Abstract
Somatic hypermutation (SHM) is an important step in antigen-driven B cell development creating B lymphocytes expressing high-affinity antibody receptors. It is known that the peripheral B lymphocyte compartments of healthy children and adults differ considerably. However, the development of SHM with age has not been studied in detail previously. Therefore, we used the immunoglobulin (Ig)κ-restriction enzyme hot-spot mutation assay (Igκ-REHMA) to gain an estimation of SHM levels in different age groups in order to relate this to the size of the memory B lymphocyte subpopulations. We show that the level of SHM increases rapidly during the first 2 years of life. This reflects the changes of the memory B cell subpopulations, but also changes in the SHM within memory B cell subsets, probably reflecting an increase of secondary memory B cell responses.
Keywords: memory B cell, peripheral B cell development, somatic hypermutation
Introduction
Somatic hypermutations (SHM) are introduced in a programmed process in the variable regions of immunoglobulin genes to generate higher-affinity B cell receptors (BCR) in memory B cells. In bone marrow, pro- and pre-B cells develop from haematopoietic stem cells, and a unique BCR is generated by V(D)J recombination of the immunoglobulin (Ig) heavy chain and light chain loci. Antigen exposure in the periphery induces activation and further differentiation into so-called switched memory B lymphocytes (smB) in germinal centre (GC) reactions in secondary lymphoid tissues by two separate processes: class-switch recombination (CSR), which leads to loss of surface IgM, and gain of surface IgG, IgA or IgE and SHM, which leads to enhanced antibody affinity [1]. Non-switched memory B lymphocytes (nsmB) mutate their immunoglobulin receptor at least partly outside a germinal centre, without CSR [2].
The peripheral B lymphocyte compartments of healthy adults and children of various ages differ considerably [3]. It has not been studied in detail how SHM develops with increasing age, nor how it is related to the development of populations of smB and nsmB cells. We used the Igκ-restriction enzyme hot-spot mutation assay (Igκ-REHMA) [4] to gain an estimation of SHM levels in healthy children of different age groups and in adults in order to relate this to the size of the B cell subsets. We compared these values to those obtained in a group of common variable immunodeficiency disorders (CVID) patients that we have published previously [5].
Material and methods
After approval by the medical ethics committees of the Jeroen Bosch Hospital and Erasmus MC, and after informed consent was obtained from the parents and patients ≥12 years, peripheral blood samples were collected from seven neonates (cord blood), 49 healthy children (mean age 5·3 years, range 1 week–15·6 years), eight healthy adults (≥16 years) [3] and 23 CVID patients [5]. The blood was collected from healthy children and adults, who underwent venipuncture or blood sampling by heel prick or finger prick for other reasons (e.g. minor surgery). Patients with an active infection, suspected or proven diseases of the immune system or on immunosuppressive therapy were excluded.
To estimate the frequency of SHM, the Igκ-REHMA assay was used. In brief, a reverse transcription–polymerase chain reaction (RT–PCR) was performed using a HEX-coupled Vk3-20U forward primer and a 6-carboxyfluorescein (FAM)-coupled Vk3-20 L reverse primer. The PCR products were digested with DdeI and Fnu4HI and run on an ABI3130XL capillary sequencer (Applied Biosystems, Carlsbad, CA, USA). Unmutated gene products could be visualized as 106- or 109-base pairs (bp) FAM-coupled fragments and mutated gene products as 244-bp FAM-coupled fragments [4]. This analysis estimates the amount of SHM in expressed Vk3/20 gene segments in a specific sequence motif (hot-spot), displayed as percentage of mutated segments within the total peripheral blood cell population. Because only B lymphocytes express these gene segments, this percentage reflects the percentage of mutated cells within the peripheral population of B lymphocytes. However, the amount of SHM within a specific B cell subpopulation cannot be estimated.
Flow cytometric immunophenotyping with directly labelled monoclonal antibodies was used to determine the following lymphocyte subpopulations: B lymphocytes (CD19+), transitional B cells (CD19+CD38++IgM++), naive B lymphocytes (CD19+CD27−IgM+IgD+), nsmB comprising natural effector cells (CD19+CD27+IgM+IgD+) and IgM-only B lymphocytes (CD19+CD27+IgM+IgD−) and smB (CD19+CD27+IgM−IgD−) [3,5].
Statistical calculations were performed with spss version 20 for Windows. We calculated the Pearson product–moment correlation between the estimated percentage of SHM and age (alpha = 0·05, two-tailed) with the logarithmic value of age in days, with cord blood designated as day 1, to cope with the non-linear, downward-bending relationship between the estimated SHM percentage and age.
Results
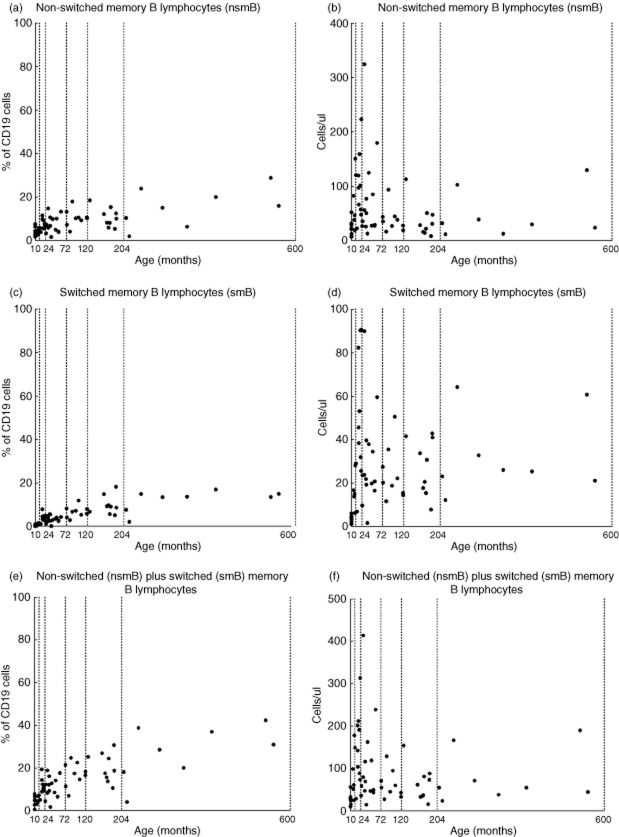
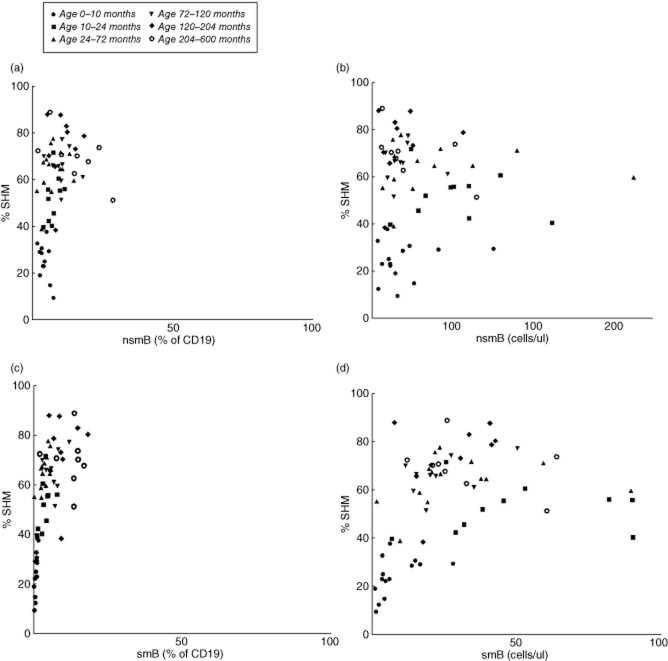
We found that the percentage of mutated hot-spots in rearranged Vk3-20 gene segments in peripheral blood using the Igκ-REHMA assay, which gives an estimate of the level of SHM, shows an increase during the first 2 years of life in healthy children (Fig. 1a), and then remains at a relatively stable level throughout childhood (mean 68%, range 38–89%, n = 40). The Pearson product–moment correlation between the estimated percentage of SHM and age was significant and high (r(64) = 0·848, P = 0·000). This pattern is clearly different from the development of the relative as well as the absolute numbers of smB and nsmB with increasing age (Fig. 2; described previously in [3]). There were also significant correlations between the percentage of SHM and the absolute numbers (r(64) = 0·354, P = 0·004) and percentages of smB (r(64) = 0·624, P = 0·001), as well as the percentages (r(62) = 0·395, P = 0·002) but not the absolute numbers of nsmB (r(62) = −0·019, P = 0·881) (Fig. 3). The strong correlation between age and the percentage of SHM is still evident after correction for the absolute numbers of smB (partial correlation estimated percentage of SHM and age = 0·804, P = 0·000) and the percentages of smB (partial correlation = 0·713, P = 0·000). The same applies to the absolute numbers of nsmB (partial correlation estimated percentage of SHM and age = 0·810, P = 0·000) and the percentages of nsmB (partial correlation = 0·767, P = 0·000). Therefore, SHM increases more than expected based on the memory B cell subset size alone, suggesting an increase in SHM frequency in single B cells.
Fig. 1.
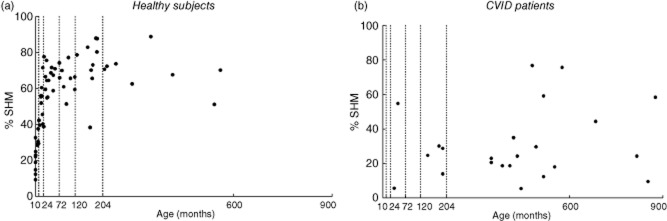
Percentage of somatic hypermutation (SHM) using the immunoglobulin κ-restriction enzyme hot-spot mutation (Igκ-REHMA) assay distributed for age. Every dot represents a healthy subject (a) or a common variable immunodeficiency disorders (CVID) patient (b). The dotted lines show the age groups used in the statistical analyses.
Fig. 2.
Percentages of non-switched memory B lymphocytes (nsmB) (a) switched memory B lymphocytes (smB) (c) and nsmB + smB (e) within the total peripheral B cell population (CD19+) distributed for age. Absolute numbers of non-switched memory B lymphocytes (nsmB) (b), switched memory B lymphocytes (smB) (d) and nsmB + smB (f) distributed for age. Every dot represents a healthy subject. The dotted lines show the age groups used in the statistical analyses.
Fig. 3.
Percentage of somatic hypermutation (SHM) using the immunoglobulin κ-restriction enzyme hot-spot mutation (Igκ-REHMA) assay distributed for percentages (a) and absolute numbers (b) of non-switched memory B lymphocytes (nsmB), and for percentages (c) and absolute numbers (d) of switched memory B lymphocytes (smB). Every dot represents a healthy subject.
As described previously, CVID patients show variable, lower estimated percentages of SHM (mean percentage SHM 31%, range 6–77%, n = 23; Fig. 1b) compared to the healthy subjects (t(85) = 4·917, P = 0·000) [5].
Discussion
SHM is an important step in the antigen-driven B cell development creating B lymphocytes expressing high-affinity antibody receptors. We show that the estimated level of SHM, using the Igκ-REHMA assay, increases steeply during the first 2 years of life. This increase was associated with changes in size of the two B lymphocyte subpopulations that contain SHM (smB and nsmB), but this could not explain the total observed increased SHM frequency. We hypothesize that the increase of SHM in childhood reflects an increase of the proportion of memory B cells originating from secondary GC-dependent B cell responses as well as an increase of the SHM frequency within the memory B cell compartment [6], reflecting immune maturation. A study in sorted (memory) B cells could confirm this hypothesis. However, this would need a great deal more blood, which poses an ethical problem in young, otherwise healthy children.
Previous studies have shown that SHM levels are decreased in certain subgroups of CVID, but this was accompanied by diminished isotype switching, decreased B cell proliferation or decreased switched memory B cell subpopulations [4,5,7,8]. So far, the clinical consequences of low levels of SHM by itself are unknown. A delayed maturation of SHM levels in the presence of normal memory B cell subset counts might therefore reflect the presence of persistent primary B cell responses and hence impaired immune maturation. We propose that if such a phenomenon exists it may be associated with recurrent infections, which has to be explored in further studies.
In conclusion, this study shows the increase of estimated percentages of SHM as measured by the Igκ-REHMA assay in the first 2 years of life. This reflects changes of the memory B cell subpopulations in young children, but also in the SHM frequency within memory B cell subsets, probably reflecting an increase of secondary memory B cell responses. Sequencing of large numbers of immunoglobulin genes at the B cell subset level in young children, e.g. by using next-generation sequencing, should be performed in future studies to confirm this hypothesis.
Acknowledgments
We would like to thank Sandra Posthumus-van Sluijs from the Department of Immunology of the Erasmus Medical Centre Rotterdam for performing the Igκ-REHMA assays. This study was supported financially by the Peribosch Foundation.
Disclosure
The authors do not have any competing interests to declare.
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schatorje EJ, Gemen EF, Driessen GJ, et al. Age-matched reference values for B-lymphocyte subpopulations and CVID classifications in children. Scand J Immunol. 2011;74:502–510. doi: 10.1111/j.1365-3083.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Permin H, Andersen V, et al. Deficiency of somatic hypermutation of the antibody light chain is associated with increased frequency of severe respiratory tract infection in common variable immunodeficiency. Blood. 2005;105:511–517. doi: 10.1182/blood-2003-12-4359. [DOI] [PubMed] [Google Scholar]
- 5.Driessen GJ, van Zelm MC, van Hagen PM, et al. B-cell replication history and somatic hypermutation status identify distinct pathophysiologic backgrounds in common variable immunodeficiency. Blood. 2011;118:6814–6823. doi: 10.1182/blood-2011-06-361881. [DOI] [PubMed] [Google Scholar]
- 6.Berkowska MA, Driessen GJ, Bikos V, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy Y, Gupta N, Le Deist F, et al. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc Natl Acad Sci U S A. 1998;95:13135–13140. doi: 10.1073/pnas.95.22.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonhomme D, Hammarstrom L, Webster D, et al. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J Immunol. 2000;165:4725–4730. doi: 10.4049/jimmunol.165.8.4725. [DOI] [PubMed] [Google Scholar]