Abstract
This is the first multi-centre retrospective survey from the United Kingdom to evaluate the aetiology and diagnostic performance of tryptase in anaphylaxis during general anaesthesia (GA). Data were collected retrospectively (2005–12) from 161 patients [mean ± standard deviation (s.d.), 50 ± 15 years] referred to four regional UK centres. Receiver operating characteristic curves (ROC) were constructed to assess the utility of tryptase measurements in the diagnosis of immunoglobulin (Ig)E-mediated anaphylaxis and the performance of percentage change from baseline [percentage change (PC)] and absolute tryptase (AT) quantitation. An IgE-mediated cause was identified in 103 patients (64%); neuromuscular blocking agents (NMBA) constituted the leading cause (38%) followed by antibiotics (8%), patent blue dye (6%), chlorhexidine (5%) and other agents (7%). In contrast to previous reports, latex-induced anaphylaxis was rare (0·6%). A non-IgE-mediated cause was attributed in 10 patients (6%) and no cause could be established in 48 cases (30%). Three serial tryptase measurements were available in 34% of patients and a ROC analysis of area under the curve (AUC) showed comparable performance for PC and AT. A ≥ 80% PPV for identifying an IgE-mediated anaphylaxis was achieved with a PC of >141% or an AT of >15·7 mg/l. NMBAs were the leading cause of anaphylaxis, followed by antibiotics, with latex allergy being uncommon. Chlorhexidine and patent blue dye are emerging important health-care-associated allergens that may lead to anaphylaxis. An elevated acute serum tryptase (PC >141%, AT >15·7 mg/l) is highly predictive of IgE-mediated anaphylaxis, and both methods of interpretation are comparable.
Keywords: anaphylaxis, general anaesthesia, neuromuscular blocking agents, patent blue dye, tryptase
Introduction
Anaphylaxis during general anaesthesia (GA) is a rare but serious event, as it often leads to significant cardiorespiratory compromise. The investigation of suspected anaphylaxis during GA is complex and challenging, as the patient is often exposed to a number of drugs within a few minutes. A systematic approach of scrutinizing the chronology of events, serum tryptase measurements and allergy testing are warranted to be able to reach a definitive diagnosis.
The true incidence of these reactions is not known in the United Kingdom, probably due to under-reporting, despite the introduction by the Medicines and Healthcare Products Regulatory Agency (MHRA) of the ‘yellow card’ system to report serious adverse reactions to drugs. Large epidemiological studies have been carried out in Australia [1] and France [2,3] [4], and the incidence of anaphylaxis has been reported as between one in 13 000 and one in 20 000–30 000 in France and Australia, respectively. By extrapolation, the incidence has been estimated to be 175–1000 reactions per annum in the United Kingdom [5]. Studies in France have shown that neuromuscular blocking agents (NMBAs) are the most common cause (55–70%), followed by latex (12·6–22·3%) and antibiotics (2·6–15·1%), with approximately 31–48% cases where the trigger is not established, despite thorough investigations [3]. A comparable data set for the United Kingdom population has not previously been available.
The main objective of this study was to investigate the demography, aetiology and severity of anaphylaxis during GA in the UK population. We also assessed the clinical utility of tryptase measurements in the diagnosis of immunoglobulin (Ig)E-mediated reactions by constructing receiver operating characteristic (ROC) curves to assess sensitivity, specificity, negative and positive predictive values (NPV and PPV). Additionally, we compared the performance of two methods for the interpretation of tryptase measurements, (i) percentage change (PC) from baseline and (ii) absolute tryptase (AT) measurement in acute phase, in identifying IgE-mediated anaphylaxis.
Materials and methods
Data collection
Data on patients with suspected perioperative anaphylaxis during GA were collated retrospectively by allergy specialists from hospital notes and electronic records of patients referred to four regional National Health Service (NHS) allergy centres in the United Kingdom (Heartlands Hospital, Birmingham; University Hospitals, Leicester; Queen's Medical Centre, Nottingham and University Hospital Southampton, Southampton) between 2005 and 2012. Data regarding age, gender, asthma status, serial serum tryptase measurements, drugs/agents involved in the reaction, diagnostic tests to identify culprit drugs and clinical features of the perioperative episode were captured systematically at the individual centres and anonymized data were transferred to the principal investigator electronically. This survey was registered and approved by the respective NHS Research and Development departments.
Investigations
Patients were evaluated systematically by an allergy specialist as per previously published guidelines [5–7]. This involved careful scrutiny of anaesthetic and drug charts, clinical features and vital parameters during the perioperative event in order to assess temporal association of events with drug administration. This enabled preparation of a shortlist of drugs and agents potentially implicated for planning allergy tests.
Skin testing
This was performed in accordance with national and international guidelines [5,6] following the perioperative event with appropriate positive (histamine) and negative (normal saline) controls. Skin prick testing (SPT) for anaesthetic agents used ‘neat’ (clinically used concentration) and 1 : 10 diluted solutions. A wheal diameter of ≥2 mm at 15 min in comparison with negative control was considered positive and significant in conjunction with the clinical history. When SPT was negative or indeterminate, intradermal tests (IDT) were performed. Concentrations for IDT were as per previously published non-irritant concentrations [5,6]. Investigations for penicillin allergy were performed as per the European Network for Drug Allergy (ENDA) position statement [8]. For drugs including ondansetron, gelofusin, midazolam and protamine, an IDT was performed at dilutions of 1 : 1000–1 : 100 (of ‘neat’ stock concentration). For IDTs, a bleb of 4–6 mm was raised by injecting 0·03–0·05 ml and a wheal diameter of at least 3 mm larger than the negative control at 20 min was considered positive. When skin testing was positive for a particular NMBA, further testing with other NMBAs was carried out to assess cross-reactivity. SPT for latex was performed using a commercial standardized extract (ALK Abello®, Hørsholm, Denmark) and where there was a high index of suspicion, a subsequent ‘prick-prick’ test with a latex glove was also undertaken.
Serum-specific IgE
Where indicated clinically (i.e. when there was strong suspicion of an allergy and SPT and IDT were negative), serum-specific IgE was performed for latex, chlorhexidine, suxamethonium, penicillin V, penicillin G and amoxicilloyl on a UniCAP® platform (Phadia, Uppsala, Sweden).
Antibiotic challenge
Supervised graded antibiotic challenges were performed for patients where the history was suggestive of an antibiotic allergy, and there was no demonstrable specific IgE by skin or blood testing.
Tryptase
Serum tryptase was measured on a UniCAP® platform (Phadia). Three serial measurements were performed, first as soon as possible following resuscitation, the second approximately 1–2 h after onset and the third ≥24 h (baseline) as per previously published guidelines [7].
Diagnosis and severity grading
In this retrospective analysis, patients with a diagnosis of IgE-mediated anaphylaxis met the World Allergy Organization (WAO) clinical criteria [9,10] for anaphylaxis, and there was presence of specific IgE to the respective drug/agent with a clear temporal association. Diagnosis of non-IgE-mediated systemic reaction/anaphylaxis was made for drugs including opiates and non-steroidal anti-inflammatory drugs when there was a clear temporal association with the respective drug, and allergy tests were negative for other drugs and agents that were potentially implicated. Severity of reactions were graded retrospectively, as per previously published criteria [6,11].
ROC curves
ROC curves were plotted using the statistical program r version 2·15·1 (with the ROCR package) for PC from baseline and AT measurements taken in acute phase. The areas under the ROC curves were then compared to evaluate which test could be considered superior. The sensitivity, specificity, PPV and NPV of PC and AT in the diagnosis of IgE-mediated reactions were determined. Patients with non-IgE-mediated reactions and those in whom a cause could not be established, despite thorough investigations, served as a control group.
Results
Demography
A total of 161 patients were referred and investigated for suspected anaphylaxis. There were 121 females (75%) and 40 males (25%). Mean age was 50 [±15 standard deviation (s.d.)] years; 24 patients (15%) had an underlying diagnosis of asthma.
Diagnostic test
The median (interquartile range) time from the perioperative episode to allergy testing was 3 (2–8) months (data available in 151 patients). One hundred and three patients (64%) had IgE-mediated reactions as evidenced by SPT [n = 25 (24%)], IDT [n = 68 (66%)], serum-specific IgE [n = 9 (9%)] and challenge tests [n = 3 (1%)]. Two of the 103 patients had a positive SPT as well as serum-specific IgE to the relevant agent. Non-IgE-mediated reactions were diagnosed in 10 patients (6%) involving the following drugs: diclofenac (n = 1), morphine (n = 7), desflurane (n = 1) and alfentanyl (n = 1). No cause could be identified in 48 patients (30%) despite full investigations and it is plausible that these patients developed entities other than anaphylaxis.
Aetiology for IgE-mediated reactions
Neuromuscular blocking agents (NMBAs) were the most common culprits [n = 61 (38%)], followed by antibiotics [n = 13 (8%)], mainly penicillin and aminopenicillin/s (Fig. 1). Within the NMBA group, atracurium followed by rocuronium were commonly implicated (Fig. 2). Interestingly, chlorhexidine (n = 8) and patent blue dye (n = 9) were implicated in ∼11% of the patient population (Fig. 1). In 12 (7%) patients ‘other drugs’ were involved, namely midazolam (n = 2), ondansetron (n = 5), latex (n = 1), local anaesthetics (n = 2), protamine (n = 1) and gelofusin (n = 1).
Fig. 1.
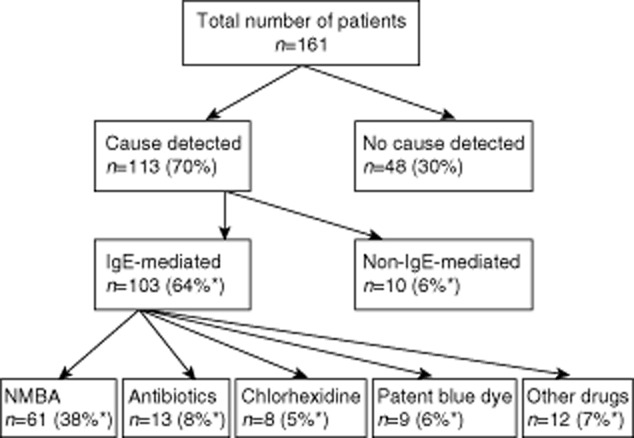
Algorithm summarizing case-series analysis and aetiology [*these percentages are shown as proportion of total cases (n = 161)].
Fig. 2.
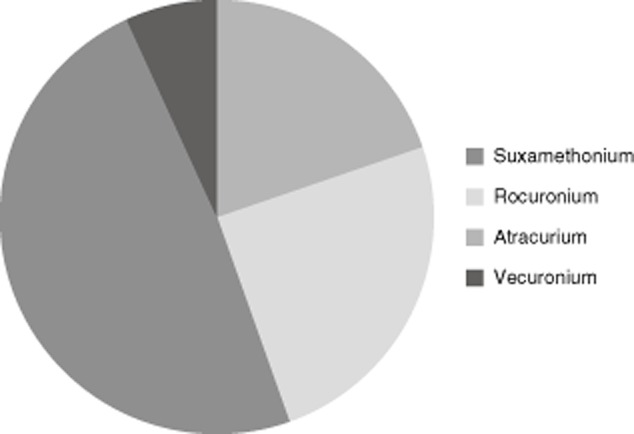
Neuromuscular blocking agents (NMBAs) implicated in anaphylaxis; suxamethonium: n = 12 (20%); rocuronium: n = 15 (25%); atracurium: n = 30 (49%); vecuronium: n = 4 (7%).
Serum tryptase
Three serial tryptase measurements were available in 55 patients and the highest measurement in the acute phase was taken as the peak value and used for calculation for percentage change from baseline. Details of number of tryptase samples available in different clinical categories in this study are summarized in Table 1.
Table 1.
Details of number of tryptase samples under each clinical category
| IgE-mediated reactions | Non-IgE-mediated reactions | No cause established | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of tryptase samples | 0 | 1† | 2‡ | 3‡ | 0 | 1† | 2‡ | 3‡ | 0 | 1† | 2‡ | 3‡ | |
| n | 17 | 29 | 21 | 36 | 2 | 4 | 1 | 3 | 14 | 16 | 2 | 16 | 161 |
Among these patients with single tryptase measurements, 27 immunoglobulin (Ig)E-mediated reactions, four non-IgE mediated reactions and 11 with no cause established were baseline tryptase measurements.
Among patients with two or three tryptase measurements, a baseline tryptase measurement was not available for five with IgE-mediated reactions, one non-IgE-mediated reaction and four with no cause established.
Severity of IgE-mediated anaphylaxis
Data on severity were available for 100 patients. There were three (3%), 19 (19%), 65 (65%) and 13 (13%) patients with grades 1, 2, 3 and 4, respectively.
Tryptase performance
The area under ROC curve values for PC from baseline and AT were similar at 0·792 [0·674, 0·910; 95% confidence interval (CI)] and 0·808 (0·695, 0·921; 95% CI), respectively (Fig. 3), with a difference of 0·016 (−0·147, 0·179; 95% CI). A previous study [3] used a determinant tryptase value of 25 μg/l to discriminate between IgE-mediated and non-IgE-mediated causes of perioperative anaphylaxis. Our study reveals a comparable performance at this ‘cut-off’ value, despite differences in allergen aetiologies (Table 2). The data set is also tabulated with the determinant values chosen as approximate quartiles, and values for sensitivity, specificity, PPV and NPV are given for both PC from baseline and AT (Table 3). Subgroup analysis for tryptase measurements in patients with IgE-mediated reactions, non-IgE-mediated reactions and where no cause was established are summarized in Table 4.
Fig. 3.
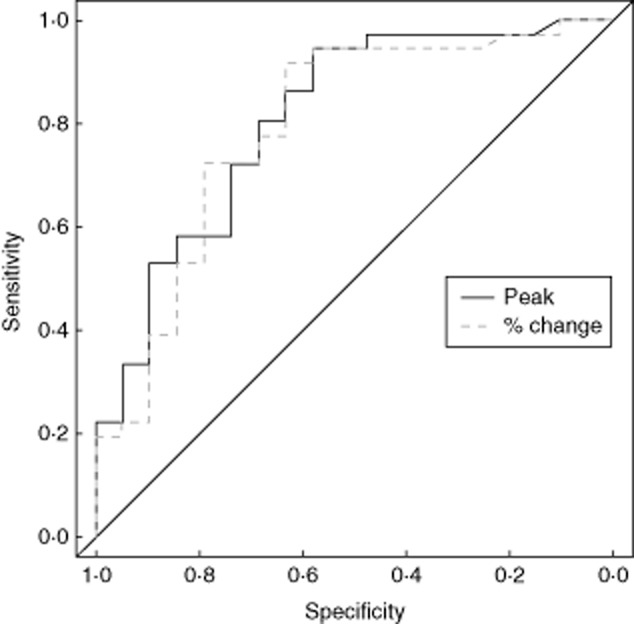
Receiver operating characteristic curves for percentage change from baseline and absolute acute phase measurement of tryptase.
Table 2.
Comparative analysis of performance of absolute tryptase between current and previously published report
| Study | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Mertes et al [3] (peak tryptase > 25 μg/l) | 64 | 89 | 93 | 54 |
| Current study (peak tryptase > 25 μg/l) | 63·9 (48·2, 79·6) | 73·7 (53·9, 93·5) | 82·1 (68·0, 96·3) | 51·9 (29·3, 67·0) |
PPV = positive predictive value; NPV = negative predictive value; values in brackets represent 95% confidence interval.
Table 3.
Sensitivity, specificity, positive and negative predictive values of percentage change and absolute acute phase measurements of tryptase shown at quartiles of data
| Quartiles: percentage change from baseline | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| >24·6 | 94·4 (87,100) | 52·6 (30·2, 75·1) | 79·1 (66·9, 91·2) | 83·3 (62·2, 100) |
| >141 | 75·0 (60·9, 89·1) | 68·4 (47·5, 89·3) | 81·8 (68·7, 95·0) | 59·1 (38·5, 79·6) |
| >506 | 52·8 (36·5, 69·1) | 84·2 (67·8, 100) | 86·4 (72·0, 100) | 48·5 (31·4, 65·5) |
| >1209 | 25·0 (10·9, 39·1) | 89·4 (75·7, 100) | 81·8 (59·0, 100) | 38·6 (24·2, 53·0) |
| Quartiles: absolute tryptase (μg/l) | ||||
| >4·47 | 94·4 (86·9, 100) | 68·4 (47·5, 89·3) | 77·3 (64·9, 89·7) | 81·8 (59·0, 100) |
| >15·7 | 75·0 (60·9, 89·1) | 68·4 (47·5, 89·3) | 81·8 (68·7, 95·0) | 59·1 (38·5, 79·6) |
| >33·6 | 52·8 (36·5, 69·1) | 84·2 (67·8, 100) | 86·4 (72·0, 100) | 48·5 (31·4, 65·5) |
| >88·0 | 27·8 (13·1, 42·4) | 94·7 (84·7, 100) | 90·9 (73·9, 100) | 40·9 (26·3, 55·4) |
PPV = positive predictive value; NPV = negative predictive value; values in brackets represent 95% confidence intervals.
Table 4.
Subgroup analysis of tryptase measurements [(Mann–Whitney U-test – IgE-mediated versus combined group (non-IgE-mediated and no cause established), absolute tryptase: P-value = 0·0002; percentage change: P-value = 0·0004]
| No cause established (n = 16) | Non-IgE-mediated reactions (n = 3) | IgE-mediated reactions (n = 36) | |
|---|---|---|---|
| Absolute tryptase (μg/l) | 4·11 (2·96, 16·6) | 18·0, IQR (10·2, 27·6) | 41·9 (16·3, 101) |
| Percentage change (%) | 22·6 (−4·17, 198) | 157, IQR (91·7, 165) | 603 (142, 1214) |
All values shown are median [interquartile range (IQR)] as data are not normally distributed. Ig = immunoglobulin.
Discussion
Our first multi-centre study of perioperative anaphylaxis in the United Kingdom has shown that there has been a notable difference in the aetiology of allergic precipitants from previously published reports [3,12], particularly with latex allergy being uncommon. Although NMBAs emerged as the most common culprits, followed by antibiotics, chlorhexidine and patent blue dye emerged as relatively newer allergens, comprising 16·5% of all cases of IgE-mediated anaphylaxis. Within the NMBA family, atracurium was the most common, followed by rocuronium and suxamethonium in our series. Among antibiotics, penicillin/amoxicillin were commonly implicated, as reported by other studies. Furthermore, our data set on absolute tryptase has shown that the value of >33·6 μg/l gives a PPV of 86·4%, comparable to the PPV of 93% from Mertes and colleagues [3], and this represents the top three quartiles of the tryptase data range. The evaluation of PC for tryptase reveals a comparable PPV of 86·4% for a > 506% change from baseline with an accompanying sensitivity of 53%.
Our data are concordant with the larger French series [3] with respect to NMBAs being the leading cause of anaphylaxis. However, in our cohort atracurium was the most common agent, as opposed to rocuronium in France, and this may be attributable at least in part to differences in anaesthetic practice. Interestingly, the proportion of NMBA-related cases in our series was about ∼20% lower, which could be explained by the emergence of newer allergens, including chlorhexidine and patent blue dye and the relatively smaller cohort studied.
An important observation we have made is that latex was implicated in only one patient (0·6% of total cases), as opposed to a large French series [3] (17% of cases) and a smaller Danish series [12] (19% of cases), where it was the second leading cause of anaphylaxis during GA. The reason for this is not known. While latex-free measures are not employed routinely in British operating theatres (except when a patient with known latex allergy is undergoing surgery), most NHS hospitals have adopted latex-free measures in other clinical areas. It is plausible that this may have reduced sensitization rates, at least in patients undergoing multiple operations or visiting clinical areas frequently.
Chlorhexidine and patent blue dye are relatively new allergens and have been reported as causing severe and protracted anaphylaxis during general anaesthesia [13–15]. Our data show a relatively high rate of 5–6% for both these agents. Our study has also highlighted the involvement of other less common drugs in anaphylaxis during GA, including ondansetron, midazolam, local anaesthetics and gelofusin, highlighting the importance of including all the relevant drugs and agents in the panel for investigation, as dictated by anaesthetic charts and temporal association with the timing of the event. Without subjecting the patients to drug provocation testing we cannot be certain that sensitization translates to clinical reactivity to these more rare drugs, but the temporal association, demonstrable sensitization and negative tests to other drugs administered concurrently suggests a causative link. For ethical reasons it was not considered appropriate to challenge patients to these drugs for confirmation, and thus they were advised to follow avoidance measures.
Currently there is no universally agreed approach to the interpretation of serial tryptase measurements in the diagnosis of anaphylaxis. In routine clinical practice, the tryptase data are interpreted in conjunction with clinical presentation, demonstrating a downward trend from peak to baseline measurement. Previous studies [3,16] have published sensitivity, specificity, PPV and NPV figures for absolute tryptase measurements of 12 and 25 μg/l during the acute phase. The performance of this test in our multi-centre cohort was evaluated in two ways, both of which showed comparable area under the ROC curves for PC from baseline and AT. Malinovsky et al. [16] (n = 39) compared acute tryptase measurements at >12 and 25 μg/l with a threefold rise in titres from baseline measurement and did not report significant advantage of the latter with respect to sensitivity, specificity, PPV and NPV. Our study (n = 161) has also shown that both methods of interpretation of serial tryptase measurements (i.e. AT and PC) are comparable. There are limitations to our data set – three serial tryptase measurements were available in only 34% of the cohort. The reason underlying the modest request rate for this test is unclear, and there is a need to raise awareness among anaesthetists about the current national guidelines and clinical utility of this biomarker. The half-life of tryptase is ∼2 h and levels may return to normal within 6–8 h, so the timing of the blood sample is important in the diagnostic interpretation. While ‘no or minimal change’ in tryptase measurement does not exclude anaphylaxis, a rising trend in the acute phase followed by a decline is highly predictive. Our data have shown high specificity (≥80%) for a > 506% change or an absolute value of >33·6 μg/l in IgE-mediated reactions, suggesting that serial tryptase measurements are a useful adjunct in the diagnostic work-up of patients following suspected perioperative anaphylaxis. There is emerging preliminary evidence that the measurement of other mast cell mediators may also become important, including chymase, carboxypeptidase and dipeptidyl peptidase 1 [17,18]. There is an urgent need for testing the efficacy of these new biomarkers in the context of anaphylaxis during GA.
In conclusion, this multi-centre study has shown that NMBAs and antibiotics are commonly implicated as the causative agents leading to perioperative anaphylaxis in the UK population. In contrast to published reports from other countries, latex allergy is a rare cause of perioperative anaphylaxis in the United Kingdom and chlorhexidine and patent blue dye are emerging as newer causative allergens for anaphylaxis during GA. Measurement of serial serum tryptase is a valuable tool in the evaluation of anaphylaxis and >33·6 μg/l or a > 506% increase from baseline derives a PPV ≥85%.
Author contributions
M. T. K. planned the study, managed patients in his centre, collated data from individual centres, analysed and interpreted the data and wrote the manuscript. J. H. and C. D. performed skin testing in their centre. G. G., M. Y. and T. C. managed patients in their respective centres and collected data. L. D. contributed to data collection and helped to set up the study by liaising with other centres. E. E., A. H. and N. K. managed patients in their respective centres. A. P. W. managed patients in his centre, contributed to manuscript writing and served as senior author. R. C. performed statistical analysis relating to ROC curves. All authors contributed to and reviewed the manuscript and ratified the final version.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Fisher M, Baldo BA. Anaphylaxis during anaesthesia: current aspects of diagnosis and prevention. Eur J Anaesthesiol. 1994;11:263–284. [PubMed] [Google Scholar]
- 2.Mertes PM, Laxenaire MC. Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France. Seventh epidemiologic survey (January 2001–December 2002) Ann Fr Anesth Reanim. 2004;23:1133–1143. doi: 10.1016/j.annfar.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Mertes PM, Laxenaire MC, Alla F. Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000. Anesthesiology. 2003;99:536–545. doi: 10.1097/00000542-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Laxenaire MC. Epidemiology of anaesthetic anaphylactoid reactions. Fourth multicenter survey (July 1994–December 1996) Ann Fr Anesth Reanim. 1999;18:796–809. doi: 10.1016/s0750-7658(00)88460-9. [DOI] [PubMed] [Google Scholar]
- 5.Ewan PW, Dugue P, Mirakian R, Dixon TA, Harper JN, Nasser SM. BSACI guidelines for the investigation of suspected anaphylaxis during general anaesthesia. Clin Exp Allergy. 2010;40:15–31. doi: 10.1111/j.1365-2222.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 6.Mertes PM, Laxenaire MC, Lienhart A, et al. Reducing the risk of anaphylaxis during anaesthesia: guidelines for clinical practice. J Invest Allergol Clin Immunol. 2005;15:91–101. [PubMed] [Google Scholar]
- 7.Association of Anaesthetists of Great Britain and Ireland (AAGBI) Suspected anaphylactic reactions associated with anaesthesia. 3rd edn. London: AAGBI and BSACI; 2003. pp. 1–20. revised. [Google Scholar]
- 8.Torres MJ, Blanca M, Fernandez J, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58:961–972. doi: 10.1034/j.1398-9995.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 9.Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127:587–593. doi: 10.1016/j.jaci.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroigaard M, Garvey LH, Gillberg L, et al. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51:655–670. doi: 10.1111/j.1399-6576.2007.01313.x. [DOI] [PubMed] [Google Scholar]
- 12.Kroigaard M, Garvey LH, Menne T, Husum B. Allergic reactions in anaesthesia: are suspected causes confirmed on subsequent testing? Br J Anaesth. 2005;95:468–471. doi: 10.1093/bja/aei198. [DOI] [PubMed] [Google Scholar]
- 13.Parkes AW, Harper N, Herwadkar A, Pumphrey R. Anaphylaxis to the chlorhexidine component of Instillagel: a case series. Br J Anaesth. 2009;102:65–68. doi: 10.1093/bja/aen324. [DOI] [PubMed] [Google Scholar]
- 14.Haque RA, Wagner A, Whisken JA, Nasser SM, Ewan PW. Anaphylaxis to patent blue V: a case series and proposed diagnostic protocol. Allergy. 2010;65:396–400. doi: 10.1111/j.1398-9995.2009.02248.x. [DOI] [PubMed] [Google Scholar]
- 15.Mertes PM, Malinovsky JM, Mouton-Faivre C, et al. Anaphylaxis to dyes during the perioperative period: reports of 14 clinical cases. J Allergy Clin Immunol. 2008;122:348–352. doi: 10.1016/j.jaci.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Malinovsky JM, Decagny S, Wessel F, Guilloux L, Mertes PM. Systematic follow-up increases incidence of anaphylaxis during adverse reactions in anesthetized patients. Acta Anaesthesiol Scand. 2008;52:175–181. doi: 10.1111/j.1399-6576.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown TA, Whitworth HS, Zhou XY, Lau L, Eren E, Walls AF. Mast cell carboxypeptidase as a confirmatory and predictive marker in allergic reactions to drugs. J Allergy Clin Immunol. 2011;127:pAB143. [Google Scholar]
- 18.Whitworth HS, Zhou XY, Lau LCK, et al. Dipeptidyl peptidase I as a serum marker of allergic reactions to food. J Allergy Clin Immunol. 2011;127:AB71. [Google Scholar]


