A, Fisher S. Extracellular matrix 5: adhesive interactions in early mammalian embryogenesis, implantation, and placentation. FASEB J. 1993;7:1320–1329. doi: 10.1096/fasebj.7.14.8224605. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype – one cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]


