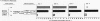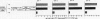Abstract
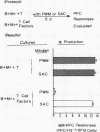
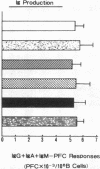
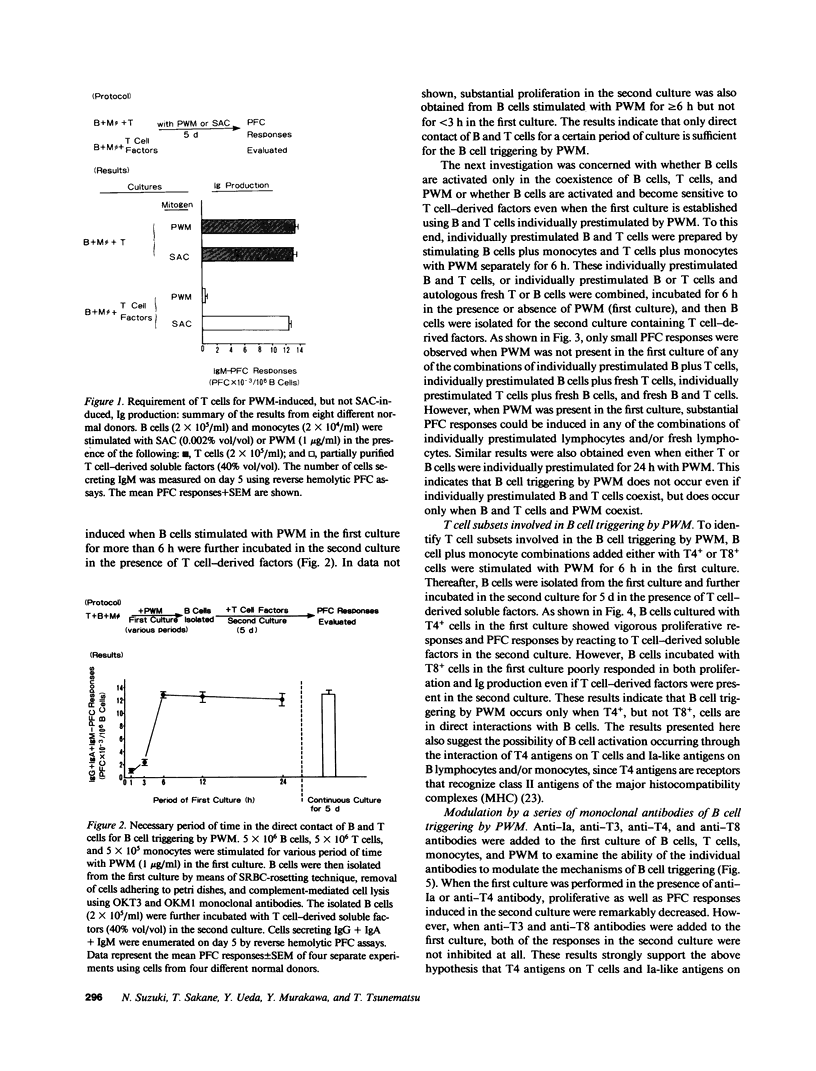
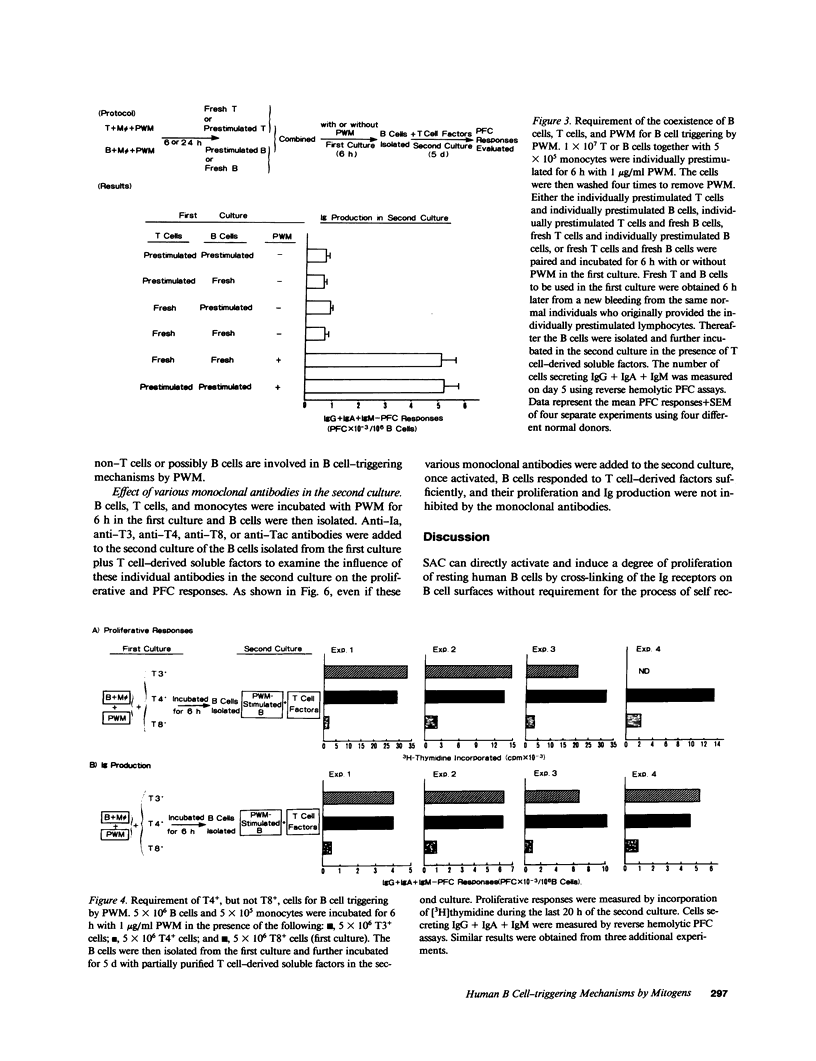
Human B cell-triggering mechanisms were investigated using the polyclonal activators Staphylococcus aureus Cowan I (SAC) and pokeweed mitogen (PWM). When the cultures of B cells, T cells, and monocytes were stimulated for 5 d by SAC or PWM, B cells could be activated by both mitogens to proliferate and secrete Ig. Even when T cells were substituted by T cell-derived soluble factors, SAC-stimulated B cells could differentiate into Ig-secreting cells. In contrast, interactions of B and T cells for at least the first 6 h of culture were necessary for the B cell triggering by PWM. Experiments that allow a more precise delineation of the B cell-triggering mechanisms by PWM demonstrated that interactions of B cells with T4+ but not T8+ cells are required for the B cell triggering; anti-Ia or anti-T4 antibody can block this triggering; in contrast, anti-T3 or anti-T8 antibody do not exert any effects on the B cell triggering. However, all these monoclonal antibodies could not modulate the ability of B cells that had been already activated by PWM to respond to T cell-derived factors. These data suggest that SAC can directly activate B cells, while cognate interactions between Ia-like antigens on B cells and T4+ cells are essential for B cell triggering by PWM. Furthermore, once B cells are triggered, they will proliferate, differentiate, and secrete Ig in response to T cell-derived factors; Ia-like antigens or T cell differentiation antigens may not be involved in the processes in this cascade.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Melchers F. T cell-dependent activation of resting B cells: requirement for both nonspecific unrestricted and antigen-specific Ia-restricted soluble factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2497–2501. doi: 10.1073/pnas.78.4.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfraissy J. F., Vazquez A., Wallon C., Desmottes R. M., Galanaud P. Helper T cell activation for the human B cell response to trinitrophenylated polyacrylamide beads: involvement of the T4 antigen. Eur J Immunol. 1984 May;14(5):426–430. doi: 10.1002/eji.1830140508. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Metzler C., Gatenby P. A., Evans R. L. Blocking of human T lymphocyte functions by anti-Leu-2 and anti-Leu-3 antibodies: differential inhibition of proliferation and suppression. J Immunol. 1983 Jun;130(6):2623–2628. [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Geha R. S., Milgrom H., Broff M., Alpert S., Martin S., Yunis E. J. Effect of anti-HLA antisera on macrophage-T-cell interactions. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4038–4041. doi: 10.1073/pnas.76.8.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kishimoto T., Yoshizaki K., Kimoto M., Okada M., Kuritani T., Kikutani H., Shimizu K., Nakagawa T., Nakagawa N., Miki Y. B cell growth and differentiation factors and mechanism of B cell activation. Immunol Rev. 1984 Apr;78:97–118. doi: 10.1111/j.1600-065x.1984.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kotani H., Takada S., Ueda Y., Murakawa Y., Suzuki N., Sakane T. Activation of immune regulatory circuits among OKT4+ cells by autologous mixed lymphocyte reactions. Clin Exp Immunol. 1984 May;56(2):390–398. [PMC free article] [PubMed] [Google Scholar]
- Landegren U., Andersson J., Wigzell H. Mechanism of T lymphocyte activation by OKT3 antibodies. A general model for T cell induction. Eur J Immunol. 1984 Apr;14(4):325–328. doi: 10.1002/eji.1830140409. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Borel Y., Mantzouranis E., Steinberg A. D., Schlossman S. F. Autoantibody to an immunoregulatory inducer population in patients with juvenile rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):753–761. doi: 10.1172/JCI110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Butler J. L., Fauci A. S. Regulation of human B-cell activation, proliferation, and differentiation by soluble factors. J Clin Immunol. 1984 Sep;4(5):337–347. doi: 10.1007/BF00917136. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985 Jan 1;161(1):181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa Y., Takada S., Ueda Y., Suzuki N., Hoshino T., Sakane T. Characterization of T lymphocyte subpopulations responsible for deficient interleukin 2 activity in patients with systemic lupus erythematosus. J Immunol. 1985 Jan;134(1):187–195. [PubMed] [Google Scholar]
- Niederhuber J. E., Allen P. Role of I-region gene products in macrophage induction of an antibody response. II. Restriction at the level of T cell in recognition of I-J-subregion macrophage determinants. J Exp Med. 1980 May 1;151(5):1103–1113. doi: 10.1084/jem.151.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhuber J. E., Frelinger J. A., Dugan E., Coutinho A., Shreffler D. C. Effects of anti-Ia serum on mitogenic responses. I. Inhibition of the proliferative response to B cell mitogen, LPS, by specific anti-Ia sera. J Immunol. 1975 Dec;115(6):1672–1676. [PubMed] [Google Scholar]
- Puck J. M., Rich R. R. Regulatory interactions governing the proliferation of T cell subsets stimulated with pokeweed mitogen. J Immunol. 1984 Mar;132(3):1106–1112. [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Schlossman S. F. A monoclonal antibody blocking human T cell function. Eur J Immunol. 1980 Oct;10(10):758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- Rogozinski L., Bass A., Glickman E., Talle M. A., Goldstein G., Wang J., Chess L., Thomas Y. The T4 surface antigen is involved in the induction of helper function. J Immunol. 1984 Feb;132(2):735–739. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Ruscetti F. W., Gallo R. C. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981 Mar;57(3):379–394. [PubMed] [Google Scholar]
- Sakane T., Kotani H., Takada S., Murakawa Y., Ueda Y. A defect in the suppressor circuits among OKT4+ cell populations in patients with systemic lupus erythematosus occurs independently of a defect in the OKT8+ suppressor T cell function. J Immunol. 1983 Aug;131(2):753–761. [PubMed] [Google Scholar]
- Sakane T., Ueda Y., Suzuki N., Niwa Y., Hoshino T., Tsunematsu T. OKT4+ and OKT8+ T lymphocytes produce soluble factors that can modulate growth and differentiation of human B cells. Clin Exp Immunol. 1985 Oct;62(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Takada S., Ueda Y., Murakawa Y., Suzuki N., Sakane T. Functional heterogeneities among concanavalin A-activated OKT4+ and OKT8+ cells by using autologous erythrocyte rosette technique. J Clin Invest. 1983 Dec;72(6):2060–2071. doi: 10.1172/JCI111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Ueda Y., Suzuki N., Murakawa Y., Hoshino T., Green I., Steinberg A. D., Horwitz D. A., Sakane T. Abnormalities in autologous mixed lymphocyte reaction-activated immunologic processes in systemic lupus erythematosus and their possible correction by interleukin 2. Eur J Immunol. 1985 Mar;15(3):262–267. doi: 10.1002/eji.1830150310. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Kaye J., Jones B. The role of B cell surface Ia antigen recognition by T cells in B cell triggering. Analysis of the interaction of cloned helper T cells with normal B cells in differing states of activation and with B cells expressing the xid defect. Eur J Immunol. 1984 Jun;14(6):553–561. doi: 10.1002/eji.1830140613. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Yokoi T., Nagaoki T., Taniguchi N. Ia-positive cells generated by PWM-stimulation within OKT4+ subset interact with OKT8+ cells for inducing active suppression on B cell differentiation in vitro. J Immunol. 1982 Jul;129(1):103–106. [PubMed] [Google Scholar]
- Yoshizaki K., Nakagawa T., Kaieda T., Muraguchi A., Yamamura Y., Kishimoto T. Induction of proliferation and Ig production in human B leukemic cells by anti-immunoglobulins and T cell factors. J Immunol. 1982 Mar;128(3):1296–1301. [PubMed] [Google Scholar]