Abstract
The biologically active form of vitamin D3, 1, 25-dihydroxyvitamin D3 (calcitriol), is a potent modulator of the immune response. We have shown previously that calcitriol modulates the immunoglobulin response in vitro and in vivo in mice and humans. To analyse the underlying molecular mechanisms we studied whether calcitriol-primed B cells modulate T cell activation and function. Human B cells were stimulated with anti-CD40 and interleukin (IL)-4 in the presence of increasing concentrations of calcitriol. After removal of calcitriol, primed B cells were co-cultured with autologous CD4+ T cells; the B cell phenotype T cell activation and their consecutive cytokine production were also assessed. Naive T cells co-cultured with calcitriol-primed naive B cells showed a reduced expansion, nuclear factor of activated T cells, cytoplasmic 2 (NFATc2) expression and cytokine production upon restimulation. CD86 expression on B cells after calcitriol priming was identified as an underlying mechanism, as T cell activation and expansion was rescued by activating anti-CD28 antibodies. Our data indicate that calcitriol-primed B cells display an impaired capacity to activate T cells. Taken together, we identified a novel B cell-dependent vitamin D immune regulatory mechanism, namely by decreased co-stimulation of calcitriol-primed B cells.
Keywords: B cells, co-stimulatory molecules, T cells, vitamin D
Introduction
The initiation of T cell responses requires T cell receptor triggering and co-stimulatory signals from antigen-presenting cells (APCs). Isolated T cell receptor stimulation leads to T cell anergy [1]. B cells have been recognized not only as antibody-producing but also as potent APCs, which play an important role in the initiation of T cell responses [2]. They encounter B cell receptor (BCR)-specific antigens in secondary lymphoid organs and internalize, process and present them to T cells [3]. Upon CD40 activation, B cells express T cell co-stimulatory molecules such as CD80 and CD86 and thus provide naive T cell activation [4,5]. Numerous studies suggest that vitamin D deficiency is associated with immunological diseases, e.g. atopic dermatitis (AD) [6] and allergic asthma [7], but also autoimmune diseases [8–11]. Calcitriol (1, 25-dihydroxyvitamin D3) is the bioactive form of vitamin D, and modulates lymphocyte function following binding to its receptor (VDR). In response to calcitriol, T cells show a reduced proliferation and production of proinflammatory cytokines, but an increased expression of interleukin (IL)-10 [12,13]. Calcitriol-treated B cells display impaired immunoglobulin (Ig)E class-switch recombination and secretion [14], but produce enhanced IL-10 [15]. In dendritic cells (DCs), calcitriol results in an inhibition of differentiation and maturation and leads to a modulation of the T cell co-stimulatory capacity promoting the generation of suppressor T cell phenotypes [16,17]. Because B cells also act as APCs to T cells we hypothesized similarly that VDR activation of B cells may result in impaired T cell activation. First, therefore, we investigated the activation profile of calcitriol-primed B cells, and secondly their impact on T cell activation.
Our data show that calcitriol-primed B cells display a diminished co-stimulatory activation profile which results in reduced expansion and cytokine production of autologous T cells, delineating a novel immunomodulatory mechanism of vitamin D in the context of B cells.
Material and methods
B and T cell isolation and cultivation in vitro
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of four to five independent donors per experiment, and B cells were purified to >98% purity using anti-CD19-coupled multi-sort magnetic beads as described previously [18]. The pre-enriched CD19+ B cells were separated after release of magnetic beads in naive/memory B cells, additionally using anti-CD27-coupled magnetic beads according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany) [19]. Naive CD4+ T cells were positively isolated from CD19neg fraction following depletion of CD45RO+, CD14+, CD25+ cells and memory CD4+ T cells upon removal of CD45RA+, CD14+ cells by using respective magnetically coupled monoclonal antibodies by magnetic cell sorting (all Miltenyi Biotec; purity > 98%). Purified B cells (1 × 106/ml), activated with anti-CD40 monoclonal antibody (mAb) (1 μg/ml, clone 626·1; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and recombinant human IL-4 (10 ng/ml; Miltenyi Biotec), were cultured in RPMI-1640 culture medium (Life Technologies, Carlsbad, CA, USA) for 2 days in the presence of 1 μmol/l calcitriol (Sigma-Aldrich, St Louis, MO, USA) [19]. After 2 days' priming phase B cells were washed three times with 50 ml phosphate-buffered saline (PBS) (PAA, Pasching, Austria) and resuspended at concentration of 5 × 105/ml in RPMI-1640 culture medium. Naive and memory T cells were labelled with 5(6)-carboxyfluorescein diacetate N-succinimidylester (CFSE; Sigma-Aldrich), as described elsewhere [20], and resuspended at a concentration of 2 × 106/ml. Based on previous titration experiments, T cells were co-cultured with B cells at ratio of 4:1 in the presence of superantigen toxic shock syndrome toxin 1 (TSST-1, 50 pg/ml; Sigma-Aldrich) or platebound anti-CD3 (1 μg/ml, clone HIT3; BD Pharmingen, San Jose, CA, USA) for 7 days in RPMI-1640 culture medium (Life Technologies). In some experiments anti-CD28 (1 μg/ml, clone 28·2; BD Pharmingen) antibodies were added to T cell–B cell co-culture.
Functional assay to determine remaining amounts of calcitriol in the co-cultures
The human promyelocytic leukaemia HL-60 cell line was used to determine the remaining amount of calcitriol upon B cell priming and to rule out a possible spillover to the T cell co-culture. The HL-60 cell line expresses CD38 upon contact with calcitriol. B cells were treated as described and rested at 37°C in 5% CO2 for 1 and 3 days in the absence of T cells and the supernatant was added to 2·5 × 105/ml HL-60 cells. Calcitriol was added in concentrations from 1 μmol/l–10 pmol/l to 2·5 × 105/ml HL-60 cells. In addition, RPMI-1640 medium supplemented with 1 μmol/l calcitriol and kept in a similar manner to B cells was used as a control for the cell-free material spillover effect. The samples were incubated for 3 days at 37°C in 5% CO2. On day 3, CD38 surface expression on HL-60 cells was determined.
Flow cytometric analysis
CD19+ B from whole blood were phenotyped using fluorochrome-conjugated monoclonal antibodies and characterized according to the surface expression of CD19, CD27, CD80, CD86 (all BD Pharmingen) and CD38 (Beckman Coulter, Krefeld, Germany). HL-60 cells were stained accordingly with antibodies against CD38 (Beckman Coulter) and CD14 (BD Pharmingen). On day 7, co-cultured cells were restimulated by 10 mg/ml phorbol 12-myristate 13-acetate (PMA), 1 mg/ml ionomycin and 2 mg/ml brefeldin A (all from Sigma-Aldrich) for 5 h. After permeabilization (Perm 2 solution; BD Pharmingen), T cells were stained intracellularly for CD4, CD45RA, IL-2, interferon (IFN)-γ, IL-4 (all BD Pharmingen) and nuclear factor of activated T cells, cytoplasmic 2 (NFATc2) (DRFZ). Flow cytometric analyses were performed using fluorescence activated cell sorter (FACS)Calibur and FACSAria II cytometer (BD Pharmingen). Data were analysed using FlowJo7 software (TreeStar, Ashland, OR, USA).
Quantitative real-time polymerase chain reaction (PCR)
B cells were harvested after 48 h activation and calcitriol-priming and RNA was isolated using the RNA isolation kit (NucleoSpin® RNA II; Macherey-Nagel GmbH & Co, Dueren, Germany), according to the manufacturer's instructions. The cDNA synthesis was performed by a reverse transcriptase-PCR with TaqMan® reverse transcription reagents (Applied Biosystems, Darmstadt, Germany). Gene expression analysis was performed using quantitative real-time PCR with LightCycler 1·5 (Roche Diagnostics, Mannheim, Germany). Primers were synthesized by TIB Molbiol (Berlin, Germany) and the sequences are listed in the Supporting information, Table S1. The expression of CD86 was normalized to the expression of hprt as a housekeeping gene.
Statistical methods
Statistical evaluations were performed with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Data displayed the percentage of observations and columns in graphs using mean ± standard deviation (s.d.). Normal distribution was judged by the Kolmogorov–Smirnov test and these parameters were tested by Student's t-test or analysis of variance. P-values ≤ 0·05 were considered statistically significant.
Results
Reduced expansion of naive T cells in the presence of calcitriol-primed naive B cells
Activated B cells can serve as potent APCs and are able to generate robust proliferative CD4+ T cell responses [21]. We first addressed whether priming of B cells with calcitriol alters T cell activation and expansion. Purified naive and memory B cells were activated by anti-CD40/IL-4 and primed with or without 1 μM calcitriol, as identified by dose–response analysis (Supporting information, Fig. S1a). Thereafter primed B cells were co-cultured with naive or memory T cells in the presence of TSST-1, which binds to major histocompatibility complex (MHC)-II of B cells and Vβ2+ T cell receptor (TCR)-bearing T cells. A concentration of 50 pg/ml of TSST-1 was added to the co-culture to ensure sufficient T cell activation and proliferation (Supporting information, Fig. S1b). After 7 days of co-culture we observed reduced percentages of expanding naive T cells upon co-culture with calcitriol-primed naive B cells (Fig. 1a,b). Accordingly, the expansion of naive T cells activated with anti-CD3 mAb in the presence of calcitriol-primed naive B cells was also reduced (Fig. 1c). However, a direct comparison revealed that the reduction of T cell expansion was more pronounced upon stimulation with TSST-1 (mean value of five experiments, 48% ± 18, P < 0·05) than with anti-CD3 mAb (mean value of five experiments, 29% ± 8, P = 0·07) (Fig. 1d). The T cell survival was comparable in the presence of activated and activated/calcitriol-primed B cells (data not shown). Upon co-culture with memory B cells, CFSE-labelled naive and/or memory T cells show no significant reduction in expansion (17% ± 3, P = 0·2 and 4% ± 14, P = 0·9). The following experiments were focused upon naive B and naive T cells.
Fig. 1.
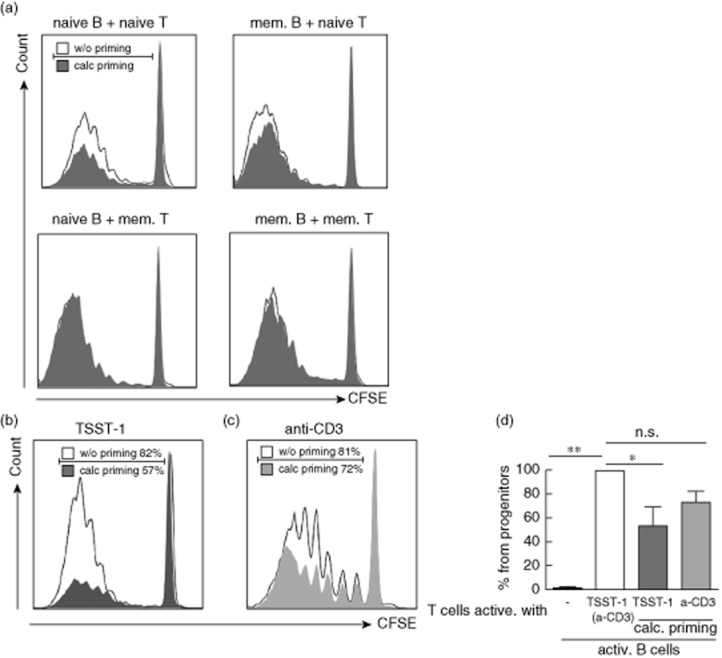
Reduced proliferation of naive but not memory CD4+ T cells in the presence of calcitriol-primed naive B cells. Carboxyfluorescein diacetate N-succinimidylester (CFSE)-labelled and proliferated T cells after 7 days co-culture with anti-CD40 (1 μg/ml) and interleukin (IL)-4 (10 ng/ml) preactivated (solid line, open bar) or preactivated and calcitriol-primed B cells (filled area, open bars). (a) Toxic shock syndrome toxin 1 (TSST-1)-induced proliferation of naive and memory T cells in the presence of naive or memory B cells. (b) T cells activated with TSST-1. (c) T cells activated with anti-CD3. Dot-blots are gated on living T lymphocytes of a representative donor. (d) Graph bars summarized results of five independent experiments and represent difference in % of progenitor T cells in the presence of activated B cells, set as 100% (open bar). Data are shown as mean ± standard deviation. *P ≤ 0·05; **P ≤ 0·01 considered significant.
Impact of calcitriol-primed B cells on T cell cytokine expression
Upon antigen-driven TCR activation, naive T cells differentiate into memory cells with characteristic patterns of cytokine expression. After 7 days of co-culture T cells were restimulated with PMA/ionomycin. NFATc2, CD40L and cytokine expression were measured by multi-colour flow cytometry in newly generated CD45RO+ memory T lymphocytes (Fig. 2a–c). Our data show a significantly decreased NFATc2 protein expression in T cells co-cultured with calcitriol-primed B cells in comparison to the controls (mean fluorescence intensity from 1598 to 1259, P < 0·05; Fig. 2d). Similar observations were obtained when analysing the frequencies of T cells expressing CD40L (from 57 to 33%, P < 0·01; Fig. 2e), IL-4 (from mean 3·8 to 1·5%, P < 0·01; Fig. 2h), IL-2 (from mean 70 to 55%, P < 0·05; Fig. 2f) and less pronounced IFN-γ (from 16·1 to 11·7%, P = 0·22; Fig. 2g) upon restimulation and previous culture with calcitriol-primed B cells. However, no significant changes in expression of investigated markers could be observed upon co-culture of naive T cells with calcitriol-primed memory B cells (data not shown).
Fig. 2.
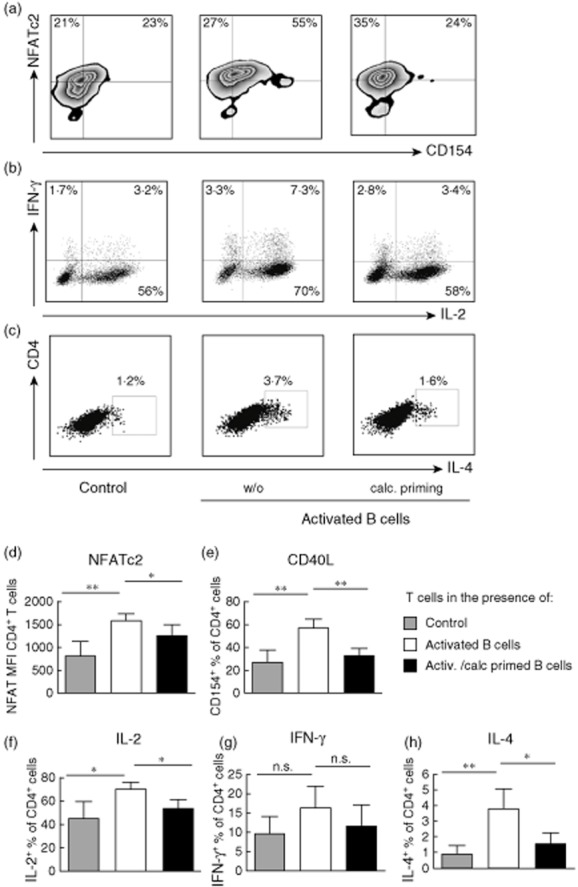
Reduced expression of nuclear factor of activated T cells, cytoplasmic 2 (NFATc2) protein, CD40L and frequencies of interleukin (IL)-2, interferon (IFN)-γ, IL-4-producing T cells upon co-culture with calcitriol-primed B cells and restimulation. Naive T cells co-cultured with anti-CD40 (1 μg/ml), IL-4 (10 ng/ml) activated naive B cells (middle column, open bars) or activated and calcitriol-primed B cells (right column, black bars). T cells alone served as control (left column, grey bars). On day 7 T cells were restimulated for 5 h with phorbol myristate acetate (PMA) (10 ng/ml) and ionomycin (1 μg/ml); for the last 4·5 h brefeldin A was added. Expression of NFATc2 protein and CD40L (a), IL-2 and IFN-γ (b) and IL-4 (c) was determined by flow cytometry. Plots are gated on newly generated CD45RAneg cells. (d,f,g,h) Graph bars summarize the results of four independent experiments. Data are shown as mean ± standard deviation. *P ≤ 0·05; **P ≤ 0·01 considered significant.
To exclude any spillover effects, we tested the B cell supernatants for a possible remaining amount of calcitriol using the HL-60 cell line, which expressed CD38 upon contact with calcitriol. We found no signs of a biological relevant calcitriol carryover (<1 nM; Supporting information, Fig. S2). Moreover, upon addition of the same calcitriol amount (10 pM–1 nM) to the T–B cell co-culture, no alteration in proliferation and frequencies of cytokine-producing T cells was observed (data not shown).
In conclusion, T cells co-cultured with calcitriol-primed B cells display reduced activation, resulting in a diminished T cell effector development.
Calcitriol-priming promotes a reduced co-stimulatory molecule expression profile in B cells
We have shown previously that activated B cells express VDR and that VDR ligation results in diminished isotype switching and increased IL-10 production [14,15,18]. We now wished to address whether or not the APC function of B cells is also altered by calcitriol, as suggested recently from data with DCs [16,22,23]. Therefore, we first determined the expression profile of the co-stimulatory molecule CD86 (B7-2) on the B cell surface but also at the mRNA level upon CD40 + IL-4 activation and calcitriol priming. Activated naive B cells showed a higher CD86 cell surface expression (8·7 ± 2·3% to 35·2 ± 12·4%, P < 0·01, Fig. 3a,b) and CD86 mRNA expression (0·29 ± 0·12% to 0·58 ± 0·26%, P < 0·05, Fig. 3c). In summary, calcitriol-priming of B cells results in a significant decrease of surface CD86 (58%, P < 0·05, Fig. 3a) and moderate mRNA (57%, P = 0·059, Fig. 3c) expression compared to activated B cells (set as 100%).
Fig. 3.
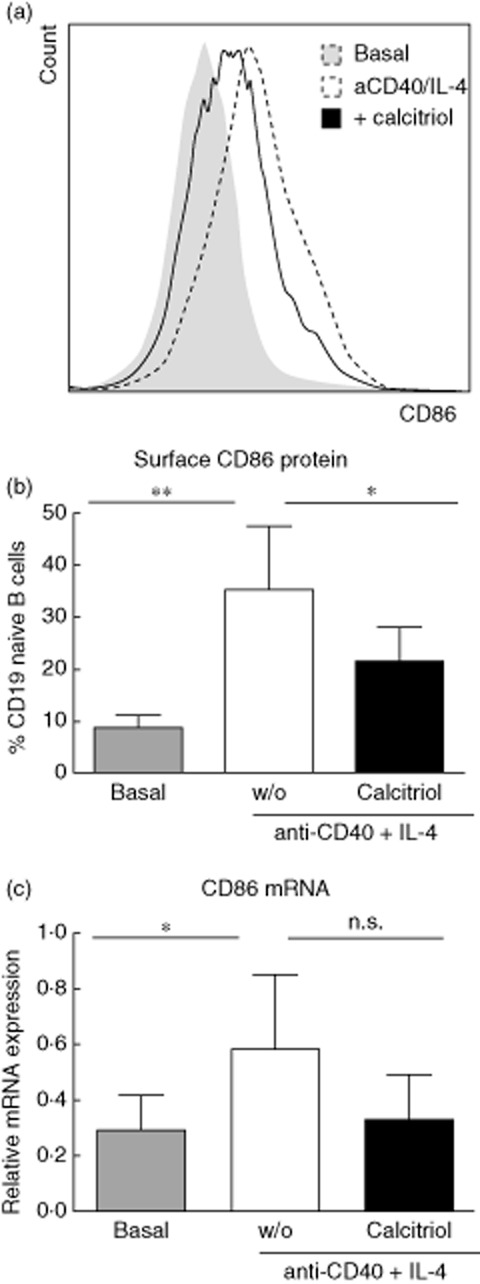
Vitamin D receptor (VDR) activation diminishes B cell co-stimulatory molecule expression. Naive B cells stimulated with anti-CD40 (1 μg/ml), interferon (IL)-4 (10 ng/ml) (dashed line), without calcitriol (solid line). Reduced expression of surface co-stimulatory CD86 molecules (a), (b) and CD86 mRNA (c) determined 48 h after incubation. (a) Overlays show living B lymphocytes of a representative donor and (b,c) summarize results from four independent experiments. Data are shown as mean ± standard deviation. *P ≤ 0·05; **P ≤ 0·01 considered significant.
Restoring of T cell activation by anti-CD28
To examine whether the reduction of the B cell co-stimulatory profile by calcitriol is biologically functional, naive T cells were co-cultured with calcitriol-primed B cells in the presence of activating anti-CD28 antibodies. As shown in Fig. 4a,b, agonistic anti-CD28 antibodies indeed enhanced activation and restored IL-2 (Fig. 4c) and IFN-γ (Fig. 4d) cytokine production in T cells when co-cultured with calcitriol-primed B cells and in comparison to the T cell–B cell co-culture without anti-CD28.
Fig. 4.
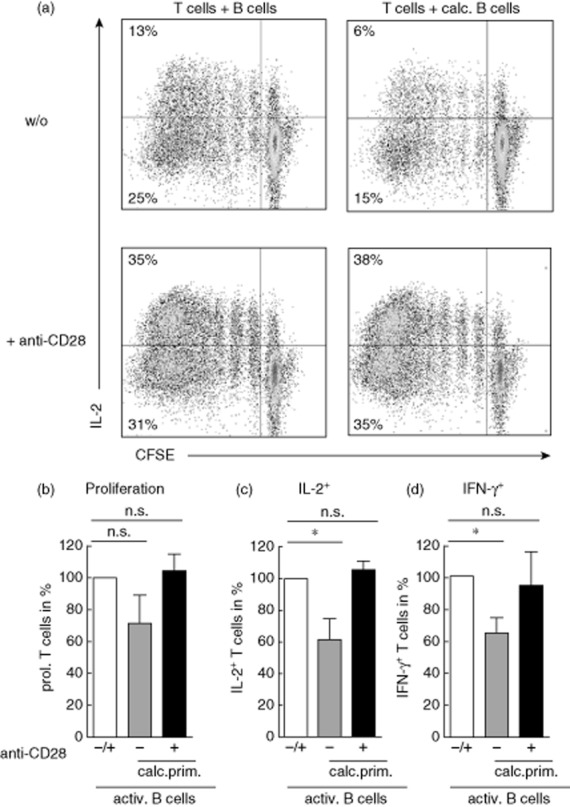
Cytokine production in T cells is restored upon addition of anti-CD28 antibodies. Carboxyfluorescein diacetate N-succinimidylester (CFSE)-labelled T cells were co-cultured with naive, anti-CD40 [(1 μg/ml), interleukin (IL)-4 (10 ng/ml)] preactivated B cells on anti-CD3 coated plates. (a) Addition of anti-CD28 (1 μg/ml) to co-culture with calcitriol-primed naive B cells enhances proliferation and restores the production of IL-2 in restimulated T cells. (b) T cell proliferation in co-culture with calcitriol-treated B cells with or without addition of anti-CD28 antibodies. T cell proliferation in the co-culture with preactivated B cells ± anti-CD28 antibodies set as 100% (open bar). (c) Similar to (b) but percentages of IL-2+ T cells. (d) Similar to (b) but percentages of interferon (IFN)-γ+ T cells. Dot-blots were gated on T lymphocytes of a representative donor and (b–d) summarize results from four independent experiments. Data are shown as mean ± standard deviation. *P ≤ 0·05 considered significant.
Discussion
In this study we show impaired naive but not memory T cell activation, cytokine production and transcription factor expression in the presence of calcitriol-primed naive B cells. Moreover, calcitriol priming resulted in a diminished co-stimulatory surface molecule expression of anti-CD40- and IL-4-activated B cells. Finally, we could prove that the reduction of T cell activation was dependent upon a calcitriol-dependent down-regulation of B cell co-stimulation signalling, as the addition of anti-CD28 restored both the T cell cytokine production and also expansion in the presence of calcitriol-primed B cells.
The induction of T cell activation and cytokine production requires simultaneous signalling of the TCR and activating co-stimulatory molecules such as CD28 [24]. In contrast, TCR signalling alone induces anergy [1]. In this study, we addressed the consequences of B cell priming with calcitriol, first regarding the expression of the co-stimulatory molecules and their impact upon T cell activation. Recently, it has been shown that VDR-mediated signals in DCs can control naive T cell activation [16]. This was associated with a reduced T cell proliferation and decreased proinflammatory cytokine expression, including IL-2 and IFN-γ, but an induction of IL-10 [12,25]. Results obtained in in-vivo models for transplantation and autoimmunity have shown that VDR ligands can act as potent inducers of regulatory APCs [26,27]. Additionally, differentiation, maturation and co-stimulatory capacity of human DCs were reduced in the presence of calcitriol or its low-calcaemic analogue in a dose-dependent manner [28]. In the present study we used anti-CD40 + IL-4-activated B cells, which can present antigens with the simultaneous expression of co-stimulatory molecules to T cells with comparable efficiency to mature DCs [5]. We demonstrated that human B cells express VDR and respond to calcitriol upon anti-CD40 + IL-4 stimulation [14,15,18,19,29]. In line with this, VDR expression is higher in naive than in memory B cells [19]. In this study, the expression of the co-stimulatory molecule CD86 was reduced by calcitriol in the naive B cell subpopulation and to a lesser extent in memory B cells. Indeed, calcitriol-mediated inhibition of T cell expansion via primed B cells was preferentially restricted to the naive, but not the memory, subset. These data are in line with findings from human monocyte-derived DCs which also down-regulate CD86 upon calcitriol [16]. As the T cell co-stimulatory molecule CD86 is a nuclear factor kappa B (NF-κB)-responsive target [30–32], and as VDR inhibits NF-κB activation in B cells and other cell types, e.g. via inhibition of nuclear translocation of p65 and p50 nuclear expression or a direct IκBα stabilization [14,19,33], this is most probably the underlying mechanism by which calcitriol-primed B cells modulate the T cell response. CD86 can activate CD28 and thereby provide critical co-stimulatory signals during antigen-specific T cell activation and cytokine responses [34]. In a murine model of rheumatoid arthritis, autoreactive T cell activation and the induction of arthritis were inhibited upon specific depletion of CD86 in B cells [35]. Blocking of the B7/CD28 ligation pathway results in changes of the immunological synapse and in reduced T cell activation proliferation and cytokine production consecutively [36,37]. Additionally, T cells co-stimulated by an activating antibody against CD28 express enhanced cytokine expression [37]. This pathway is currently targeted in the therapy of rheumatoid arthritis by using a dimeric recombinant cytotoxic T lymphocyte antigen 4 (CTLA4)/IgG Fc fusion protein, abatacept, which blocks B7/CD28 interaction and DC-dependent T cell activation [38]. In consequence, the circulating frequencies of Th1, Th2 and Th17 cells in the blood are reduced [39]. These findings support our observation that the addition of anti-CD28 to the B–T cell co-culture could mimic antigen-presenting help and was sufficient to restore T cell activation and cytokine production.
T cells co-cultured in the presence of calcitriol-primed B cells showed reduced proinflamatory cytokine production and expression of activation marker CD154 (CD40L) upon restimulation.
T cell activation, as a prerequisite for proliferation, differentiation into effector cells and cytokine expression, is dependent upon several transcription factors, including calcineurin-dependent NFAT [40]. The engagement of co-stimulatory molecule signalling increases NFAT activation mainly via enhanced nuclear import of NFAT [41] and shortening the mean time for the first T cell division [42]. We observed lower amounts of NFATc2 protein in T cells co-cultured with calcitriol-primed B cells. Additionally, the level of NFATc2 mirrored the CD40L expression and cytokine production upon restimulation. Thus, T cell activation was most probably reduced following contact with the calcitriol-primed B cells.
As published previously, VDR activation induces IL-10 production in B cells [15]. However, in this setting IL-10 was not induced in B cells, due to the type of stimulation and time-frame [43]. Accordingly, the concentrations of secreted IL-10 were at the detection limits and neutralizing IL-10 antibodies did not affect the co-culture (data not shown).
VDR activation in T cells has a direct impact upon proliferation [44] and transcriptional modulation of cytokine-producing T cells, as also observed in this study [45]. However, in our setting calcitriol was not added to the T–B cell co-culture. We tested the co-cultures for possible calcitriol spillover effects to exclude any direct effects of calcitriol on T cells. Indeed, we did not find any biologically relevant remaining calcitriol concentrations (<1 nM). In addition, small amounts of calcitriol were titrated into the co-cultures without any measurable effects using up to 1 nM. Thus, we conclude that the direct modulation of T cell activation is most probably dependent upon VDR activation in B cells.
Taken together, in this study we demonstrate that T cell activation, cytokine and transcription factor expression were impaired in the presence of calcitriol-primed naive B cells. We describe a novel mechanism by which the bioactive form of vitamin D controls T cell activation, namely via reduced co-stimulation primed B cells.
Acknowledgments
Special thanks to Timo Lischke for technical support. This work was supported by a grant to M. W. from the Deutsche Forschungsgemeinschaft (DFG – SFB650/TP5).
Disclosure
The authors declare that they have no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. T cell proliferation following 7-day co-culture with activated B cells. Carboxyfluorescein diacetate N-succinimidylester (CFSE) dilution was determined in CD4+ T cells after 7-day co-culture activated [anti-CD40, 1 μg/ml + interleukin (IL)-4, 10 ng/ml, for 48 h] B cells. (a) T cell proliferation detected upon 7-day co-culture with activated and calcitriol primed, as indicated in the figure, B cells. (b) T cell proliferation upon 7-day co-culture with activated B cells in the presence of increasing concentrations of toxic shock syndrome toxin 1 (TSST-1). Data represent four to five independent experiments and analysed using the analysis of variance (anova) test. Data are shown as mean ± standard deviation. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001 are considered significant.
Fig. S2. No relevant calcitriol spillover from B cell culture into T–B cell co-culture. The human promyelocytic leukaemia HL-60 cell line was incubated with calcitriol 1 μmol/l–100 pmol/l (upper plots) or supernatants from anti-CD40 (1 μg/ml), interleukin (IL)-4 (10 ng/ml) preactivated and calcitriol (as indicated in the Figure)-treated B cells; 48 h preactivation following washing and additional incubation with RPMI-1640 medium for 1 and 3 days. Supernatants were tested for calcitriol spillover with calcitriol-dependent expression of CD38 by the HL-60 cell line.
Table S1. Primer sequences for quantitative polymerase chain reaction (qPCR).
References
- 1.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 2.Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453–1461. [PubMed] [Google Scholar]
- 3.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadi T, Flies A, Efebera Y, Sherr DH. CD40 Ligand-activated, antigen-specific B cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008;124:129–140. doi: 10.1111/j.1365-2567.2007.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011;164:1078–1082. doi: 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 7.Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heine G, Lahl A, Muller C, Worm M. Vitamin D deficiency in patients with cutaneous lupus erythematosus is prevalent throughout the year. Br J Dermatol. 2010;163:863–865. doi: 10.1111/j.1365-2133.2010.09948.x. [DOI] [PubMed] [Google Scholar]
- 9.Thudi A, Yin S, Wandstrat AE, Li QZ, Olsen NJ. Vitamin D levels and disease status in Texas patients with systemic lupus erythematosus. Am J Med Sci. 2008;335:99–104. doi: 10.1097/MAJ.0b013e318134eeb6. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66:S135–138. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 12.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeke F, van Etten E, Gysemans C, Overbergh L, Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med. 2008;29:376–387. doi: 10.1016/j.mam.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32:3395–3404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210–2218. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- 16.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen AW, Holmstrom K, Jensen SS, et al. Phenotypic and functional markers for 1alpha,25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin Exp Immunol. 2009;157:48–59. doi: 10.1111/j.1365-2249.2009.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milovanovic M, Heine G, Hallatschek W, Opitz B, Radbruch A, Worm M. Vitamin D receptor binds to the epsilon germline gene promoter and exhibits transrepressive activity. J Allergy Clin Immunol. 2010;126:1016–1023. doi: 10.1016/j.jaci.2010.08.020. 23 e1–4. [DOI] [PubMed] [Google Scholar]
- 19.Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-dihydroxyvitamin D3 impairs NF-kappaB activation in human naive B cells. Biochem Biophys Res Commun. 2011;407:699–702. doi: 10.1016/j.bbrc.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 21.Harp CT, Lovett-Racke AE, Racke MK, Frohman EM, Monson NL. Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clin Immunol. 2008;128:382–391. doi: 10.1016/j.clim.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 23.Bartels LE, Hvas CL, Agnholt J, Dahlerup JF, Agger R. Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int Immunopharmacol. 2010;10:922–928. doi: 10.1016/j.intimp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 25.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1alpha,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose-titration study. J Steroid Biochem Mol Biol. 2013;136:160–165. doi: 10.1016/j.jsbmb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann B, Heine G, Babina M, et al. Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy. 2011;66:540–548. doi: 10.1111/j.1398-9995.2010.02513.x. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- 31.Zou GM, Hu WY. LIGHT regulates CD86 expression on dendritic cells through NF-kappaB, but not JNK/AP-1 signal transduction pathway. J Cell Physiol. 2005;205:437–443. doi: 10.1002/jcp.20420. [DOI] [PubMed] [Google Scholar]
- 32.Giannoukakis N, Bonham CA, Qian S, et al. Prolongation of cardiac allograft survival using dendritic cells treated with NF-kB decoy oligodeoxyribonucleotides. Mol Ther. 2000;1:430–437. doi: 10.1006/mthe.2000.0060. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem. 2013;288:19450–19458. doi: 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Cozar FJ, Molina IJ, Cuadrado MJ, Marubayashi M, Pena J, Santamaria M. Defective B7 expression on antigen-presenting cells underlying T cell activation abnormalities in systemic lupus erythematosus (SLE) patients. Clin Exp Immunol. 1996;104:72–79. doi: 10.1046/j.1365-2249.1996.d01-648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J Immunol. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 36.Wetzel SA, McKeithan TW, Parker DC. Live-cell dynamics and the role of costimulation in immunological synapse formation. J Immunol. 2002;169:6092–6101. doi: 10.4049/jimmunol.169.11.6092. [DOI] [PubMed] [Google Scholar]
- 37.Petro TM, Chen SS, Panther RB. Effect of CD80 and CD86 on T cell cytokine production. Immunol Invest. 1995;24:965–976. doi: 10.3109/08820139509060721. [DOI] [PubMed] [Google Scholar]
- 38.Mayer E, Holzl M, Ahmadi S, et al. CTLA4-Ig immunosuppressive activity at the level of dendritic cell/T cell crosstalk. Int Immunopharmacol. 2013;15:638–645. doi: 10.1016/j.intimp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieper J, Herrath J, Raghavan S, Muhammad K, Vollenhoven R, Malmstrom V. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol. 2013;14:34. doi: 10.1186/1471-2172-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 41.Diehn M, Alizadeh AA, Rando OJ, et al. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gett AV, Hodgkin PD. A cellular calculus for signal integration by T cells. Nat Immunol. 2000;1:239–244. doi: 10.1038/79782. [DOI] [PubMed] [Google Scholar]
- 43.Milovanovic M, Heine G, Zuberbier T, Worm M. Allergen extract-induced interleukin-10 in human memory B cells inhibits immunoglobulin E production. Clin Exp Allergy. 2009;39:671–678. doi: 10.1111/j.1365-2222.2009.03233.x. [DOI] [PubMed] [Google Scholar]
- 44.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi S, Pantalena LC, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. T cell proliferation following 7-day co-culture with activated B cells. Carboxyfluorescein diacetate N-succinimidylester (CFSE) dilution was determined in CD4+ T cells after 7-day co-culture activated [anti-CD40, 1 μg/ml + interleukin (IL)-4, 10 ng/ml, for 48 h] B cells. (a) T cell proliferation detected upon 7-day co-culture with activated and calcitriol primed, as indicated in the figure, B cells. (b) T cell proliferation upon 7-day co-culture with activated B cells in the presence of increasing concentrations of toxic shock syndrome toxin 1 (TSST-1). Data represent four to five independent experiments and analysed using the analysis of variance (anova) test. Data are shown as mean ± standard deviation. *P ≤ 0·05; **P ≤ 0·01; ***P ≤ 0·001 are considered significant.
Fig. S2. No relevant calcitriol spillover from B cell culture into T–B cell co-culture. The human promyelocytic leukaemia HL-60 cell line was incubated with calcitriol 1 μmol/l–100 pmol/l (upper plots) or supernatants from anti-CD40 (1 μg/ml), interleukin (IL)-4 (10 ng/ml) preactivated and calcitriol (as indicated in the Figure)-treated B cells; 48 h preactivation following washing and additional incubation with RPMI-1640 medium for 1 and 3 days. Supernatants were tested for calcitriol spillover with calcitriol-dependent expression of CD38 by the HL-60 cell line.
Table S1. Primer sequences for quantitative polymerase chain reaction (qPCR).


