Abstract
Sarcoidosis is a granulomatous disorder of unknown aetiology. The presence of Mycobacterium tuberculosis catalase-peroxidase (mKatG) in sarcoidosis tissue has been reported. T helper type 1 (Th1) responses against mKatG have previously been observed. However, little is known about interleukin (IL)-17 and Th17 responses in sarcoidosis. Here, we investigated the levels of IL-17 and frequencies of IL-17-producing cells responding to mKatG in sarcoidosis patients with different prognosis. Peripheral blood and bronchoalveolar lavage (BAL) cells were obtained from sarcoidosis patients with or without Löfgren's syndrome (often associated with spontaneous recovery), and also stratified according to human leucocyte antigen (HLA) type. Cells producing IL-17 and interferon (IFN)-γ after stimulation with mKatG were enumerated by enzyme-linked immunospot (ELISPOT). The level of IL-17 in the BAL fluid of sarcoidosis patients and healthy controls was measured by quantitative immuno-polymerase chain reaction (qIPCR). We also performed flow cytometry and immunohistochemistry for further characterization of IL-17 expression. Patients with Löfgren's syndrome had a higher frequency of IL-17-producing cells responding to mKatG in BAL fluid compared to patients without Löfgren's syndrome (P < 0·05). The HLA-DR3+ sarcoidosis patients with Löfgren's syndrome (known to have a particularly good prognosis) also had a clearly higher level of IL-17 in BAL fluid compared to healthy controls and sarcoidosis patients without Löfgren's syndrome (P < 0·01) and (P < 0·05), respectively. No such difference between patient groups was observed with regard to IFN-γ and not with regard to either cytokine in peripheral blood. These findings suggest that IL-17-producing cells may be a useful biomarker for the prognosis of sarcoidosis and play a role in the spontaneous recovery typical of patients with Löfgren's syndrome.
Keywords: cytokines, IL-17, inflammation, sarcoidosis, T cells
Introduction
Sarcoidosis is a systemic inflammatory disorder. It is characterized by granuloma that most commonly affect the lungs. The pathogenesis of sarcoidosis includes the accumulation of lymphocytes and macrophages in the alveoli, thus involving innate as well as adaptive immune mechanisms [1,2]. It is known that T cells, and in particular CD4+ T cells, are activated in the lower airways of sarcoidosis patients and sarcoidosis is currently considered to be a T helper type 1 (Th1)-mediated disease [1].
Sarcoidosis patients display phenotypic heterogeneity. One group of sarcoidosis patients, having what is known as Löfgren's syndrome, displays an acute onset. Patients in this group have erythema nodosum and/or bilateral ankle arthritis, with bilateral hilar lymphadenopathy but with good prognosis [3,4]. Spontaneous recovery within 2 years is particularly high in Löfgren's syndrome patients who also express the human leucocyte antigen (HLA) allele DRB1*0301 (DR3) [4], and these patients virtually always have accumulations of CD4+ T cells with a particular T cell receptor (TCR) alpha chain variable gene segment (AV2S3) (TCR-AV2S3) in the lungs [5].
The aetiology of sarcoidosis is still not known. However, associations with environmental factors, genetic susceptibility and bacterial triggers have been reported [1]. The presence of mycobacterial DNA in the lungs of sarcoidosis patients has been shown [6] and a specific foreign protein, Mycobacterium tuberculosis catalase-peroxidase (mKatG), has been identified in sarcoidosis tissue [7]. Th1 responses against mKatG and purified protein derivative (PPD) of M. tuberculosis have been observed in bronchoalveolar lavage (BAL) and blood cells from patients [8].
Th17 cells were identified as a distinct subset of T cells less than a decade ago [9]. The hallmark of this subset of T cells is production of interleukin (IL)-17 (synonymous with IL-17A), a pleiotropic cytokine with widespread effects. IL-17 is emerging as an essential player in host defence in several mammalian organs, including the lungs of humans [10,11]. IL-17 and IL-17-producing cells are thought to play a key role in chronic inflammation such as mycobacterial infection, autoimmune disorders and also granuloma formation [12,13].
The involvement of IL-17 and IL-17-producing T cells in sarcoidosis was indicated recently [14,15]. In the current study, we characterized the presence and level of IL-17 and antigen-specific IL-17-producing cell responses to the mycobacterial antigen mKatG in patients with sarcoidosis. In particular, we compared the expression of IL-17 in sarcoidosis patients with or without Löfgren's syndrome, with consideration of HLA types HLA-DR3+ and HLA-DR3–. We hypothesize that differences in IL-17 responses are associated with different clinical outcomes.
Materials and methods
Additional materials and methods details are available in the Supporting information (Appendix S1).
Study subjects and characterization
Sarcoidosis patients referred to the Respiratory Medicine Unit (Karolinska University Hospital, Solna, Stockholm, Sweden) for primary diagnostic investigation were enrolled into this study. The diagnosis was established using the criteria from the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) [16]. Patient characteristics, including results of pulmonary function tests and BAL cell differentials, are given in Table 1. In our study patients were divided into two groups: Löfgren's syndrome patients and those without Löfgren's syndrome. Patients were also categorized as being HLA allele DRB1*0301 (DR3)-positive or DR3-negative. All DR3+ patients except one had lung accumulated CD4+ TCR AV2S3+ T cells, in line with previous studies [17]. Of 61 unique sarcoidosis-enrolled patients, four were treated with oral corticosteroids [one enrolled in an enzyme-linked immunospot assay (ELISPOT) and three enrolled in quantitative immuno-PCR (qIPCR) experiments; three had Löfgren's syndrome and one without Löfgren's syndrome]. BAL and blood samples from healthy control subjects were obtained for some experiments. Informed, written consent was obtained from all subjects and the study was approved by the Regional Ethical Review Board (Stockholm, Sweden).
Table 1.
Clinical and bronchoalveolar lavage (BAL) fluid characteristics of patients included in the study. Data are shown as median (min–max)
| qIPCR | ELISPOT | Flow cytometry | ||||||
|---|---|---|---|---|---|---|---|---|
| Löfgren patients DR3+ | Löfgren patients DR3– | Non-Löfgren patients | Healthy controls | Löfgren patients | Non-Löfgren patients | Löfgren patients | Non-Löfgren patients | |
| Patients' characteristics | ||||||||
| Number of individuals | 12 | 11 | 13 | 12 | 15 | 13 | 3 | 2† |
| Sex (male/female) | 9/3 | 7/4 | 12/1 | 7/5 | 10/5 | 11/2 | 1/2 | 2/0 |
| Age (years) | 33 (30–55) | 39 (30–57) | 51 (32–69) | 24 (21–35) | 39 (30–55) | 47 (32/66) | 35 (30–48) | 47 (42–52) |
| Smoking (yes/ex/never) | 0/6/6 | 1/4/6 | 1/4/8 | 0/0/12 | 2/6/5/2 | 2/2/9 | 2/0/1 | 0/0/2 |
| Chest X-ray stage (0/I/II/III/IV/n.d.)‡ | 0/4/5/0/0/3 | 0/5/3/0/0/3 | 0/3/5/0/1/4 | _ | 0/9/5/0/0/1 | 0/5/6/0/1/1 | 0/1/1/0/0/1 | 0/0/1/0/0/1 |
| HLA (DR3+/DR3–/n.d.) | 12/0 | 0/10/1 | 2/8/3 | 1/10/1 | 8/7 | 3/8/2 | 2/1 | 0/1/1 |
| Pulmonary function tests§ | ||||||||
| VC (% of predicted) | 90 (79–123) | 87 (57–101) | 75 (66–115) | – | 90 (79–117) | 83·5 (66–107) | 96 (89–103) | 67·5 (62–78) |
| FEV1 (% of predicted) | 92** (66–111) | 93** (73–102) | 87*** (53–107) | 114 (86–130) | 90 (66–111) | 90 (53–107) | 94 (89–99) | 63 (60–66) |
| DLCO (% of predicted) | 93·5 (58–139) | 93 (75–112) | 88 (61–110) | – | 97 (58–139) | 80 (61–110) | 85·5 (84–87) | 76·5 (60–93) |
| BAL differential cell counts | ||||||||
| % Macrophages | 71·9* (38·6–94) | 76 (42·6–95·4) | 66* (39·7–84·6) | 86·7 (66·2–97·2) | 72·2 (38·6–95·4) | 59 (39·7–84·6) | 77·2 (60–84·6) | 82·4 (78·2–86·6) |
| % Lymphocytes | 25·4* (5·8–59·8) | 22 (4–56·4) | 27·6* (14·2–58·2) | 10·9 (2·4–28·4) | 24·6 (4–59·8) | 29·6 (14·6–56·7) | 21·4 (13·2–39·6) | 16·3 (12·4–20·2) |
| % Neutrophils | 1 (0–2·6) | 1 (0·2–4) | 1 (0–19·5) | 1·5 (0·2–5) | 1 (0·4–5·8) | 1·7 (0·6–19·5) | 1·2 (0·2–1·4) | 1·2 (0·8–1·6) |
| % Eosinophils | 0·2 (0–0·8) | 0·2 (0–1·6) | 0·6 (0–2·9) | 0·2 (0–1·4) | 0·2 (0–1·4) | 0·4 (0–2·9) | 0·2 (0·2–0·8) | 0 |
| T cell markers | ||||||||
| CD4/CD8 ratio | 10·95*** (0·9–26) | 8* (1·4–17) | 5·1* (2–21) | 1·7 (0·9–5·2) | 7·3 (1·9–26·4) | 9·6 (2–25) | 4·8 (4–8·6) | 6·6 (3·6–9·6) |
| %TCR AV2S3+ CD4 cells | 34·5**** (15–47) | 2·9 (1·6–9·9) | 3·4 (1·8–10) | 3·7 (1·6–5·6) | 16 (1·6–50–4) | 6·2 (1·7–22) | 11 (7·5–35) | 3·8 (3·2–4·5) |
P < 0·05 versus healthy controls.
P < 0·01 versus healthy controls.
P < 0·001 versus healthy controls.
P < 0·001 versus all three groups.
In this group we have two BAL and two blood samples from three different individuals.
Stage 0 = normal; stage I = bilateral hilar lymphoma (BHL); stage II = BHL with parenchymal infiltrates; stage III = parenchymal infiltrates; stage IV = fibrotic changes and volume reduction.
DLCO = diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 s; VC = vital capacity; qIPCR = quantitative immuno-polymerase chain reaction; HLA = human leucocyte antigen; ELISPOT = enzyme-linked immunospot assay; n.d. = not done.
Sample preparation
BAL was performed as described previously [18]. BAL cells were prepared and differential counts were determined for each sample. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized peripheral blood.
ELISPOT
ELISPOT for detection of interferon (IFN)-γ and IL-17 following mKatG stimulation was performed on BAL and PBMC cells from 28 sarcoidosis patients and PBMCs of 15 healthy controls.
qIPCR
Due to the low absolute concentration of extracellular IL-17 protein in BAL fluid, the level (i.e. concentration) of soluble IL-17 protein was measured in the cell-free BAL fluid of 12 healthy controls, 13 sarcoidosis patients without Löfgren's syndrome, 12 DR3+ and 11 DR3– sarcoidosis patients with Löfgren's syndrome by qIPCR.
Intracellular cytokine staining and flow cytometry
Intracellular staining of IFN-γ and IL-17 was performed on BAL and whole blood samples from three sarcoidosis patients with Löfgren's syndrome and two patients without Löfgren's syndrome. Antigen stimulation was performed with PPD from M. tuberculosis (Statens Serum Institut, Copenhagen, Denmark). Stimulation with the combination of Staphylococcus enterotoxin A (SEA) and S. enterotoxin B (SEB) (both from Sigma-Aldrich, Schnelldorf, Germany) was also used for detection of IFN-γ- and IL-17-producing cells.
Immunohistochemistry
For visualization of IL-17-producing cells in lung tissue of sarcoidosis patients, immunohistochemistry was performed on contiguous 4-μm thick transbronchial paraffin-embedded sections from four sarcoidosis patients with Löfgren's syndrome, four sarcoidosis patients without Löfgren's syndrome and two healthy controls.
Statistical analysis
Statistical analysis of the data was performed by GraphPad Prism version 5·02 (GraphPad Software Inc., San Diego, CA, USA). The Mann–Whitney U-test or unpaired t-test was used for comparisons between two groups and Wilcoxon's signed-rank test was used for comparisons of dependent samples. A one-way analysis of variance (anova) test followed by Dunn's or Tukey's post-test was performed for data analysis of more than two groups. Correlation analysis was performed by Spearman's rank correlation test. A P-value of less than 0·05 was considered to define statistical significance.
Results
Stronger IL-17 responses specific to mKatG in BAL cells of Löfgren's patients
BAL cells from sarcoidosis patients with and without Löfgren's syndrome showed significant IFN-γ and IL-17 responses to mKatG compared with cell culture medium alone, as determined by ELISPOT (Supporting information, Fig. S1). Löfgren's syndrome patients had a significantly higher frequency of IL-17-producing cells in BAL following mKatG stimulation compared to non-Löfgren's syndrome patients after background deduction (P < 0·05) (Fig. 1a). Comparing the two groups of patients after stimulation with anti-CD3 antibody did not show any significant differences with regard to the IL-17 production. No such differences between patient groups were observed with regard to IFN-γ after stimulation with mKatG or phytohaemagglutinin (PHA) as a positive control (Fig. 1a).
Fig. 1.
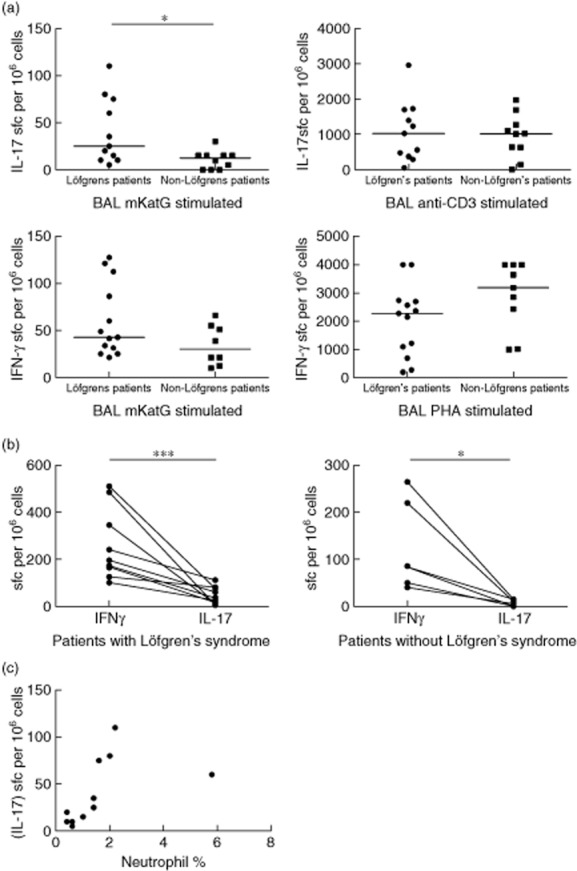
Enzyme-linked immunospot assay assay for detection of interleukin (IL)-17- and interferon (IFN)-γ-producing cells in bronchoalveolar lavage (BAL) samples. (a) IL-17- and IFN-γ-producing cells in bronchoalveolar lavage (BAL) samples from patients with and without Löfgren's syndrome after stimulation with Mycobacterium tuberculosis catalase-peroxidase (mKatG), PHA or anti-CD3. (b) Paired analysis of IL-17- and IFN-γ-producing cells reacting to mKatG in BAL samples from patients with or without Löfgren's syndrome. (c) Correlation of neutrophil percentage and number of mKatG-reactive IL-17-producing cells in BAL fluid of sarcoidosis patients with Löfgren's syndrome. Graphs show mean numbers of spots per 106 cells per well after deduction of background. The correlations were analysed using Spearman's rank test (r = 0·84; P < 0·01; *P < 0·05; ***P < 0·001).
In response to mKatG, IL-17 was produced by fewer BAL cells than IFN-γ in both Löfgren's and non-Löfgren's patients (Fig. 1b). However, when we calculated the ratio of IL-17- to IFN-γ- producing cells in response to mKatG, we found the median IL-17/IFN-γ ratio to be twice as high in Löfgren's syndrome patients, although this difference did not reach statistical significance (Supporting information, Fig. S2). Associating mKatG-specific IL-17 responses with BAL cell differential counts, we detected a strong and statistically significant correlation between the neutrophil percentage and IL-17-producing mKatG reactive cells in the BAL fluid of Löfgren's syndrome patients (r = 0·84, P < 0·01) (Fig. 1c). There were no correlations between other BAL cell differential results either in IL-17-producing mKatG-reactive cells or in soluble IL-17 in BAL fluid.
Löfgren's and non-Löfgren's patients have similar T cell reactivity to mKatG in peripheral blood
ELISPOT assays for detection of IFN-γ- and IL-17-producing cells in response to mKatG were also carried out on PBMCs from sarcoidosis patients and healthy controls. We divided our patients into those with and those without Löfgren's syndrome. Healthy controls were considered as PPD+ and PPD– according to the PPD skin test. Both groups of patients had blood T cell reactivity to mKatG by IL-17 and IFN-γ responses significantly higher than media control (P < 0·001 and P < 0·01) (Supporting information, Fig. S3a and S3b). There were no significant differences in IFN-γ- and IL-17-producing cells after mKatG stimulation of PBMCs from Löfgren's compared to non-Löfgren's patients after background deduction (Fig. 2). After in-vitro PPD stimulation, both IFN-γ- and IL-17-producing cells occurred at significantly higher frequencies in PBMC of PPD+ compared to PPD– controls (Supporting information, Fig. S4). However, we did not detect any significant differences with regard to mKatG responses between PPD+ and PPD– healthy controls.
Fig. 2.
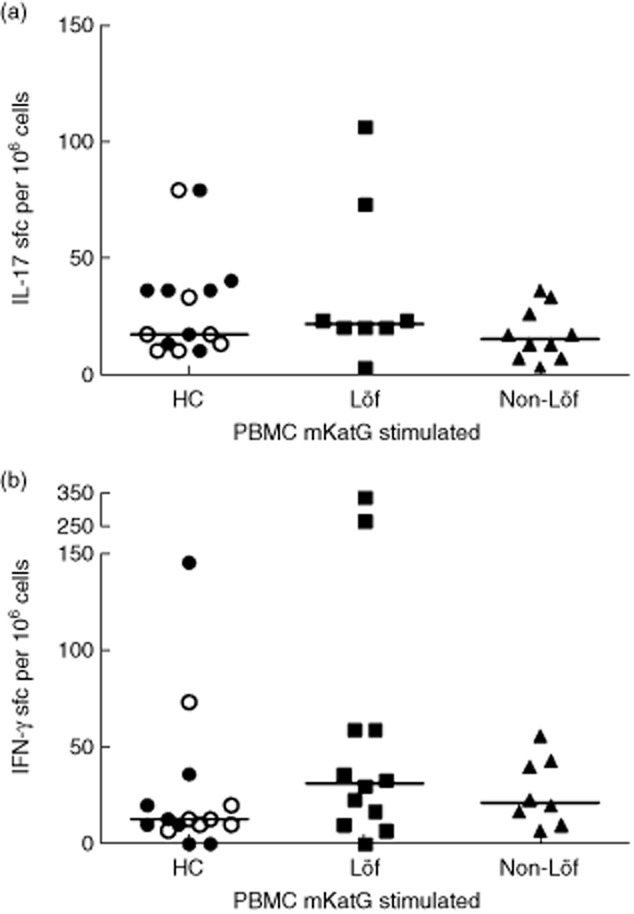
Enzyme-linked immunospot assay for evaluation of interleukin (IL)-17- and interferon (IFN)-γ-producing cells in response to Mycobacterium tuberculosis catalase-peroxidase (mKatG) in peripheral blood mononuclear cells (PBMCs). Results for cytokine-producing cells in PBMCs from purified protein derivative (PPD)– (open symbols) and PPD+ (filled symbols) healthy controls and sarcoidosis patients with and without Löfgren's syndrome are shown. Graphs show mean numbers of spots from 106 cells per well after deduction of background.
Similar IL-17 responses to mKatG regardless of sampling at the site of inflammation or periphery
BAL T cells responded with IFN-γ production to a significantly higher extent than blood T cells after mKatG stimulation in both groups of Löfgren's and non-Löfgren's patients (P < 0·05). In contrast, there was no significant difference between the frequencies of IL-17-producing cells in BAL compared to PBMCs after mKatG stimulation, although in Löfgren's patients there was a tendency towards higher expression of IL-17 by BAL cells compared to PBMCs [median 35 versus 20 spot-forming cells (SFC) per 106 cells]. In non-Löfgren's patients an opposite trend was observed, with higher IL-17 expression in PBMC than in BAL cells (median 17 versus 10 SFC per 106 cells) (Fig. 3).
Fig. 3.
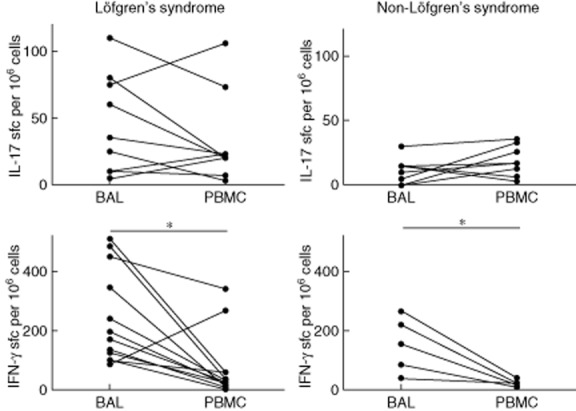
Paired analysis of bronchoalveolar lavage (BAL) and blood cytokine responses to Mycobacterium tuberculosis catalase-peroxidase (mKatG) detected with enzyme-linked immunospot for interleukin (IL)-17 and interferon (IFN)-γ production. Results for sarcoidosis patients with or without Löfgren's syndrome are presented in separate graphs. Graphs show mean numbers of spots from 106 cells per well after deduction of background. *P < 0·05.
Higher level of soluble IL-17 in bronchoalveolar lavage fluid (BALF) of HLA-DR3+ Löfgren's syndrome patients
HLA-DR3+ sarcoidosis patients with Löfgren's syndrome had a significantly higher concentration of IL-17 in BAL fluid compared to healthy controls and non-Löfgren's syndrome patients (P < 0·01 and P < 0·05, respectively) (Fig. 4a). Within the Löfgren's syndrome group, HLA-DR3+ patients had higher IL-17 levels than HLA-DR3– patients, although this difference was not significant. Because the presence of Löfgren's syndrome as well as HLA-DR3 predicts a good prognosis, we also compared patient groups where both these factors were either present or absent, i.e. DR3+ Löfgren's patients and DR3– non-Löfgren's patients. We hypothesized that this would reflect opposite ends of the ‘prognostic spectrum’ in sarcoidosis. The IL-17 levels were substantially higher in the DR3+ Löfgren's patients (P < 0·01) (Fig. 4b).
Fig. 4.
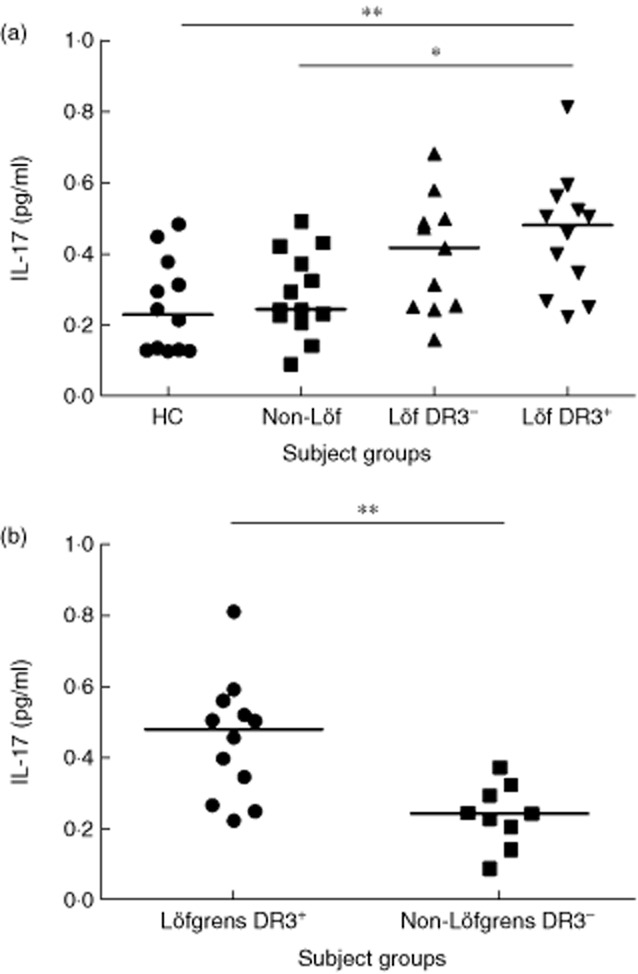
(a) Interleukin (IL)-17 levels in bronchoalveolar lavage (BAL) fluid determined by immuno-polymerase chain reaction (IPCR) in samples from healthy controls (HC), sarcoidosis patients without Löfgren's syndrome (non-Löf) and with Löfgren's syndrome. Sarcoidosis patients with Löfgren's syndrome were divided into two subgroups; human leucocyte antigen (HLA)-DR3+ and HLA-DR3–. (b) Comparison of IL-17 levels between. DR3+ Löfgren's patients and DR3– non-Löfgren's patients. *P < 0·05; **P < 0·01.
Potential for IL-17 production by T cell subsets
The capacity for IFN-γ and IL-17 production in BAL and blood CD4+ and CD8+ T cells in response to PPD and superantigen (combination of SEA and SEB) was studied by flow cytometry in three Löfgren's syndrome and two non-Löfgren's syndrome patients (Fig. 5a).
Fig. 5.
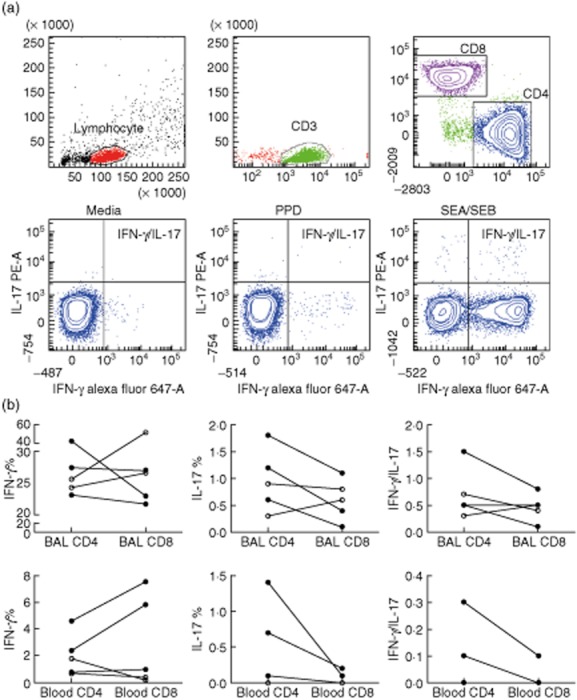
a, Flow cytometry plots of in-vitro-stimulated bronchoalveolar lavage (BAL) fluid cells of a patient with Löfgren's syndrome. Successive gating was performed on lymphocytes, CD3+, CD4+ and CD8+ T cells. Cytokine expression in CD4+ T cells in response to medium alone, purified protein derivative (PPD) and Staphylococcus enterotoxin A (SEA)/S. enterotoxin B (SEB) were evaluated by intracellular staining for detection of interferon (IFN-)γ and interleukin (IL)-17. (b) Paired analysis show frequencies of CD4+ and CD8+ T cells expressing either IFNγ, IL-17 or both cytokines simultaneously in response to superantigen (SEA/SEB) stimulation. Samples were from BAL (b; top row) and blood (b; bottom row) of sarcoidosis patients with Löfgren's syndrome (filled symbols) and sarcoidosis patients without Löfgren's syndrome (open symbols). Note different scales on axes.
Both BAL CD4+ and CD8+ T cells responded to superantigen by IL-17 production and we could detect a tendency towards a higher frequency of IL-17 + CD4+ (Th17) cells after superantigen stimulation in Löfgren's syndrome compared to non-Löfgren's syndrome (median 1·2 versus 0·6% of BAL CD4+ cells) (Fig. 5b).
The observed cytokine responses to superantigen in both Löfgren's and non-Löfgren's syndrome patients indicated that there are subsets of BAL CD4+ and CD8+ T cells capable of producing both IFN-γ and IL-17 simultaneously and thus have a hybrid IFN-γ/IL-17 phenotype (Fig. 5b). The majority of IL-17-producing cells in both CD4 and CD8 subsets also produced IFN-γ (median 83% for both CD4 and CD8 cells). In this limited number of patients the observed frequencies of these hybrid cells were similar in both groups of patients (Supporting information, Table S1 and Fig. 5b).
In blood samples, as in BAL samples, we detected CD4+ and CD8+ T cells producing IFN-γ and/or IL-17 in response to the superantigens in both Löfgren's and non-Löfgren's syndrome patients (Fig. 5b).
In patients with elevated numbers of BAL CD4+ TCR AV2S3+ cells, there was a tendency towards higher IFN-γ production but lower IL-17 production in AV2S3+ cells, in both BAL and blood (Supporting information, Table S2).
IL-17 expression in sarcoid lung tissue
Immunostaining of IL-17 was performed on lung biopsy sections from sarcoidosis patients for visualizing IL-17-producing cells. The lung biopsies from sarcoidosis patients contained typical non-necrotizing granulomas composed of macrophages, epithelioid cells and multi-nucleated giant cells in the central region with an accumulation of lymphocytes in the peripheral region (or central region). We observed IL-17 expression in tissue from all four sarcoidosis patients with Löfgren's syndrome (Fig. 6a), but IL-17-producing cells detected in only two of four non-Löfgren's patients (Fig. 6b). We did not detect any IL-17 expression in tissue from two healthy controls (Fig. 6c). Contiguous slides stained with isotype antibodies served as controls (Fig. 6e–h). A few positive cells were detected in both patient groups, but we did not find any marked difference in the number of IL-17-producing cells in patients with and without Löfgren's syndrome. Positive immunoreactivity for IL-17 was seen typically In inflammatory cells in the periphery of the granuloma and also surrounding the granuloma. In the granuloma compartment, the main IL-17-producing cells seemed to be lymphocytes infiltrating in the peripheral parts. In addition, some lung biopsies from non-granuloma compartments displayed immunoreactivity for IL-17 in what appeared to be alveolar macrophages (Fig. 6d).
Fig. 6.
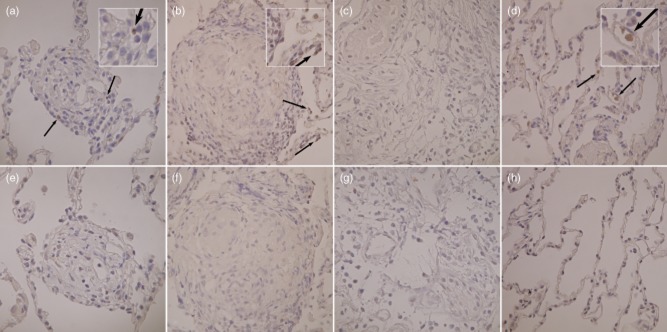
Immunohistochemical analysis of interferon (IL)-17 in lung tissue. (a) Sample from granuloma of a sarcoidosis patient with Löfgren's syndrome. (b) Granuloma of a sarcoidosis patient without Löfgren's syndrome. (c) Respiratory bronchiole from healthy control and (d), non-granulomatous alveolar section from a sarcoidosis patient with Löfgren's syndrome. (e–h) Contiguous corresponding slides were stained with rabbit immunoglobulin (Ig)G as a negative control, respectively. IL-17-producing cells are indicated by arrows. Magnification: ×400.
Discussion
In this study, we investigated the presence of IL-17 and IL-17-producing cells in sarcoidosis patients, focusing on the ability of bronchoalveolar lavage and peripheral blood T cells from patients with and without Löfgren's syndrome to respond to the mycobacterial antigen mKatG by production of IFN-γ and IL-17. We demonstrated the presence of T cells producing IFN-γ as well as IL-17 in response to the mycobacterial antigen mKatG in pulmonary sarcoidosis. IL-17-producing cells that were reactive to mKatG were found in the affected organ, the lung and also in peripheral blood. This implies that the observed Th17 responses against the mycobacterial protein mKatG may contribute to the local as well as systemic inflammation in sarcoidosis, although they generally occur at lower frequencies than corresponding Th1 responses. Importantly, we found that sarcoidosis patients with Löfgren's syndrome have higher levels of IL-17 and more IL-17-producing cells responding to mKatG locally at the site of inflammation. This suggests that IL-17-producing cells may be a useful biomarker for the prognosis of sarcoidosis, and that they may play a protective role in the spontaneous recovery typical of patients with Löfgren's syndrome.
There is strong evidence for the involvement of mycobacterial antigens in the pathogenesis of sarcoidosis. Song et al. previously identified the candidate antigen mycobacterial protein mKatG in sarcoidosis tissues and lymph nodes by mass spectrometry [7]. In collaboration with Moller's group, we previously showed mKatG-reactive IFN-γ-producing effector T cells to be present in sarcoidosis patients in a manner expected for a pathogenic antigen [8]. In another study, we showed that mKatG has the potential to stimulate the CD4+ TCR AV2S3+ T cells, which accumulate in the lungs of HLA-DR3+ patients (typically associated with Löfgren's syndrome and a very good prognosis). Moreover, we found a more pronounced multi-functional cytokine profile, with simultaneous production of IFN-γ and TNF, in the mKatG-specific BAL CD4+ T cells of DR3+ Löfgren's syndrome patients [19]. Mycobacterial antigen [6 kDa early secretory antigenic target (ESAT-6)]-specific Th17 responses in sarcoidosis have been reported recently by Richmond et al. [20]. Here, for the first time, we demonstrate mKatG-specific IL-17 responses in patients with pulmonary sarcoidosis. Comparing patient subgroups, we observed higher frequencies of mKatG-specific IL-17-producing cells in the lungs of sarcoidosis patients with Löfgren's syndrome. This finding is compatible with IL-17-producing cells playing a role in the elimination of presumed antigen leading to spontaneous recovery that is characteristic for patients with Löfgren's syndrome.
One of the most frequently documented functions of IL-17 is neutrophil recruitment and infiltration, and in our study we found an association between neutrophil percentage and the frequency of IL-17-producing mKatG-reactive cells in the BAL fluid of patients with Löfgren's syndrome. The significance of this, however, is not clear, and sarcoidosis is not a disorder where neutrophils are believed to play a major role in the pathogenesis. Although neutrophils have been considered to be proinflammatory cells, which are associated with chronic disease and fibrosis, it is known that some types of neutrophils exhibit anti-inflammatory or healing characteristics [21].
We found higher levels of soluble IL-17 in the BALF of DR3+ sarcoidosis patients with Löfgren's syndrome compared with non-Löfgren's patients. This finding is interesting, as the combination of being DR3+ and having Löfgren's syndrome is associated with the very best prognosis – virtually all such patients recover within 2 years [4]. The properties of the HLA-DR3 molecule in presenting sarcoidosis-specific antigen(s) such as mKatG for T cells, followed by induction of Th17 cells in the lungs of DR3+ sarcoidosis patients with Löfgren's syndrome, provide a likely explanation for this association.
In sarcoidosis, the relative roles of Th1 and Th17 cells and the interplay between these immune cells are not well understood. We speculate that the higher expressions of IL-17 and mKatG-specific IL-17-producing cells in sarcoidosis patients with Löfgren's syndrome are potent inducers of Th1 responses, similar to what has been observed in animal studies [22,23], and contribute to a more rapid and diverse effector T cell response, which then helps to protect against unknown antigen(s).
IL-17 has been reported to be important in the pathogenesis of several inflammatory disorders, in particular autoimmune diseases such as rheumatoid arthritis (RA), multiple sclerosis (MS), psoriasis and inflammatory bowel disease (IBD) [24]. In addition to Th17 cells, other types of innate and adaptive lymphocytes can also produce IL-17. Clearly, IL-17-mediated immune responses are essential for the protection against several bacterial and some fungal pathogens in mammalian models, but responses against self-antigens or excess immune reactions can lead to hyperinflammatory responses and tissue damage [25]. Most studies have shown a proinflammatory role for Th17 cells, and one consequence of IL-17 expression in sarcoidosis patients is likely be the recruitment and activation of inflammatory innate immune cells at the site of inflammation [26]. However, in some circumstances a protective or non-pathogenic role for Th17 cells has been observed [27–30]. We know from previous studies that the levels of some cytokines, such as transforming growth factor (TGF)-β1 that may influence differentiation of Th17 cells, differ between sarcoidosis patients with or without Löfgren's syndrome [31].
Based on flow cytometry, our findings indicate that, in sarcoidosis patients, there is a subset of T cells which has the ability to produce both IFN-γ and IL-17 simultaneously. In fact, we found that the majority of Th17 cells can also produce IFN-γ. The existence of such ‘hybrid’ T cells has been reported previously in other inflammatory diseases and also in sarcoidosis [14,32]. Importantly, there is evidence that hybrid Th1/Th17 T cells are more pathogenic than conventional Th17 cells [33]. However, our flow cytometric analyses were performed on samples from only a few individuals, so these findings need to be corroborated.
The role of IL-17 in the formation and maturation of granuloma has been demonstrated, e.g. by characterizing the deficient sequestration of bacilli Calmette–Guérin (BCG) in IL-17 knock-out mice [12,23]. The presence of IL-17 in granuloma of sarcoidosis tissue has been reported previously by Ten Berge et al. [14] and Facco et al. [14,15]. We also detected IL-17-producing cells in the sarcoidosis granuloma, although to a much more limited extent than in Facco et al.'s study, which showed expression of IL-17 by a majority of granuloma cells [15]. We found IL-17-producing cells to be present in the periphery of granulomas, i.e. they co-localize with lymphocytes, while other studies found IL-17 to be expressed preferentially by multi-nucleated giant cells and T cells localized inside the granuloma. These discrepancies might be due to differences regarding the exact specificity or concentration of antibody used. The finding that IL-17-producing cells are part of sarcoidosis granulomas further implicate them in the pathogenesis of this disease as probable contributors to the sequestration and, in some patients, the elimination of assumptive antigen(s).
Hypothetically, targeting IL-17 signalling may have a therapeutic potential in some sarcoidosis patients, similar to what has been carried out in rheumatoid arthritis [34] and psoriasis [35]. However, given the increased IL-17 responses among a subgroup of sarcoidosis patients with good prognosis that we demonstrate here, it remains uncertain as to whether inhibiting this signalling will offer clinical benefits for patients. Therefore, a more detailed understanding about the pathogenic or protective role of Th17 cells in various subsets of sarcoidosis patients is needed.
In summary, for the first time we have provided evidence to show that IL-17 levels in the airways and frequencies of mKatG-specific IL-17-producing cells are higher in a subgroup of patients with Löfgren's syndrome: a subgroup signified by a very good clinical prognosis. These findings, in patients characterized typically by spontaneous disease resolution, are thus compatible with a role for Th17 cells and IL-17 per se in the elimination of antigen together with the previously described multi-functional Th1 cells specific for mKatG. Our findings underline the potential importance of IL-17 as a prognostic tool in sarcoidosis patients. However, more studies are needed to elucidate the more precise mechanistic role of IL-17 and antigen-specific Th17 cells in subgroups of sarcoidosis patients.
Acknowledgments
This study was supported by The Swedish Heart Lung Foundation, The Swedish Research Council, through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, Novartis collaborative grant, Torsten and Ragnar Söderberg's Foundation, The King Oscar II Jubilee Foundation, Karolinska Institutet and The Mats Kleberg Foundation.
Author contributions
M. O., J. W. and J. G. performed the study design, and J. W. and J. G. the study conception; M. O. and P. G. (qIPCR) collected data and performed the experiments; A. E. performed bronchoalveolar lavages; D. M. provided recombinant mKatG; M. O., J. W., J. G., D. M. (ELISPOT), C. O. H. (IHC), A. L. (qIPCR) and P. G. (qIPCR) contributed to the analysis and interpretation of data; M. O. wrote the manuscript; J. G. and A. L. drafted the manuscript; J. W. supervised the writing of the manuscript; J. G. and A. E. supplied clinical advice and patient inclusion and characterization.
Disclosures
The authors have no financial conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Paired analysis of cytokine responses to Mycobacterium tuberculosis catalase-peroxidase (mKatG) compared to response to media alone (i.e. without any stimulation). Graphs show results for bronchoalveolar lavage (BAL) samples from patients with or without Löfgren's syndrome. (a) Mean number of interleukin (IL)-17 and (b) mean number of interferon (IFN)-γ-producing cells after stimulation with mKatG and in media control, respectively. Graphs show mean numbers of spots from 106 cells per well. *P < 0·05; **P < 0·01.
Fig. S2. The ratio of interleukin (IL)-17- to interferon (IFN)-γ-producing cells in response to mKatG in bronchoalveolar lavage (BAL) samples was calculated for each patient. Data are shown for nine patients with Löfgren's syndrome and six patients without Löfgren's syndrome.
Fig. S3. Paired analysis of cytokine responses to Mycobacterium tuberculosis catalase-peroxidase (mKatG) compared to response to media alone (i.e. without any stimulation). Graphs show results for peripheral blood mononuclear cell (PBMC) samples from patients with or without Löfgren's syndrome. (a) Mean number of interleukin (IL)-17 and (b) mean number of interferon (IFN)-γ-producing cells after stimulation with mKatG and in media control, respectively. Graphs show mean numbers of spots from 106 cells per well. **P < 0·01; ***P < 0·001.
Fig. S4. Enzyme-linked immunospot assay for evaluation of interferon (IFN)-γ- and interleukin (IL)-17-producing cells in response to purified protein derivative (PPD) in of healthy individuals. Blood samples were obtained from healthy controls with PPD– and PPD+ reactions in the skin, respectively. Graphs show mean numbers of spots from 106 cells per well after deduction of background. *P < 0·05; **P < 0·01.
Table S1. T cell responses to superantigens Staphylococcus enterotoxin A (SEA)/S. enterotoxin B (SEB) analysed in three sarcoidosis patients with Löfgren's syndrome and two sarcoidosis patients without Löfgren's syndrome. Percentages of bronchoalveolar lavage (BAL) and blood CD4+ or CD8+ T cells expressing interferon (IFN)-γ, interleukin (IL)-17 and both cytokines simultaneously in response to SEA/SEB were analysed by flow cytometry. Values depict median (min–max) percentage of cytokine expression after deduction of background. Two of three Löfgren's syndrome patients participating in our study were DR3+, and one was DR13+ with a T cell receptor (TCR) AV2S3 expansion and included in the DR3+ group. The reason for this is that in our experience DR13+ patients with AV2S3 expansions always carry the human leucocyte antigen (HLA)-DRB3*0101 allele, whose protein product has a very similar structure to that of HLA-DRB1*0301, and indeed these molecules have been shown to be able to present similar antigenic peptides (J. Grunewald, Thorax 2002; 57:348–352). Two of three Löfgren's syndrome patients had a lung expansion of TCR AV2S3+ CD4+ T cells and one DR3+ patient in a rare case had an elevated number of AV2S3+ CD4+ T cells (7·5%) (≥10·5% of CD4+ cells in BAL fluid defined as a expanded) [J. Grunewald, Eur J Immunol 1992; 22 (1):129–135]. Data are shown as median (min–max).
Table S2. In three patients with Löfgren's syndrome who displayed elevated numbers of bronchoalveolar lavage (BAL) CD4+ T cell receptor (TCR) AV2S3+ cells (see Supporting information, Table S1 legend), the capacity for induced cytokine production by AV2S3+ and AV2S3– cells in BAL and blood was compared. There was a tendency to a higher frequency of interferon (IFN)-γ-producing cells in response to Staphylococcus enterotoxin A (SEA) and S. enterotoxin B (SEB) within the AV2S3+ subset in both BAL and blood samples. In contrast, the tendency was towards higher IL-17 responses in AV2S3– cells (in both BAL and blood samples). Frequencies of simultaneous IFN-γ- and IL-17-producing cells in response to SEA and SEB were similar in BAL AV2S3+ and AV2S3– cells. Data are shown as median (min–max).
Appendix S1. Materials and methods.
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2013;S0140–6736:60680–60687. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen ES, Moller DR. Sarcoidosis – scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 4.Grunewald J, Eklund A. Lofgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. 2009;179:307–312. doi: 10.1164/rccm.200807-1082OC. [DOI] [PubMed] [Google Scholar]
- 5.Grunewald J, Janson CH, Eklund A, et al. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992;22:129–135. doi: 10.1002/eji.1830220120. [DOI] [PubMed] [Google Scholar]
- 6.Saboor SA, Johnson NM, McFadden J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet. 1992;339:1012–1015. doi: 10.1016/0140-6736(92)90535-b. [DOI] [PubMed] [Google Scholar]
- 7.Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ES, Wahlstrom J, Song Z, et al. T cell responses to mycobacterial catalase–peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181:8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 10.Glader P, Smith ME, Malmhall C, et al. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. Eur Respir J. 2010;36:1155–1164. doi: 10.1183/09031936.00170609. [DOI] [PubMed] [Google Scholar]
- 11.Kolls JK. CD4(+) T-cell subsets and host defense in the lung. Immunol Rev. 2013;252:156–163. doi: 10.1111/imr.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto Yoshida Y, Umemura M, Yahagi A, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 13.Vanaudenaerde BM, Verleden SE, Vos R, et al. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med. 2011;183:977–986. doi: 10.1164/rccm.201007-1196PP. [DOI] [PubMed] [Google Scholar]
- 14.Ten Berge B, Paats MS, Bergen IM, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxf) 2012;51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 15.Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2010;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 16.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 17.Grunewald J, Wahlstrom J, Berlin M, Wigzell H, Eklund A, Olerup O. Lung restricted T cell receptor AV2S3+ CD4+ T cell expansions in sarcoidosis patients with a shared HLA-DRbeta chain conformation. Thorax. 2002;57:348–352. doi: 10.1136/thorax.57.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahlstrom J, Dengjel J, Winqvist O, et al. Autoimmune T cell responses to antigenic peptides presented by bronchoalveolar lavage cell HLA-DR molecules in sarcoidosis. Clin Immunol. 2009;133:353–363. doi: 10.1016/j.clim.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Wiken M, Ostadkarampour M, Eklund A, et al. Antigen-specific multifunctional T-cells in sarcoidosis patients with Lofgren's syndrome. Eur Respir J. 2012;40:110–121. doi: 10.1183/09031936.00166110. [DOI] [PubMed] [Google Scholar]
- 20.Richmond BW, Ploetze K, Isom J, et al. Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-gamma expression. J Clin Immunol. 2013;33:446–455. doi: 10.1007/s10875-012-9817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 22.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 23.Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guérin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 24.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota K, Ahlfors H, Duarte JH, Stockinger B. Regulation and function of innate and adaptive interleukin-17-producing cells. EMBO Rep. 2012;13:113–120. doi: 10.1038/embor.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 27.Ke Y, Liu K, Huang GQ, et al. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182:3183–3190. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverpil E, Glader P, Hansson M, Linden A. Impact of interleukin-17 on macrophage phagocytosis of apoptotic neutrophils and particles. Inflammation. 2011;34:1–9. doi: 10.1007/s10753-010-9201-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idali F, Wiken M, Wahlstrom J, et al. Reduced Th1 response in the lungs of HLA-DRB1*0301 patients with pulmonary sarcoidosis. Eur Respir J. 2006;27:451–459. doi: 10.1183/09031936.06.00067105. [DOI] [PubMed] [Google Scholar]
- 32.Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012;12:592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- 33.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 35.Waisman A. To be 17 again – anti-interleukin-17 treatment for psoriasis. N Engl J Med. 2012;366:1251–1252. doi: 10.1056/NEJMe1201071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Paired analysis of cytokine responses to Mycobacterium tuberculosis catalase-peroxidase (mKatG) compared to response to media alone (i.e. without any stimulation). Graphs show results for bronchoalveolar lavage (BAL) samples from patients with or without Löfgren's syndrome. (a) Mean number of interleukin (IL)-17 and (b) mean number of interferon (IFN)-γ-producing cells after stimulation with mKatG and in media control, respectively. Graphs show mean numbers of spots from 106 cells per well. *P < 0·05; **P < 0·01.
Fig. S2. The ratio of interleukin (IL)-17- to interferon (IFN)-γ-producing cells in response to mKatG in bronchoalveolar lavage (BAL) samples was calculated for each patient. Data are shown for nine patients with Löfgren's syndrome and six patients without Löfgren's syndrome.
Fig. S3. Paired analysis of cytokine responses to Mycobacterium tuberculosis catalase-peroxidase (mKatG) compared to response to media alone (i.e. without any stimulation). Graphs show results for peripheral blood mononuclear cell (PBMC) samples from patients with or without Löfgren's syndrome. (a) Mean number of interleukin (IL)-17 and (b) mean number of interferon (IFN)-γ-producing cells after stimulation with mKatG and in media control, respectively. Graphs show mean numbers of spots from 106 cells per well. **P < 0·01; ***P < 0·001.
Fig. S4. Enzyme-linked immunospot assay for evaluation of interferon (IFN)-γ- and interleukin (IL)-17-producing cells in response to purified protein derivative (PPD) in of healthy individuals. Blood samples were obtained from healthy controls with PPD– and PPD+ reactions in the skin, respectively. Graphs show mean numbers of spots from 106 cells per well after deduction of background. *P < 0·05; **P < 0·01.
Table S1. T cell responses to superantigens Staphylococcus enterotoxin A (SEA)/S. enterotoxin B (SEB) analysed in three sarcoidosis patients with Löfgren's syndrome and two sarcoidosis patients without Löfgren's syndrome. Percentages of bronchoalveolar lavage (BAL) and blood CD4+ or CD8+ T cells expressing interferon (IFN)-γ, interleukin (IL)-17 and both cytokines simultaneously in response to SEA/SEB were analysed by flow cytometry. Values depict median (min–max) percentage of cytokine expression after deduction of background. Two of three Löfgren's syndrome patients participating in our study were DR3+, and one was DR13+ with a T cell receptor (TCR) AV2S3 expansion and included in the DR3+ group. The reason for this is that in our experience DR13+ patients with AV2S3 expansions always carry the human leucocyte antigen (HLA)-DRB3*0101 allele, whose protein product has a very similar structure to that of HLA-DRB1*0301, and indeed these molecules have been shown to be able to present similar antigenic peptides (J. Grunewald, Thorax 2002; 57:348–352). Two of three Löfgren's syndrome patients had a lung expansion of TCR AV2S3+ CD4+ T cells and one DR3+ patient in a rare case had an elevated number of AV2S3+ CD4+ T cells (7·5%) (≥10·5% of CD4+ cells in BAL fluid defined as a expanded) [J. Grunewald, Eur J Immunol 1992; 22 (1):129–135]. Data are shown as median (min–max).
Table S2. In three patients with Löfgren's syndrome who displayed elevated numbers of bronchoalveolar lavage (BAL) CD4+ T cell receptor (TCR) AV2S3+ cells (see Supporting information, Table S1 legend), the capacity for induced cytokine production by AV2S3+ and AV2S3– cells in BAL and blood was compared. There was a tendency to a higher frequency of interferon (IFN)-γ-producing cells in response to Staphylococcus enterotoxin A (SEA) and S. enterotoxin B (SEB) within the AV2S3+ subset in both BAL and blood samples. In contrast, the tendency was towards higher IL-17 responses in AV2S3– cells (in both BAL and blood samples). Frequencies of simultaneous IFN-γ- and IL-17-producing cells in response to SEA and SEB were similar in BAL AV2S3+ and AV2S3– cells. Data are shown as median (min–max).
Appendix S1. Materials and methods.


