Abstract
Although it is widely believed that interleukin (IL)-27 is anti-inflammatory, its role in controlling human immune responses is not fully established. In particular, its interactions with T helper type 17 (Th)17 cytokines are unclear. Our aims were to establish the relationships between IL-27 and proinflammatory cytokines, including IL-17A, in human sera and cultures of peripheral blood mononuclear cells. Plasma IL-27 levels in 879 healthy humans from 163 families varied widely, but with relatively low heritability (19%). Despite IL-27 including a subunit encoded by Epstein–Barr virus-induced gene 3 (EBI3), there was no correlation of levels with serological evidence of infection with the virus. Although IL-27 has been reported to inhibit IL-17A production, we demonstrated a strong positive correlation in sera, but lower correlations of IL-27 with other proinflammatory cytokines. We verified that IL-27 inhibited IL-17A production by human peripheral blood T cells in vitro, but not that it stimulated IL-10 secretion. Importantly, addition of IL-17A decreased IL-27 production by stimulated T cells but had the opposite effect on resting T cells. Together, these data suggest a model whereby IL-27 and IL-17A exerts complex reciprocal effects to boost inflammatory responses, but restrain resting cells to prevent inappropriate activation.
Keywords: cytokines, human, IL-17A, IL-27, T cells
Introduction
Interleukin (IL)-27 is a heterodimeric cytokine that is composed of the subunits p28 and Epstein–Barr-induced gene 3 (EBI3) [1]. Dendritic cells (DCs) and macrophages produce interleukin (IL)-27 subunits in response to stimulation with Toll-like receptor (TLR)-3, -4 or -9 agonists signalling via myeloid differentiation primary response gene 88 (MyD88) [2]. The IL-27 receptor (IL-27R) is also heterodimeric and consists of WSX-1 (leucocyte-restricted expression) and gp130 (ubiquitous expression), with the highest level of IL-27R expression found on activated T cells and natural killer (NK) cells [3]. IL-27 receptor ligation activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, particularly STAT-1 and STAT-3 [2]. Since the identification of IL-27, when it was initially proposed to be proinflammatory, there has been a growing consensus that its main role is anti-inflammatory.
Initial data following the identification of IL-27 suggested that the cytokine had proinflammatory properties and was able to induce production of interferon (IFN)-γ by naive human or murine T cells [1]. However, mice lacking IL-27 receptor subunits suffer lethal inflammation in response to infections, indicating that IL-27 has an immunosuppressive phenotype [4]. For example, WSX-1−/− IL-27 receptor-deficient mice infected with Toxoplasma gondii, despite producing a protective T cell response and limiting parasite replication, die of a T cell-mediated inflammatory disease [4]. EBI3-deficient mice demonstrate enhanced delayed-type hypersensitivity (DTH) responses [5]. Furthermore, consistent with an anti-inflammatory role for IL-27, WSX-1−/− mice are more susceptible to experimental autoimmune encephalomyelitis (EAE) and generate more T helper type 17 (Th17) cells [6], effects that have been attributed to inhibition of granulocyte–macrophage colony-stimulating factor (GM-CSF) production by IL-27 [7].
It has been suggested that the anti-inflammatory properties of IL-27 result from effects on T cells, particularly naive T cells. For example, IL-27 has been demonstrated in mice to increase the production of the suppressive cytokine IL-10 by CD4+ T cells, and to decrease the production of IL-17A and GM-CSF [8]. IL-27 activation of STAT-1 has been shown to inhibit IL-17 production [6], and recent data suggest that the effect includes induction of programmed death ligand 1 (PD-L1) to block bystander cell differentiation to a Th17 phenotype [9]. Other mechanisms by which IL-27 inhibits IL-17 have been identified, including direct suppression of RAR-related orphan receptor (ROR)γt and RORα, and the promotion of T cells secreting IL-10 [10,11]. This report also showed that IL-27 suppresses both developing and committed Th17 cells and other Th17-associated cytokines such as IL-22 and IL-21 [12].
In many human autoimmune diseases, IL-27 and IL-17 have been shown to be involved in disease progression or failure of resolution. In mouse models of experimental autoimmune uveitis (EAE), WSX-1 mice have reduced clinical scores [13] and in EAE up-regulated expression of IL-27p28 and EBI3 by antigen-presenting cells (APC) in the central nervous system (CNS) [14]. In active Crohn's disease, mucosal expression of IL-27p28 and EBI3 is higher than in non-inflammatory bowel disease (IBD) control subjects, and in rheumatoid arthritis (RA) increased levels of IL-27 are found in the synovial fluid compared to osteoarthritis patients [2]. IL-17 serum levels have been shown to be elevated in patients with RA [15], systemic lupus erythematosus [16] and Crohn's disease [17] compared to healthy controls. IL-17 mRNA is increased in mononuclear cells in the blood of multiple sclerosis patients. This overlap in disease associations, as well as the effects of IL-27 on Th17 differentiation, indicates that the inter-relationships between these two cytokines requires clarification.
Despite the extensive interest in the immune properties of IL-27, and its potential role in disease, much of the biology of the cytokine has yet to be determined, particularly in humans. Variations in IL-27 concentrations between individuals may influence immune responsiveness and contribute to disease susceptibility, but the circulating levels in healthy human populations have not been measured systematically. Our first aim was therefore to measure plasma IL-27 in a large cohort of healthy human donors and to address the open question as to whether any individual variation results from genetic or environmental factors. Given the inter-relationships between IL-27 and other cytokines in mice, particularly with IL-17, we also wished to determine whether their levels are associated in humans, and to identify any reciprocal effects on T cells of varying the concentrations of these cytokines.
Materials and methods
Donors
Healthy volunteer donors were recruited from the University of Aberdeen, and samples for preparation of serum or peripheral blood mononuclear cells (PMBC) taken by venepuncture, respectively, into plain or lithium heparin Vacutainers (Becton Dickinson, Oxford, UK). The NHS Grampian Ethical Committee approved the study, and all donors gave informed consent. Plasma samples for analyses of heritability of cytokine levels were obtained from a cohort recruited in 1993–96 as part of a study into the genetic basis of hypertension, as described previously [18,19]. Ethical approval for participant recruitment was obtained from the Central Oxford Research Ethics Committee. Families with at least four members and at least one member with idiopathic hypertension were recruited. All participants were of self-reported British Caucasian ancestry and aged more than 18 years. Blood was immediately placed on ice and transported to a central facility for centrifugation, generally within 2 h of blood draw. Plasma was frozen at −80°C until assays were performed.
EBV and CMV serostatus assays
Semi-quantitative determination of IgG antibodies to Epstein–Barr virus (EBV) nuclear antigen (EBNA)-1 in human plasma was performed by indirect enzyme immunoassay Is-EBNA-1 IgG Test Kit (Diamedix Corporation, Hialeah, FL, USA), according to the manufacturer's instructions. The qualitative detection of immunoglobulin (Ig)G antibodies to cytomegalovirus (CMV) in human serum was performed by bioenzyme-linked immunosorbent assay (bioELISA) CMV IgG (Biokit, Barcelona, Spain), according to the manufacturer's instructions.
Cell culture
PBMC were isolated from whole blood by density gradient centrifugation [20]. CD4+ T cells, CD45RA+ naive CD4+ T cells or CD45RO+ memory CD4+ T cells were isolated using commercially available negative selection kits (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the fractions was 85–95%, as determined by flow cytometric analysis using anti-CD4 phycoerythrin-cyanin7 (PE-Cy7), anti-CD45RO PE-Cy5 or anti-CD45RA fluorescein isothiocyanate (FITC). PBMCs, CD4+ T cells, naive CD4+ T cells or memory CD4+ T cells were cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) containing 10% fetal calf serum (FCS), 2% penicillin streptomycin and 1% L-glutamine at a concentration of 1 × 106 cells/ml in 24-well plates. T cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (precoated at 0·1 μg/ml; R&D Systems, Minneapolis, MN, USA). Where indicated, recombinant human (rh) IL-27 or rhIL-17A (both R&D Systems) was added.
Co-culture assays
PBMC without T cells and T cell-only cultures were created using a CD2-positive isolation kit (Miltenyi Biotec). The purity of the fractions was 90% for T cells only and PBMC without T cells 99%, as determined by flow cytometric analysis using anti-CD3 FITC. Both PBMC without T cells and T cells were cultured in RPMI-1640 containing 10% FCS, 2% penicillin streptomycin and 1% L-glutamine at a concentration of 1 × 106 cells/ml in 24-well plates. T cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (precoated at 0·1 μg/ml). Where indicated, 100 ng/ml rhIL-27 or rhIL-17A was added for 24 h. T cells and PBMC without T cells were washed and the T cells were then added back to the PBMC without T cells and cultured for a further 3 days before IL-17A or IL-27 ELISAs were performed.
Cytokine assays
ELISA determined cytokine levels in serum samples, with cell-based ELISAs (celELISA) [21] used to measure cytokine production by PBMC after 5 days of culture (unless different timing specified). The levels of IFN-γ, IL-10, IL-4 or transforming growth factor (TGF)-β1 were measured, using antibody pairs from BD Pharmingen (San Jose, CA, USA), and IL-17A using antibodies from eBioscience (San Diego, CA, USA). The concentrations of IL-27 in cultures or sera were determined using the Duoset ELISA Development System for human IL-27 (R&D Systems). If cytokine levels were below the assay sensitivity level, values of 4 pg/ml for IL-10 and IL-17A and 8 pg/ml for IL-27, IFN-γ and TGF-β1 were allocated, representing half of the lowest detectable values for the respective cytokines. In the cases where the level of cytokine detected was above the range of the standard curve, then the highest standard concentration was assigned.
Real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Total RNA was extracted from cultured PBMC with the RNAeasy Mini Kit and QIAshredder (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. First-strand cDNA was synthesized from isolated RNA using Superscript® III First Strand Synthesis Supermix (Life Technologies, Carlsbad, CA, USA). qRT–PCR was used to quantify transcripts with the StepOnePlus System (Applied Biosystems, Carlsbad, CA, USA) using primers designed using the Universal ProbeLibrary Assay Design Center (Roche, Indianapolis, IN, USA) for TBX21, related orphan receptor C (RORC), STAT-3, STAT-4 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The housekeeping gene, GAPDH, was used to normalize mRNA abundance.
Statistical analysis
The heritability of plasma IL-27 levels and evidence for genetic association with genotyped single nucleotide polymorphisms (SNPs) was calculated using merlin software [22].
The comparisons of serum concentrations were analysed using the non-parametric Mann–Whitney U-test. The associations between cytokine serum concentrations were determined by Spearman's rank correlation. Changes in cytokine production were analysed by Wilcoxon's signed-rank tests. Two-tailed P-values lower than 0·05 were considered significant.
Results
Peripheral blood cytokine concentrations
The initial aim was to establish the range of serum IL-27 concentrations in healthy human sera. Measurements from 43 volunteer donors indicated that, while most values lay between 100 and 1000 pg/ml (n = 27), a substantial minority of individuals had very high concentrations (>2000 pg/ml; n = 12). Our initial hypothesis was that such variation was genetically determined. We therefore measured plasma IL-27 concentrations from 879 healthy individuals from 163 families who had already been genotyped at multiple loci [23]. Again, a minor proportion of donors exhibited high values, with 10% more than four times the median IL-27 concentration (>2000 pg/ml), with 4·4% >6000 pg/ml (Fig. 1). Several environmental covariates (smoking, exercise, sex) were tested for association with IL-27 levels, of which only non-smoking versus other and no exercise versus some exercise were correlated significantly and positively. However, together these only accounted for 1·6% of variance. Heritability was calculated using merlin software at 19·4%.
Fig. 1.
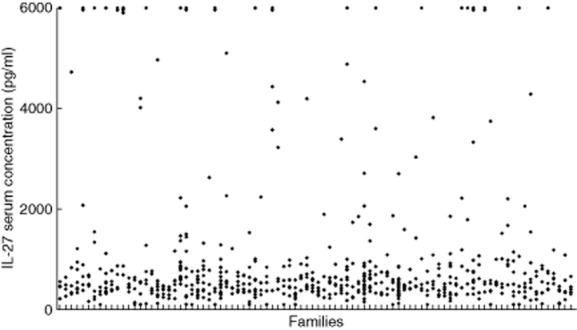
Plasma concentrations of interleukin (IL)-27 in donors grouped by family. The concentration of IL-27 in plasma from 879 individuals from 163 families was determined by enzyme-linked immunosorbent assay (ELISA). Each dash on the horizontal axis indicates a family, each diamond a family member. The graph shows results from families with five or more tested members (n = 625).
Given the relatively low heritability of IL-27 levels, we investigated chronic infection as a possible environmentally determined factor. A number of pieces of circumstantial evidence led us to focus on EBV. The EBI3 subunit of IL-27 is induced by EBV [24]; >90% of individuals are infected and the virus has a profound and persistent effect on the immune system [25]. When we tested whether or not infection with EBV was correlated with IL-27 levels using the family samples (Fig. 2a), no correlation was observed (P = 0·91, Mann–Whitney). Previous infection with CMV was also investigated, as this virus is also known to exert widespread and persistent effects on the immune system [26]. However, there was also no difference between the two donor groups for IL-27 (P = 0·98, Fig. 2b).
Fig. 2.
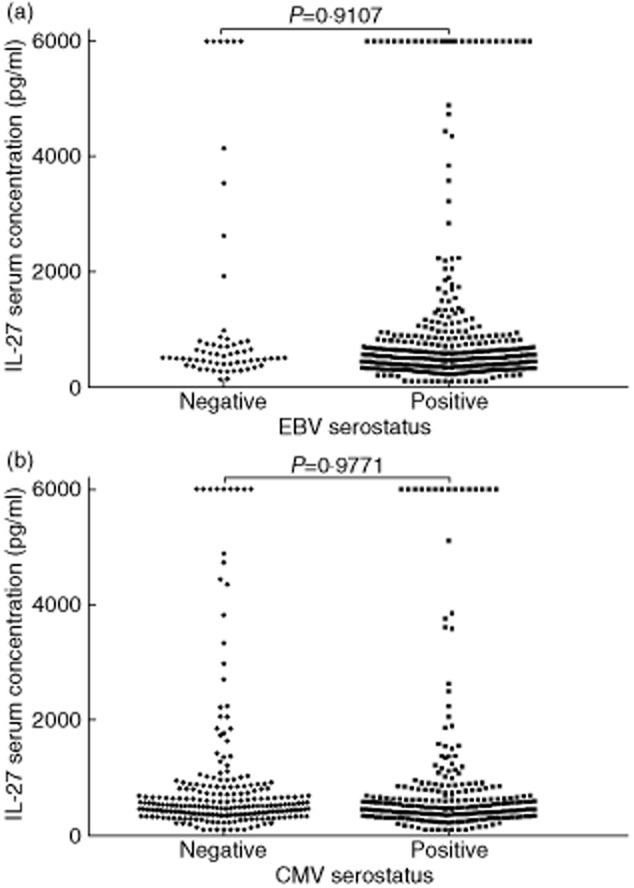
Comparison of interkeukin (IL)-27 plasma concentrations between Epstein–Barr virus (EBV) and cytomegalovirus (CMV)-seropositive and -seronegative donors. The seropositivity for EBV and CMV was determined by indirect enzyme immunoassay and bioELISA, respectively, and IL-27 concentration by enzyme-linked immunosorbent assay (ELISA). (a) EBV-seropositive (n = 380) and -seronegative (n = 63) demonstrate no difference in IL-27 concentrations. (b) CMV-seropositive (n = 240) and -seronegative (n = 233) demonstrate no difference in IL-27 concentrations. P-values determined by Mann–Whitney U-test.
We next tested whether high IL-27 values were indicative of an ongoing inflammatory response, by measuring IL-6, TNF-α and C-reactive protein (CRP) levels in the above familial samples. However, the correlations between IL-27 values and each of the three parameters (IL-6 R2 = 0·00011, P = 0·744; TNF-α R2 = 0·019, P ≤ 0·001; CRP R2 = 0·00038, P = 0·539) were low (Supporting information, Fig. S1). Correlations of IL-27 with IL-10, IFN-γ and in particular IL-17A were also determined in a more restricted set of donors, (Fig. 3), as these cytokines have been described previously to interact with IL-27 [27]. Furthermore, IL-17A levels have been associated with some of the same autoimmune diseases as IL-27 [28]. The presence of high concentrations of IL-27, and published evidence that IL-27 decreases the concentration of IL-17A produced by T cell cultures [6], suggested that high levels of IL-27 would be associated with low levels of IL-17A. In contrast to this prediction, a strong positive correlation was seen between IL-27 and IL-17A concentrations in the panel of sera (R2 = 0·65, P ≤ 0·001, Fig. 3a). IL-27 and IL-10 concentrations were also correlated positively, but less strongly (R2 = 0·34, P ≤ 0·001, Fig. 3b), while IL-27 and IFN-γ were only poorly correlated (R2 = 0·17, P = 0·008) (Fig. 3c). The inter-relationships between these cytokines were also illustrated by the findings that IL-17A was also correlated positively with IL-10 (R2 = 0·59, P ≤ 0·001) (Supporting information, Fig. S2a) and, less strongly, with IFN-γ (R2 = 0·34, P ≤ 0·001) (Supporting information, Fig. S2d).
Fig. 3.
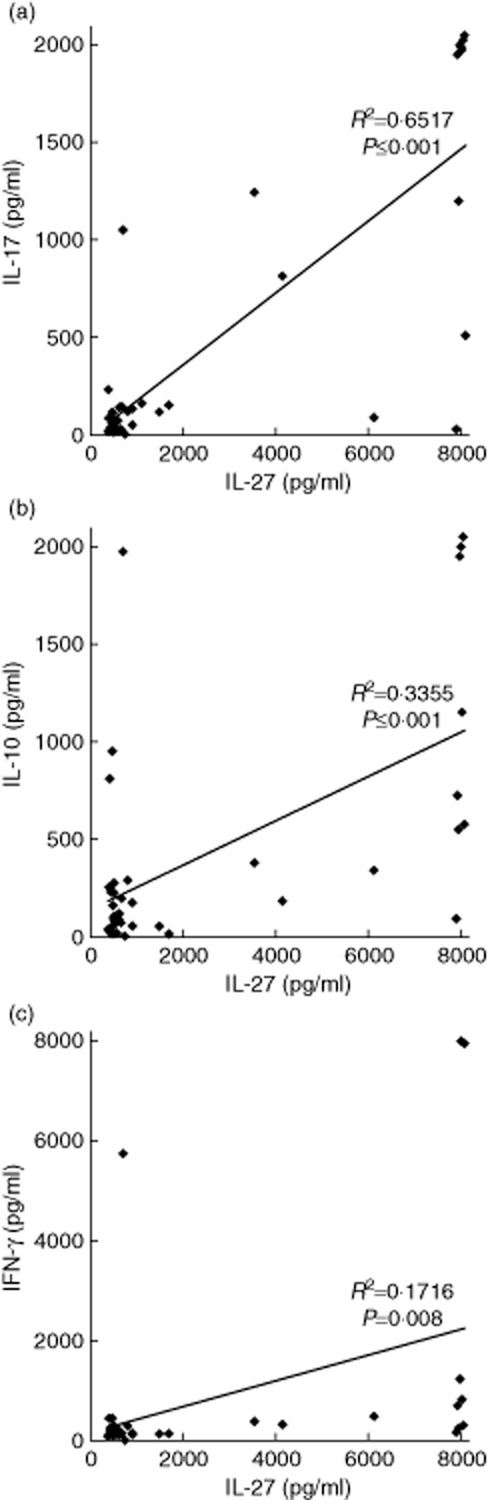
Correlation between cytokine serum concentrations in healthy donors. Enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-27, IL-17A, IL-10 and interferon (IFN)-γ were performed on serum samples from healthy donors. (a) IL-27 concentration compared to IL-17A concentration (n = 42). (b) IL-27 concentration compared to IL-10 concentration (n = 40). (c) IL-27 concentration compared to IFN-γ (n = 40). Spearman's rank correlation coefficients are shown.
IL-27 affects the production of IL-17A, IL-10 and IFN-γ by PBMC cultures
The positive correlation between IL-27 and IL-17A in sera raised the question as to whether the relationship was causal, so we tested the effects of each cytokine on production of the other in cultures of PBMC. We first demonstrated that rhIL-27 addition decreased the production of IL-17A in human PBMC cultures (Fig. 4). All experiments were performed on PBMC from donors with low (<1000 pg/ml) IL-27 serum concentrations. IL-17A concentrations were measured on days 1, 3, 5 and 7 of culture to investigate if there were any differences in early responses to rhIL-27 compared to established activation, as some studies have suggested that the effects of IL-27 differ when comparing committed and naive T cells [8]. In unstimulated cultures, IL-17A levels increased slightly in response to rhIL-27, but the increase was not significant (0 versus 100 ng/ml: day 1, P = 0·317, day 3, P = 0·317, day 5, P = 0·285, day 7, P = 0·917, Wilcoxon's signed-rank tests; Fig. 4a). However, in the stimulated cultures on all days tested, rhIL-27 addition resulted in a decrease in production of IL-17A (Fig. 4b). This effect was only seen when 10 ng/ml or more rhIL-27 was added. These results confirm that rhIL-27 decreases the production of IL-17A in our system.
Fig. 4.
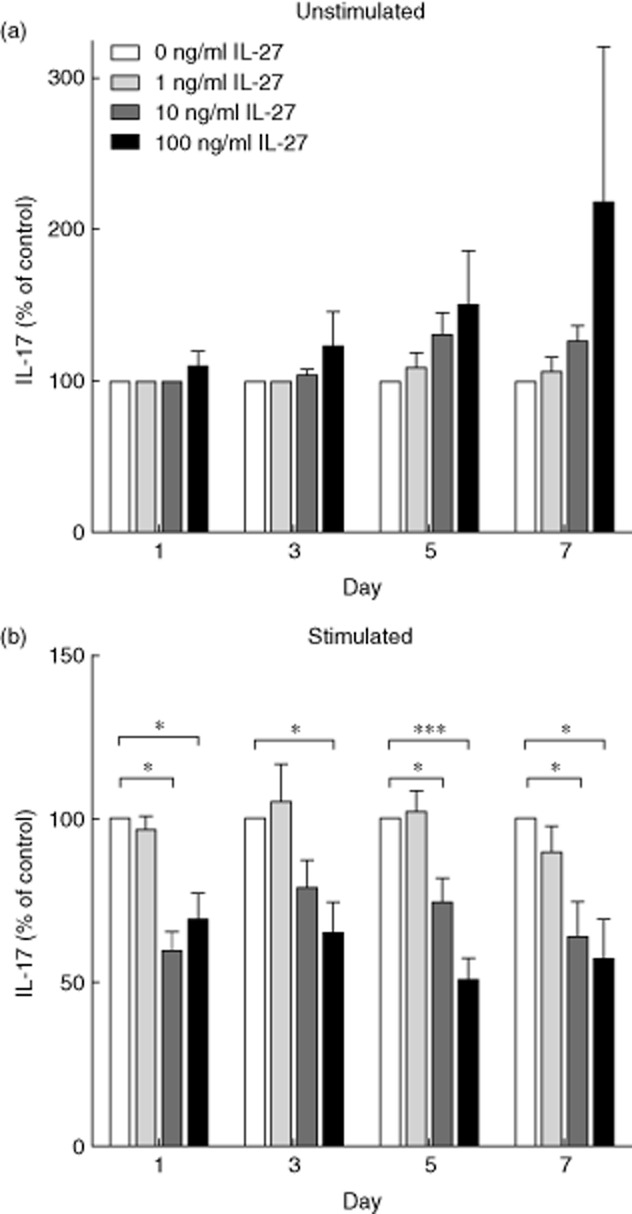
Interleukin (IL)-17A concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. PBMC were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-27 (0, 1, 10 or 100 ng/ml). On days 1, 3, 5 and 7 the concentration of IL-17A in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) PBMC were left unstimulated; no significant differences were observed in IL-17A production (n = days 1, 8; days 3, 8; days 5, 11; days 7, 8). (b) T cells within the PBMC were stimulated by plate-bound anti-CD3 and anti-CD28. Significant differences were observed on all days (n = days 1, 8, days 3m 8, days 5, 17, days 7m 8); day 1, 0 versus 10 ng/ml P = 0·043; 0 versus 100 ng/ml P = 0·028. Day 3, 0 versus 100 ng/ml P = 0·028. Day 5, 0 versus 10 ng/ml P = 0·028; 0 versus 100 ng/ml P < 0·001. Day 7, 0 versus 10 ng/ml P = 0·043; 0 versus 100 ng/ml P = 0·017. The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test, *P < 0·05; ***P < 0·001.
We also measured the production of IL-10 and IFN-γ in unstimulated and stimulated cultures on day 5, when these cytokine responses peak [29] (Fig. 5). In unstimulated cultures, the concentration of IL-10 did not change significantly in response to the addition of rhIL-27 (0 versus 100 ng/ml, P = 0·131), but the production of IFN-γ increased significantly (0 versus 10 ng/ml, P = 0·043, 0 versus 100 ng/ml, P = 0·008) (Fig. 5a). However, in stimulated cultures, the concentration of IL-10 changed with the addition of rhIL-27 with, in contrast to other reports [27,30], IL-10 levels falling rather than rising, even at low concentrations (for example, 1 ng/ml) of added rhIL-27 (Fig. 5b). The production of IFN-γ did not alter significantly with the addition of rhIL-27 to stimulated cultures (0 versus 100 ng/ml, P = 0·807). The concentrations of cytokines TGF-β and IL-4 were also measured in response to rhIL-27 addition and did not display any consistent changes (data not shown).
Fig. 5.
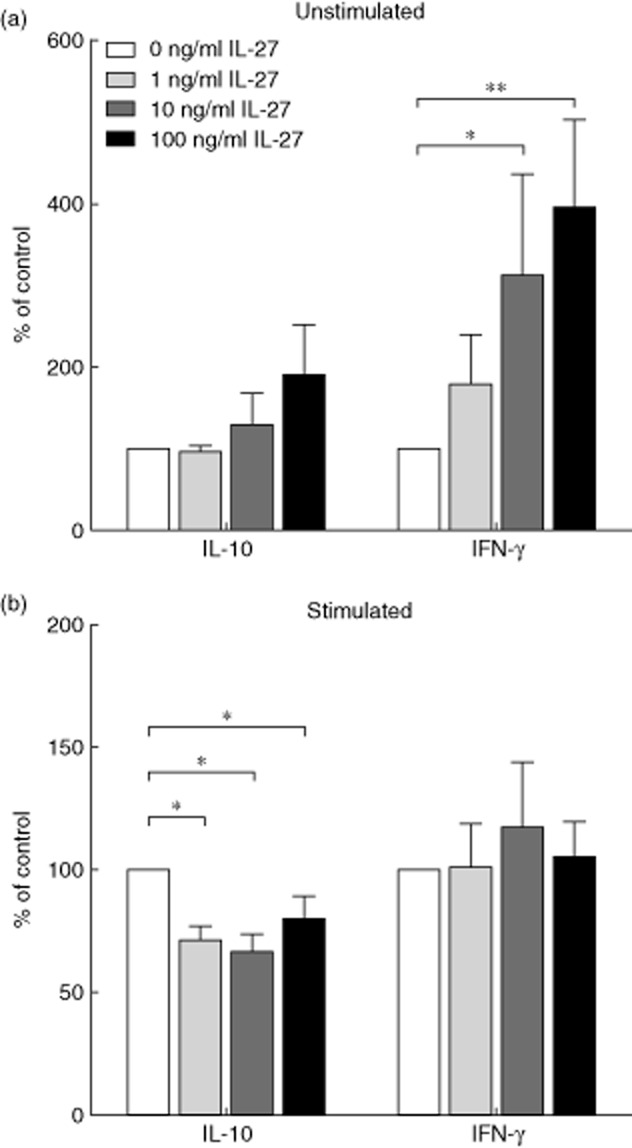
Interleukin (IL)-10 and interferon (IFN)-γ concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. PBMC were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-27 (0, 1, 10 or 100 ng/ml). On the fifth day of culture the concentration of IL-10 and IFN-γ in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) PBMC were left unstimulated; no significant differences were observed in IL-10 production. (n = 0 ng/ml 14; 1 ng/ml 5, 10 ng/ml 5; 100 ng/ml 14). Significant differences in IFN-γ were observed: 0 versus 10 ng/ml P = 0·043; 0 versus 100 ng/ml P = 0·008. (n = 0 ng/ml 11; 1 ng/ml 5; 10 ng/ml 5; 100 ng/ml 11). (b) T cells within the PBMC were stimulated by plate-bound anti-CD3 and anti-CD28. Significant differences were observed in IL-10 production: 0 versus 1 ng/ml P = 0·012; 0 versus 10 ng/ml P = 0·012; 0 versus 100 ng/ml P = 0·020 (n = 0 ng/ml 16; 1 ng/ml 8, 10 ng/ml 8; 100 ng/ml 16). No significant differences were observed in IFN-γ production (n = 0 ng/ml 13; 1 ng/ml 8; 10 ng/ml 8; 100 ng/ml 13). The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test, *P < 0·05; **P < 0·01.
In order to indicate which transcriptional pathways are involved in these changes, we performed qPCR for the transcription factors T-bet, ROR-γt, STAT-3 and STAT-4 (Supporting information, Fig. S3). The results are consistent with the effects of IL-27 on IFN-γ or IL-17A production being mediated via T-bet and ROR-γt, respectively. Thus, reflecting the changes in IFN-γ production illustrated in Fig. 5, T-bet mRNA levels increased after IL-27 treatment of unstimulated PBMC, while there was there was less effect in anti-CD3 anti-CD28-stimulated cultures. Furthermore, IL-27 inhibited the induction of ROR-γt by anti-CD3 anti-CD28, while there was little inhibition in unstimulated PBMC, consistent with changes to IL-17A levels shown in Fig. 4. STAT-3 and STAT-4 mRNA levels showed no changes after IL-27 treatment of stimulated or unstimulated PBMC cultures, although these data do not exclude involvement of these transcription factors via, for example, phosphorylation status.
The cell type that decreased its production of IL-17A as a result of the addition of rhIL-27 was not determined from the initial PBMC cultures. Most of the literature on the decrease of IL-17A production by the addition of IL-27 suggests that this effect only occurs when added to naive CD4+ T cells [8]. To establish whether the decreases in IL-17A levels were a consequence of changing production by CD4+ T cells, either naive or memory, PBMC were fractionated into CD4+, CD4+CD45RA+ (naive) and CD4+CD45RO+ (memory) cultures. None showed any marked changes in the production of IL-10, IFN-γ, TGF-β or IL-4 (data not shown) when rhIL-27 was added. Although rhIL-27 decreased the concentration of IL-17A produced from both naive and memory CD4+ T cells (Supporting information, Fig. S4), the changes were not statistically significant. These data are not conclusive, but suggest that both naive and memory CD4+ T cells may decrease their production of IL-17A as a result of culturing with rhIL-27.
IL-17A affects the production of IL-27 by PBMC cultures
The decreased production of IL-17A in response to rhIL-27, but a positive correlation between the serum concentrations of the two cytokines, appeared paradoxical and raised the possibility that IL-17A may influence IL-27 production. To investigate this possibility, the effects of rhIL-17A addition on the production of IL-27 by PBMC in resting and stimulated cultures were examined over a period of 7 days (Fig. 6). The culture conditions were the same as for the IL-27 addition experiments. The PBMC IL-27 responses to rhIL-17A depended on the activation status of the T cells. In unstimulated cultures, the addition of rhIL-17A to the culture medium increased the production of IL-27. In contrast, when stimulated with anti-CD3 and anti-CD28 in the presence of rhIL-17A, during early responses (before day 5) the production of IL-27 decreased, but later in the culture period these differences were lost. During these experiments the production of IL-10 and IFN-γ were measured on day 5 and no significant differences were identified (0 versus 100 ng/ml: IL-10 unstimulated, P = 0·144; stimulated, P = 0·345; IFN-γ unstimulated, P = 0·893; stimulated, P = 0·225; Fig. 7). Taken together, these data suggest that rhIL-17A has opposing effects on the production of IL-27, depending on the activation status of the T cells within the culture. The apparent counter regulation of IL-27 and IL-17 raises the question as to whether these effects are mediated via APC or T cells, Both populations appear to be necessary, as criss-cross experiments with reciprocal cytokine treatment of purified T cells and PBMC depleted of T cells showed that neither fraction responded as strongly as unfractionated PBMC (data not shown).
Fig. 6.
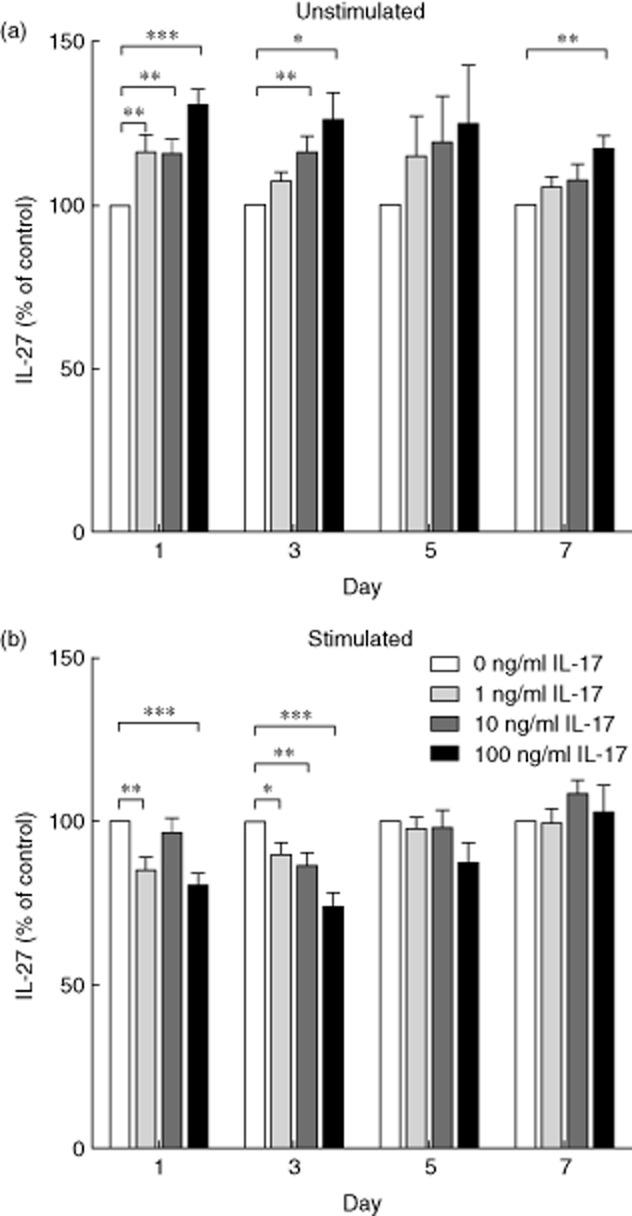
Interleukin (IL)-27 concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-17A. PBMC were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-17A (0, 1, 10 or 100 ng/ml). On days 1, 3, 5 and 7 the concentration of IL-27 in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) PBMC were left unstimulated, significant differences were observed on days 1, 3 and 7. Day 1, 0 versus 1 ng/ml P = 0·003; 0 versus 10 ng/ml P = 0·005; 0 versus 100 ng/ml P < 0·001. Day 3, 0 versus 10 ng/ml P = 0·005; 0 versus 100 ng/ml P = 0·012. Day 7, 0 versus 100 ng/ml P = 0·003. n = 15. (b) T cells within the PBMC were stimulated by plate-bound anti-CD3 and anti-CD28. Significant differences were observed on days 1 and 3. Day 1, 0 versus 1 ng/ml P = 0·003; 0 versus 100 ng/ml P,0·001. Day 3, 0 versus 1 ng/ml P = 0·027; 0 versus 10 ng/ml P = 0·006; 0 versus 100 ng/ml P < 0·001. n = 15. The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test, *P < 0·05; **P < 0·01, ***P < 0·001.
Fig. 7.
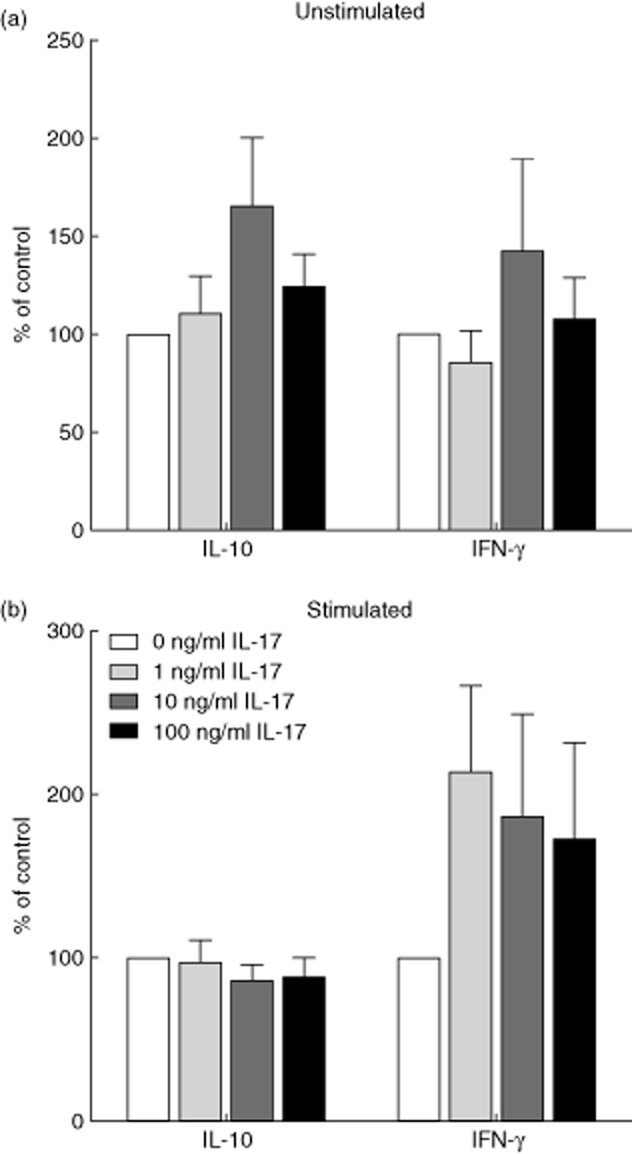
Interleukin (IL)-10 and interferon (IFN)-γ concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-17A. PBMC were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-27 (0, 1, 10 or 100 ng/ml). On the fifth day of culture the concentration of IL-10 and IFN-γ in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) PBMC were left unstimulated; no significant differences were observed for either cytokine, n = 5. (b) T cells within the PBMC were stimulated by plate-bound anti-CD3 and anti-CD28. No significant differences were observed, n = 5. The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test.
Discussion
In this study we report that plasma concentrations of IL-27 are highly variable between human subjects. Genetic influence was assessed to not be the main determinant and IL-27 levels failed to correlate with the presence of antibodies to either EBV or CMV. When investigating the relationship between IL-27 and IL-17A, we found a positive correlation between the serum levels of these cytokines. However, we also confirmed previous reports that rhIL-27 inhibits IL-17A production by T cells in vitro. These apparently paradoxical findings led us to examine the effects of IL-17A on IL-27 production. The results reveal a complex relationship between the two cytokines, with rhIL-17A increasing production of IL-27 in unstimulated cultures, but exerting the opposite effect when T cells were stimulated.
Several studies have indicated associations between IL-27 serum concentrations and autoimmune disease. Patients with systemic luous erythematosus (SLE) have significantly lower IL-27 serum concentrations than healthy controls [31]. Over-expression of WSX-1 in a mouse model of SLE has been shown to be associated with the development of autoimmune disease [32]. In contrast, elevated IL-27 serum concentrations have been found in patients with psoriasis compared with healthy controls, with the severity of disease correlating with IL-27 concentration [33]. Similarly, in patients with systemic sclerosis there are higher concentrations of IL-27 in the serum compared to healthy controls, concentrations correlating with extent of fibrosis [34]. Ischaemic heart disease has also been shown to associate with increased concentrations of serum IL-27 [35]. However, these associations may be secondary to disease-associated immune processes and IL-27 concentration may not be a causal or contributory factor.
Few studies have been performed on heritability of cytokine levels [36,37]. We found heritability of plasma IL-27 concentrations to be 19%, indicating that genetic factors are not the main determinants of the high levels we observed in certain subjects. This is notable, given that polymorphisms within IL-27 genes have been associated with several diseases, including inflammatory bowel disease, chronic obstructive pulmonary disease, asthma and colorectal cancer [33].
Given the EBV-inducible nature of one of the IL-27 subunits (EBI3), one hypothesis to be tested was whether infection with the virus was a factor in IL-27 serum concentrations. EBV has been shown previously to affect cytokine responses, with the peripheral T and NK cell populations losing expression of IL-15Rα after infectious mononucleosis (IM) even when tested 14 years later [25]. Co-infection with CMV is thought to alter the immune response to EBV [26], so we also tested for CMV serostatus. However, neither EBV nor CMV infection status was correlated with IL-27 levels. Although hepatitis B virus infection has been shown to be associated with elevated serum IL-27 levels [38], our data indicate that EBV and CMV do not have this effect.
The identification of high IL-27 concentrations in subjects without raised levels of IL-6, TNF-α or CRP indicates that these subjects do not have a generalized immune activation. However, IL-27 and IL-17A are correlated strongly within serum, suggesting a complex relationship between IL-27 and IL-17A that may well involve mutual regulation. The reduction in IL-17A production by activated PBMC cultures in response to rhIL-27 addition reported here is in line with numerous reports on the effects of IL-27 [8,10,27]. Our data demonstrate that both naive and memory CD4 cells respond in this way, supporting a previous study [12]. These findings of reduced IL-17 production by T cells do not exclude a possible effect on non-CD4+ T cells, but given that the highest expression of IL-27R is found on activated T cells [3], it is likely that they dominate the IL-27 response in PBMC cultures.
Despite demonstrating a decrease in IL-17A production with the addition of rhIL-27 to PBMC cultures, indicating that rhIL-27 was functional in our laboratory, we failed to measure an increase in IL-10 production. An increase in IL-10 production has been shown previously to occur in the presence of IL-27 in mouse [11,30] and human [27] T cell cultures, which is now reflected in the consensus as to the role of IL-27 [33]. None the less, despite closely replicating the conditions of Murugaiyan et al. [27], we failed to identify a similar increase in IL-10. Furthermore, other studies have also not detected statistically significant increases in IL-10 production in response to IL-27. In murine T cell cultures, IL-27 was shown to decrease or not affect the concentration of IL-10 produced by CD4+ T cells activated by plate-bound anti-CD3 and soluble anti-CD28 [12,39]. It has also been shown that there are site-specific reductions in DNA methylation in murine, but not human, IL10 genes in response to IL-27 [40]. Taken together, these results indicate that induction of IL-10 from CD4+ T cells by IL-27 in humans is not robust. Furthermore, Yoshimura et al. [39] and Liu and Rohohsky-Kochan [12], as well as our study, detected decreases in IL-17 production in response to IL-27, despite not identifying increases in IL-10, suggesting that the reduction in IL-17A production is not a result of the inhibitory effect of IL-10.
A recent study showed that IL-17 injected into murine synovial joints results in increased IL-27 expression, and that in collagen-induced arthritis mice this response was increased further [41]. In this study we demonstrate that rhIL-17A affects the production of IL-27 in human PBMC cultures. If T cells within a PBMC culture are left unstimulated addition of rhIL-17A results in an increase in IL-27, but when T cells are stimulated rhIL-17A has the opposite effect. This indicates that the relationship between IL-27 and IL-17A in humans is not unidirectional and is the first evidence that activation status is a key factor in determining the response to IL-17A. One model to explain this paradox is that IL-27 and IL-17A exert complex reciprocal effects to boost inflammatory responses, but restrain resting cells to prevent inappropriate activation. This model may help to elucidate the pathogenesis of the growing number of autoimmune diseases in which Th17 responses are implicated [42].
Acknowledgments
This work was supported by the NHS Grampian and the University of Aberdeen. M. A. F., L. R. and N. B. performed the experiments; B. K. supervised the genetic analyses; M. A. F., R. N. B. and M. A. V. designed the study and wrote the paper. We are thankful to Shuo Han (University of Aberdeen) for help in initiating the qPCR.
Disclosure
None.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Correlation between cytokine serum concentrations in healthy donors. Enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-27, IL-6, tumour necrosis factor (TNF)-α and C-reactive protein (CRP) were performed on serum samples from familial donors. (a) IL-27 concentration compared to IL-6 concentration (n = 1133). (b) IL-27 concentration compared to TNF-α concentration (n = 1133). (c) IL-27 concentration compared to CRP (n = 1000). Spearman's rank correlation coefficients are shown.
Fig. S2. Correlation between cytokine serum concentrations in healthy donors. Enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-17A, IL-10, tumour necrosis factor (TNF)-α, IL-6 and interferon (IFN)-γ were performed on serum samples from healthy and familial donors. (a) IL-17A concentration compared to IL-10 concentration (n = 40). (b) IL-17A concentration compared to TNF-α concentration (n = 428). (c) IL-6 concentration compared to TNF-α (n = 1184). (d) IL-17A concentration compared to IFN-γ concentration (n = 40). (e) IL-7A concentration compared to IL-6 concentration (n = 428). (f) IL-10 concentration compared to IFN-γ concentration (n = 40). Spearman's rank correlation coefficients are shown.
Fig. S3. Gene expression of Tbet (TBX21), RAR-related orphan receptor gamma t (ROR-γt) (RORC), signal transducer and activator of transcription 3 (STAT-3) and STAT-4 by peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. PBMC were isolated from healthy donors and cultured in the presence of 100 ng/ml rhIL-27. The cultures were stimulated with plate-bound anti-CD3 and anti-CD28. On the fifth day of culture mRNA was isolated, converted to cDNA and then quantitative polymerase chain reaction (qPCR) performed. The graph demonstrates the relative expression of the each gene normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) compared to unstimulated 0 ng/ml ± standard error of the mean; n = 3.
Fig. S4. Interleukin (IL)-17A, IL-10 and interferon (IFN)-γ concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. T cells were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-27 (0, 10 or 100 ng/ml). The different T cell cultures were stimulated with plate-bound anti-CD3 and anti-CD28. On the fifth day of culture the concentration of IL-17A, IL-10 and IFN-γ in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) CD4+ T cells. (b) Naive CD45RA+CD4+ T cells. (c) Memory CD45RO+CD4+ T cells. No significant differences were observed; n = 3. The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test.
References
- 1.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Bosmann M, Ward PA. Modulation of inflammation by interleukin-27. J Leukoc Biol. 2013;94:1159–1165. doi: 10.1189/jlb.0213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 4.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 5.Tong H, Miyazaki Y, Yamazaki M, et al. Exacerbation of delayed-type hypersensitivity responses in EBV-induced gene-3 (EBI-3)-deficient mice. Immunol Lett. 2010;128:108–115. doi: 10.1016/j.imlet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 7.Young A, Linehan E, Hams E, et al. Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. J Immunol. 2012;189:2079–2083. doi: 10.4049/jimmunol.1200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirahara K, Ghoreschi K, Yang XP, et al. Interleukin-27 Ppriming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Rohowsky-Kochan C. Interleukin-27-mediated suppression of human Th17 cells is associated with activation of STAT1 and suppressor of cytokine signaling protein 1. J Interferon Cytokine Res. 2011;31:459–469. doi: 10.1089/jir.2010.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonoda KH, Yoshimura T, Takeda A, Ishibashi T, Hamano S, Yoshida H. WSX-1 plays a significant role for the initiation of experimental autoimmune uveitis. Int Immunol. 2007;19:93–98. doi: 10.1093/intimm/dxl125. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201–1207. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 16.Mok MY, Wu HJ, Lo Y, Lau CS. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol. 2010;37:2046–2052. doi: 10.3899/jrheum.100293. [DOI] [PubMed] [Google Scholar]
- 17.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaukrodger N, Mayosi BM, Imrie H, et al. A rare variant of the leptin gene has large effects on blood pressure and carotid intima-medial thickness: a study of 1428 individuals in 248 families. J Med Genet. 2005;42:474–478. doi: 10.1136/jmg.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunnington MS, Mayosi BM, Hall DH, et al. Novel genetic variants linked to coronary artery disease by genome-wide association are not associated with carotid artery intima-media thickness or intermediate risk phenotypes. Atherosclerosis. 2009;203:41–44. doi: 10.1016/j.atherosclerosis.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stott LM, Barker RN, Urbaniak SJ. Identification of alloreactive T-cell epitopes on the Rhesus D protein. Blood. 2000;96:4011–4019. [PubMed] [Google Scholar]
- 21.Devereux G, Hall AM, Barker RN. Measurement of T-helper cytokines secreted by cord blood mononuclear cells in response to allergens. J Immunol Methods. 2000;234:13–22. doi: 10.1016/s0022-1759(99)00185-4. [DOI] [PubMed] [Google Scholar]
- 22.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin – rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 23.Vickers MA, Green FR, Terry C, et al. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res. 2002;53:1029–1034. doi: 10.1016/s0008-6363(01)00534-x. [DOI] [PubMed] [Google Scholar]
- 24.Devergne O, Hummel M, Koeppen H, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauce D, Larsen M, Curnow SJ, et al. EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15. Blood. 2006;108:11–18. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]
- 26.Khan N, Hislop A, Gudgeon N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 27.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takatori H, Kanno Y, Chen Z, O'Shea JJ. New complexities in helper T cell fate determination and the implications for autoimmune diseases. Mod Rheumatol. 2008;18:533–541. doi: 10.1007/s10165-008-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plebanski M, Saunders M, Burtles SS, Crowe S, Hooper DC. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing different CD45 isoforms. Immunology. 1992;75:86–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 31.Li TT, Zhang T, Chen GM, et al. Low level of serum interleukin 27 in patients with systemic lupus erythematosus. J Investig Med. 2010;58:737–739. doi: 10.231/JIM.0b013e3181d88f7b. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama N, Nakashima H, Yoshimura T, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor alpha (WSX-1) Ann Rheum Dis. 2008;67:1461–1467. doi: 10.1136/ard.2007.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshizaki A, Yanaba K, Iwata Y, et al. Elevated serum interleukin-27 levels in patients with systemic sclerosis: association with T cell, B cell and fibroblast activation. Ann Rheum Dis. 2011;70:194–200. doi: 10.1136/ard.2009.121053. [DOI] [PubMed] [Google Scholar]
- 35.Jafarzadeh A, Nemati M, Rezayati MT. Serum levels of interleukin (IL)-27 in patients with ischemic heart disease. Cytokine. 2011;56:153–156. doi: 10.1016/j.cyto.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Worns MA, Victor A, Galle PR, Hohler T. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels – a study in twins. Genes Immun. 2006;7:600–605. doi: 10.1038/sj.gene.6364330. [DOI] [PubMed] [Google Scholar]
- 37.Sas AA, Jamshidi Y, Zheng D, et al. The age-dependency of genetic and environmental influences on serum cytokine levels: a twin study. Cytokine. 2012;60:108–113. doi: 10.1016/j.cyto.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Wang HL, Zhang HY, Zhai ZL, Zhou X. The correlation between hepatitis B virus infection and IL-27. Biomed Mater Eng. 2012;22:187–193. doi: 10.3233/BME-2012-0706. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura T, Takeda A, Hamano S, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 40.Hedrich CM, Ramakrishnan A, Dabitao D, Wang F, Ranatunga D, Bream JH. Dynamic DNA methylation patterns across the mouse and human IL10 genes during CD4+ T cell activation; influence of IL-27. Mol Immunol. 2010;48:73–81. doi: 10.1016/j.molimm.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baek SH, Lee SG, Park YE, Kim GT, Kim CD, Park SY. Increased synovial expression of IL-27 by IL-17 in rheumatoid arthritis. Inflamm Res. 2012;61:1339–1345. doi: 10.1007/s00011-012-0534-7. [DOI] [PubMed] [Google Scholar]
- 42.Hall AM, Zamzami OM, Whibley N, et al. Production of the effector cytokine interleukin-17, rather than interferon-gamma, is more strongly associated with autoimmune hemolytic anemia. Haematologica. 2012;97:1494–1500. doi: 10.3324/haematol.2011.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation between cytokine serum concentrations in healthy donors. Enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-27, IL-6, tumour necrosis factor (TNF)-α and C-reactive protein (CRP) were performed on serum samples from familial donors. (a) IL-27 concentration compared to IL-6 concentration (n = 1133). (b) IL-27 concentration compared to TNF-α concentration (n = 1133). (c) IL-27 concentration compared to CRP (n = 1000). Spearman's rank correlation coefficients are shown.
Fig. S2. Correlation between cytokine serum concentrations in healthy donors. Enzyme-linked immunosorbent assays (ELISAs) for interleukin (IL)-17A, IL-10, tumour necrosis factor (TNF)-α, IL-6 and interferon (IFN)-γ were performed on serum samples from healthy and familial donors. (a) IL-17A concentration compared to IL-10 concentration (n = 40). (b) IL-17A concentration compared to TNF-α concentration (n = 428). (c) IL-6 concentration compared to TNF-α (n = 1184). (d) IL-17A concentration compared to IFN-γ concentration (n = 40). (e) IL-7A concentration compared to IL-6 concentration (n = 428). (f) IL-10 concentration compared to IFN-γ concentration (n = 40). Spearman's rank correlation coefficients are shown.
Fig. S3. Gene expression of Tbet (TBX21), RAR-related orphan receptor gamma t (ROR-γt) (RORC), signal transducer and activator of transcription 3 (STAT-3) and STAT-4 by peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. PBMC were isolated from healthy donors and cultured in the presence of 100 ng/ml rhIL-27. The cultures were stimulated with plate-bound anti-CD3 and anti-CD28. On the fifth day of culture mRNA was isolated, converted to cDNA and then quantitative polymerase chain reaction (qPCR) performed. The graph demonstrates the relative expression of the each gene normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) compared to unstimulated 0 ng/ml ± standard error of the mean; n = 3.
Fig. S4. Interleukin (IL)-17A, IL-10 and interferon (IFN)-γ concentrations in peripheral blood mononuclear cell (PBMC) cultures in response to the addition of recombinant human interleukin (rhIL)-27. T cells were isolated from healthy donors and cultured in the presence of varying concentrations of rhIL-27 (0, 10 or 100 ng/ml). The different T cell cultures were stimulated with plate-bound anti-CD3 and anti-CD28. On the fifth day of culture the concentration of IL-17A, IL-10 and IFN-γ in the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). (a) CD4+ T cells. (b) Naive CD45RA+CD4+ T cells. (c) Memory CD45RO+CD4+ T cells. No significant differences were observed; n = 3. The graph demonstrates percentage level compared to 0 ng/ml ± standard error of the mean. Wilcoxon's signed-rank test.


