Abstract
Purpose of review
We review literature concerning the epidemiology of HIV-associated tuberculosis (HIV-TB), focussing on articles published between 2007-2008.
Recent findings
An estimated 1.37 million new cases of HIV-TB occurred in 2007, representing 15% of the total global burden of TB. In addition, an estimated 456,000 HIV-TB deaths accounted for 23% of global HIV/AIDS mortality. Sub-Saharan Africa is the worst affected region with 79% of the disease burden. The epicentre of the co-epidemic lies in the south of the continent, with South Africa alone accounting for over one quarter of all cases. A critical overlap between HIV and the global multi-drug resistant TB (MDR-TB) epidemics is emerging. Although it is as yet unclear whether HIV is driving a disproportionate increase in MDR-TB cases at a population level, HIV has nevertheless been a potent risk factor for institutional outbreaks, especially in South Africa and Eastern Europe. Increasing data have highlighted the risk of TB among HIV-infected health-care workers in resource-limited settings. However, many studies also show the major benefits to be derived from antiretroviral therapy in high- and low-income countries.
Summary
HIV-TB remains a major challenge to global health that requires substantial increases in resource allocation and concerted international action.
Keywords: HIV, tuberculosis, antiretroviral, epidemiology
Introduction
From the early stages of the HIV epidemic, a strong association with tuberculosis (TB) was apparent [1] and HIV subsequently emerged as one of the key factors undermining global TB control [2]. The epicentre of this co-epidemic is in southern Africa and where TB incidence rates have risen to unprecedented levels [3**]. The situation has been further compounded by the intersection with the growing global epidemic of multi-drug resistant TB (MDR-TB) and extensively drug-resistant TB (and XDR-TB), especially in Eastern Europe and South Africa. In this paper, we review the current global disease burden, recent epidemiological studies that provide insights into the epidemic and the impact of the increasing availability of antiretroviral therapy (ART) in both high- and low-resource settings.
Global burden of HIV-Associated TB
Between 1990 and 2003, HIV infection was one of the key factors underlying an approximately 1% annual increase in the global TB incidence. World Health Organization (WHO) estimates that global TB incidence peaked at 142 cases per 100,000 population in 2004 and that it is now decreasing slowly [3**]. However, as a result of global population growth, the absolute number of TB cases globally continued to rise in 2007. Trends in recent years also suggest that the annual number of incident cases of HIV-associated TB (HIV-TB) peaked at 1.39 million cases in 2005 and is now decreasing [3**].
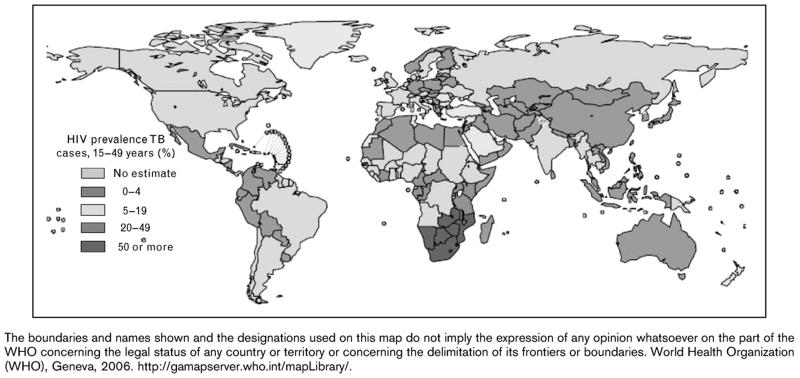
In 2007 there were an estimated 9.3 million new cases of TB worldwide. Among these there were 1.37 million (14.8%) cases of HIV-TB and approximately 456,000 HIV-TB deaths [3**]. Sub-Saharan Africa accounted for the vast majority (79%) of cases, with the co-epidemic being particularly concentrated in the countries of southern Africa countries where HIV prevalence is highest (Figures 1 and 2). The South-East Asian region accounted for 11% of cases [3**].
Figure 1. Country estimates of the prevalence of HIV infection in new cases of tuberculosis (TB) diagnosed in 2005.
Source: World Health Organization (WHO), Geneva, 2006. Available at: http://gamapserver.who.int/mapLibrary/ The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the WHO concerning the legal status of any country or territory or concerning the delimitation of its frontiers or boundaries.
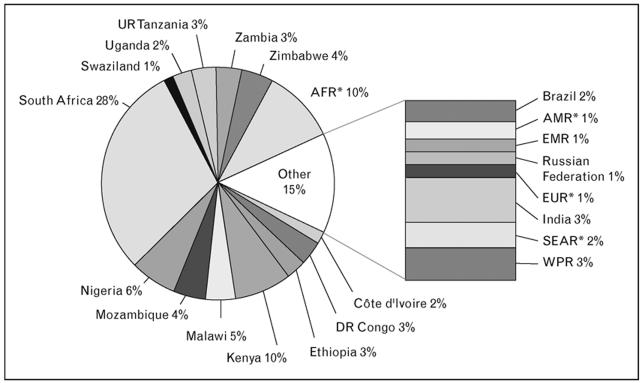
Figure 2. Geographical distribution of estimated HIV-associated TB cases in 2006.
For each country or region, the estimated number of cases is shown as a percentage of the global total. AFR* represents all countries in the WHO African region except for those shown separately. AMR* is the WHO Region of the Americas, excluding Brazil. EUR* is the WHO European Region, excluding the Russian Federation. SEAR* is the WHO South-East Asia Region, excluding India. Source: WHO, 2008 [4*].
These estimates released by WHO in 2009 represent a substantial increase from previous estimates, with an approximately two-fold greater disease burden [3**,4*]. These new estimates have arisen because of the substantial increase in HIV-testing among TB patients, particularly in Africa where the proportion of TB patients being tested for HIV has increased greatly in recent years, reaching 37% of TB patients in 2007 [3**]. Thus, much more reliable data on the prevalence of HIV in TB patients are now available. Using these data, the relative risk of HIV-infected people developing TB compared to HIV-negative people has been revised. Previously this ratio was estimated to be approximately 6 in populations with a generalized HIV epidemic but has now been revised to 20.6 (95%CI, 20.4-34.9) [3**,4*]. These revised estimates indicate that the scale of the challenge of HIV-TB is considerably greater than previously thought.
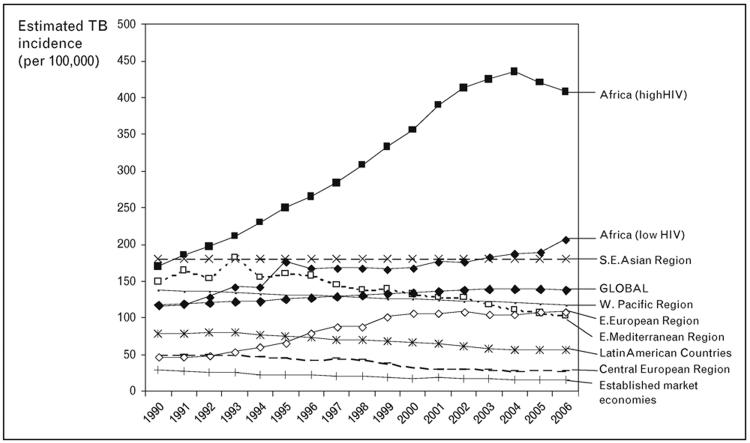
Regional trends in the overall rate of HIV-TB are uncertain at present. However, overall TB notification rates in many countries in southern Africa have started to decrease between 2003 and 2006 (Figure 3) [4**]. These trends are perhaps most likely to reflect the natural evolution of the HIV epidemic; the contribution, if any, of other potential factors such as scale-up of ART are as yet unknown. South Africa and Swaziland are the exception to this trend, with rates continuing to rise in 2007 and this may reflect the later development of the HIV epidemic in these countries [3**]. With just 0.7% of the world’s population, South Africa accounted for 28% of the global burden of HIV-associated TB in 2006 (Figure 2) [4*].
Figure 3. Trends in estimated tuberculosis incidence rates (per 100,000 per year) for the period 1990-2006.
Data are from the 134 countries with the most reliable surveillance systems. Global data and data from nine sub-regions are shown. Source: WHO, 2008 [4*].
HIV-TB is also an important public health challenge in Eastern Europe. Here, the overlap of the HIV and antituberculosis drug resistance epidemics is an important factor in the rising TB rates observed in the region (Figure 3). A study of 25 of the highest burden countries in the WHO European Region found that the proportion of TB cases testing positive increased from 2.1% in 2004 to 3.3% in 2005, with Ukraine accounting for much of this [5*]. The highest incidence rates of HIV-TB were in Portugal, followed by Ukraine, Estonia, the Russian Federation and Latvia. England and Wales were not included in this study but here HIV prevalence among TB cases has increased from 3.1% in 1999 to 8.3% in 2003 [6*]. HIV-coinfected cases contributed almost one third to the increase in overall TB notifications in England and Wales in this period and the majority were non-UK born. In marked contrast, the United States experienced a three-fold decrease in the number of HIV-TB cases between 1993 and 2004, coinciding with improvements in TB control and advances in HIV diagnosis and treatment [7].
HIV-associated TB and Mortality
Patients with HIV-TB have high mortality risk [8-13] and TB is a leading cause of death in HIV-infected patients in TB endemic countries, including those with free access to ART such as in Brazil [14*]. TB is also associated with an increased risk of AIDS-related deaths in men and women living in the United States [15*,16]. WHO estimates that there were a total of 456,000 HIV-TB deaths in 2007 [3**]. This numbers equates to 33% of the number of incident HIV-TB cases that year. Moreover, this represents 23% of the estimated 2 million deaths from HIV/AIDS in 2007. Such deaths are routinely classified as ‘HIV deaths’ rather than ‘TB deaths’ in the International Statistical Classification of Diseases (ICD-10).
Deaths from HIV-TB have exacted a huge toll on the worst-affected communities in sub-Saharan Africa. In a rural South African community with high HIV prevalence, there has been an increasing trend in TB mortality since 1994 in HIV-infected but not HIV-uninfected TB patients, especially in young adults [12]. In recent years the excess HIV-TB mortality has been 1.6-fold greater in women compared to men. Observed regional differences in death rates in Ugandan and Malawian patients with HIV-TB are likely be due to differences in patient age and stage of HIV epidemic [9].
Consistent with previous randomized clinical trials, co-trimoxazole prophylaxis significantly reduced mortality risk in HIV-infected pulmonary TB patients in Zambia [8]. Together with post-mortem data from South Africa [17*], these data highlight bacterial sepsis as a likely frequent cause of death in these patients.
HIV and Recurrent TB
A systematic review of 32 studies [18] and recently published data from Rio de Janeiro, South African gold mines and San Francisco [19**,20*,21*] confirm that HIV-TB patients are at increased risk of recurrent disease, especially those with low CD4 cell counts. Consistent with an earlier study [22], molecular epidemiological data from South African gold mines [20*] suggest that exogenous reinfection accounts for approximately two-thirds of recurrent disease. This contrasts with data from the low TB burden setting of San Francisco where the relapse rate among HIV-infected patients was substantially reduced by increased duration of TB treatment, suggesting the importance of relapse in this setting [18*]. Increased duration of TB treatment, use of antiretroviral treatment and secondary isoniazid prophylaxis are all strategies that may reduce recurrence rates [19**,21*,23].
Epidemiological studies in sub-Saharan Africa
The WHO DOTS strategy has proven insufficient to control TB in high HIV prevalence communities in southern Africa [24]. Adjunctive interventions are needed but must be based upon sound epidemiological data derived from community-based studies.
A study of an HIV-seroconverter cohort of gold-miners found that TB risk did not increase during the HIV seroconversion period but that TB risk rapidly increased three-fold within the first 2 years of HIV infection. Thereafter, TB incidence increased steadily with time such that by 11 years from seroconversion nearly half the HIV-infected men had developed TB [25**].
In a township in Cape Town, South Africa, the annual TB notification rate reached 1,500/100,000 in 2004 [24] and exceeded 2,000/100,000 in 2006 [26**]. This rate is almost unprecedented in era of multi-drug TB treatment and has been driven by high HIV prevalence in the context of poverty and over-crowding [24]. Despite a reasonably well-functioning DOTS service in this community achieving a 67% case-finding rate in HIV-uninfected adults, a cross-sectional survey found a very high prevalence of undiagnosed TB (5%) among HIV-infected people and a case finding proportion of just 37% [27*]. TB disease duration (and period of infectiousness) in the community was similar among HIV-infected and uninfected individuals, contrasting with earlier studies from South African gold miners and Zimbabwean factory workers in which disease duration was substantially shorter in HIV-infected people [28, 29**]. Various factors may underlie this difference, including health-seeking behaviour, access to care, efficiency of TB treatment services and stage of evolution of the HIV epidemic. Studies from Cape Town and Harare, however, agree that a significant proportion of patients with culture-positive TB identified through active screening have sub-clinical disease [27*,29**].
The high prevalence of undiagnosed TB is the key driver of transmission in these communities. The annual risk of infection among school children in a poor community in Cape Town was found to be approximately 4-5% [26**]. As a result, approximately 50% were infected by the age of 15 years and were thus primed for development of TB in the event of subsequent HIV acquisition in early adult life. Among adults in South African townships and gold mines, the prevalence of TB infection is 77-89% [30-32]. Enhanced case finding such as that described in a work place intervention in Harare, Zimbabwe [29**], may be a key intervention to reduce high transmission rates.
HIV-TB is generally less infectious and the impact of this disease burden on overall transmission in high burden communities is not clear. Consistent with previous data, the study by Middelkoop et al. [26**] provides no evidence that the HIV-TB epidemic has resulted in increased TB transmission to children, which largely occurs in the home. However, in some settings within communities, large numbers of HIV-TB cases may outweigh any effect of reduced infectiousness and result in increased transmission among adults. For example, Glynn et al. [25**] suggested that increased secondary transmission accounted for rising TB rates in both HIV-infected and non-infected workers in a South African gold mine, which contrasts with an earlier study [33]. Collectively these data suggest that the contribution of HIV-TB to transmission is variable and may be age-specific.
HIV and MDR- and XDR-TB Epidemics
Approximately 425,000 MDR-TB cases occur annually worldwide, representing nearly 5% of the world’s annual TB burden [34]. The interrelationship between the MDR-TB and HIV epidemics has been comprehensively reviewed elsewhere [35**]. By fuelling increased TB incidence rates, HIV may also be contributing to increases in absolute numbers of MDR-TB cases. However, the evidence to support a disproportionate association between the two diseases at a population level has not been conclusive [35**]. Nevertheless, a more recent study from the Ukraine (where the rates of MDR-TB are among the highest in the world) found a significant independent association between HIV and MDR-TB (adjusted odds 1.7, 95%CI 1.3-2.3) [36*]. It is also notable that more than half of the XDR-TB patients reported in the United States between 1993-2007 were HIV-infected [37].
HIV infection is associated with institutional outbreaks of MDR-TB as first described in industrialized countries in the late 1980s and early 1990s [35**]. The risk associated with newly expanding HIV care and treatment services in resource-limited settings was vividly exemplified by the Tugela Ferry hospital outbreak in rural Kwazulu Natal in South Africa in 2005 and 2006 [38]. Surveillance during this outbreak found that 39% of patients had MDR-TB and 6% had XDR-TB. Of those with XDR-TB, only half had previously received TB treatment, two-thirds had a recent hospital admission and genotyping found that 85% of strains were similar. All those tested were HIV-infected [38] and 52 of 53 patients died with median survival of 16 days from diagnosis; two deaths were among health care workers.
Further molecular epidemiological studies of patients with more than one TB episode confirm that exogenous reinfection was frequently the source of drug resistant disease at this hospital in Kwazulu Natal [39*]. The causal strain has been identified as F15/LAM4/KZN and this has been associated with MDR-TB in the province as early as 1994 and with XDR-TB from 2001 [40*]. The lack of drug susceptibility testing and drug resistance surveillance permitted the evolution of the XDR-TB strain to go undetected until the Tugela Ferry outbreak.
The Tugela Ferry outbreak developed in the context of a very poorly functioning provincial TB control programme. Moreover, there was a critical lack of TB infection control measures within this health facility where the prevalence of both HIV and TB were high [41]. Evidence is growing that this was not a sporadic localised outbreak. Cases have been identified in patients attending approximately 60 different health facilities in Kwazulu Natal Province and in all 9 provinces of South Africa [42]. Such outbreaks threaten to overwhelm public health programmes and undermine the successes of ART. However, the extent to which HIV-associated MDR- and XDR-TB will lead to a rise in drug resistant TB in the general community remains to be determined.
Nosocomial TB transmission and infection control
Studies of the infectiousness of hospital in-patients with HIV-TB have been conducted in Peru using an air sampling system that exposed guinea pigs to exhaust air within an animal facility above the ward [43,44**]. These studies found that the infectiousness of patients receiving treatment varied greatly and that a small number of inadequately treated HIV patients with MDR-TB were responsible for almost all transmission events [43, 44**]. Patients enrolling in ART clinics in resource-limited settings have a high prevalence of untreated TB [45, 46]. Patients with sputum smear-positive disease are most infectious and yet represent a minority of the disease burden and can be rapidly diagnosed by sputum examination. In contrast, there are often substantial delays in diagnosis among the large number of those with smear-negative culture-positive pulmonary TB and such patients are a potentially important source of nosocomial transmission [47].
The risk of TB among health-care workers in low- and middle-income countries is an increasingly recognised problem [48]. In Zimbabwe, nursing students had an extremely high risk of acquiring TB infection (19.3/100PYs) [49*]. In a systematic review, the median annual incidence of TB infection attributable to health-care work in low-resource settings was 5.8% compared to 1.1% in high-income countries [50]. This has major implications for countries where a significant proportion of health-care workers are HIV-infected. In Kenya, for example, HIV-infected health-care workers were found to have a much higher risk of developing TB (adjusted odds 29.1; 95%CI, 5.1-167) [51**].
TB infection control in health facilities in resource-limited settings has been hugely neglected, but in the era of rapid expansion of HIV care and treatment services and increasing MDR-TB prevalence, this is increasingly recognised as a high priority [52*]. Simple low-cost interventions such as increased natural ventilation, upper-room ultra-violet lights and negative air ionization may be very effective [53*,54**]. An epidemiological modelling study based on the Tugela Ferry outbreak of XDR-TB suggested that if no TB infection control measures were instituted about 1300 cases of XDR-TB would occur by the end of 2012 [55**]. However, implementation of a combination of administrative, environmental and personal infection control measures was estimated to nearly halve this number of cases.
Tuberculosis and antiretroviral therapy
In recent years, access to ART has been rapidly scaled up in resource-limited settings where the burden of TB is highest. Increasing data from around the world indicate the substantial impact that ART has on HIV-TB.
Impact of ART on survival of TB/HIV patients
The apparent complexity of concurrent administration of ART during TB treatment may have resulted in either the under-utilization or delayed initiation of this key intervention. However, increasing data indicate the huge survival benefit of ART for patients with HIV-associated TB. In the Netherlands, there has been a 54% reduction in the adjusted odds of death among those with HIV-TB during the ART era [10]. In Thailand [56, 57] and Spain [58], adjusted mortality risk is estimated to be reduced by 80-93% and 63%, respectively. In Malawi, adults and children with TB receiving ART have good outcomes similar to non-TB patients [59, 60]. Surprisingly, a further retrospective observational study from Malawi found no short-term survival benefit conferred by ART started during the continuation phase of TB treatment but this study may well be subject to ART allocation bias [61]. Furthermore, most deaths occurred in the intensive phase of TB treatment [61], suggesting the need for early ART initiation [62]. Consistent with data from South Africa [45], patients developing incident TB during ART in Malawi have much poorer survival [63]. This reflects the fact that TB develops opportunistically in those patients with poor CD4 cell recovery and who already have poor survival [64].
TB and mortality in ART services
TB is a leading cause of early mortality in ART services in sub-Saharan Africa [65] although much of the true burden of disease may remain unascertained. In an ART service in South Africa, TB was associated with a 2-3-fold increased crude mortality risk [45]. However, some data from ART services in Uganda, Malawi and South Africa suggest that TB is not an independent predictor of mortality, which instead appears to be largely driven by low baseline CD4 cell counts [46,66,67]. Since patients with HIV-TB typically have advanced immunodeficiency and very high morality risk, early initiation of ART is often warranted [62]. The precise timing of this remains unclear and randomised controlled trials are awaited [68].
Impact of ART on TB prevention
The WHO DOTS strategy alone is insufficient to control the HIV-TB epidemic. ART, however, is a potent intervention for TB prevention. Adding to earlier data [69], recent studies have shown a 54-74% reduction in TB rates associated with use of ART in an adult cohorts in Spain [70, 71] and at a population level in Brazil [72**,73]. Recurrence rates were halved by ART in a study from Brazil [19]. Furthermore, in a retrospective hospital-based paediatric cohort in South Africa, the extremely high TB incidence rates (53.3 cases/100PYs) decreased by 88% during ART [74].
TB incidence rates decline with increasing duration of ART [45,46] and risk reductions observed in cohorts in high- and low-incomes countries are similar [75*]. Despite this, it remains unclear whether ART will improve TB control at the population level [69, 76]. A study from Brazil found that much HIV-TB occurs prior to HIV diagnosis [77] and early HIV diagnosis and treatment are clearly needed for TB prevention. In addition, since TB rates do not return to background during ART [45], concurrent adjunctive interventions are needed. Isoniazid preventive therapy in a routine clinical service reduces the risk of HIV-TB substantially [78]. More recent retrospective observational data from Brazil suggest that isoniazid preventive therapy and ART used together may have an additive effect [72**]. Concurrent use of these treatments was not associated with an increased risk of hepatitis in South African gold miners [79]. In eastern Europe where rates of HIV-associated drug-resistant disease are high, a system dynamics simulation model suggested that very high coverage (>75%) with ART combined with high TB treatment success rates would need to be achieved to substantially impact mortality rates associated with TB [80].
TB-associated immune reconstitution disease
The early phase of ART may be complicated by the development of immune reconstitution disease (IRD), alternatively known as immune reconstitution inflammatory syndrome (IRIS). Although this is associated with a wide variety of opportunistic infections, mycobacterial diseases are the most common [81]. TB accounted for 41% of IRD events in a prospective South African cohort [82*].
TB IRD was reviewed in 2008 [83*] and may present as either the clinical deterioration of pre-existing TB following ART initiation (‘paradoxical’ IRD) or the clinical presentation of sub-clinical TB present at the time of ART initiation (‘unmasking’ TB). Between 8-43% of TB patients who commence ART develop paradoxical IRD, with a tendency for lower rates to be observed in studies in resource-limited settings [83*]. The reasons for this apparent difference are not clear but recently published consensus case definitions may help standardisation of data [84*].
Although just 12% of TB patients developed IRD in a study from South Africa, the proportion was much higher (32%) among the sub-set who initiated ART in the first 2 months of TB treatment [85**]. In adjusted analyses, those initiating ART in the first month of TB treatment had a 70-fold higher risk of TB IRD compared to those initiating ART after at least 3 months of TB treatment. A further prospective study from the US found a rate of just 15% but highlighted the considerable morbidity and frequent need for interventions [86].
For a long time ‘unmasking’ TB IRD has remained a poorly defined phenomenon but has recently been extensively reviewed [87*,88]. Both papers provide a similar conceptual framework that proposes how ART modifies the presentation of TB. This may cause a temporal clustering of cases in the initial weeks of treatment – a phenomenon referred to as ‘unmasking’. In a sub-set of these cases, there may also be an increase in the severity of manifestations due to an overt hyper-inflammatory response – a phenomenon referred to as ‘unmasking TB IRD’ [87, 88]. Although reports of the latter in the literature are relatively few, a recent case report illustrates how this is occasionally fatal [89].
Conclusion
HIV-TB accounts for a huge burden of morbidity and mortality, which, in light of revised WHO estimates in 2009 (3**), has previously been underestimated. The African continent bears the brunt of the vast majority of this disease burden and associated mortality. However, rates are also increasing in some middle- and high-income countries.
While much needs to be done to improve diagnosis and treatment outcomes, it is also clear that this epidemic cannot be controlled solely through treatment of infectious TB cases. There is a great need for scale-up of preventive interventions [90*] such as the WHO ‘3I’s strategy’ (intensified case finding, isoniazid preventive therapy and infection control) and ART. In addition a much stronger focus on prevention needs to include concerted action with regard to HIV/AIDS prevention and addressing social determinants of disease such as poverty, malnutrition and overcrowding [90*]. To accelerate progress towards epidemiological impact targets set for 2015, HIV-TB must move up the global public health agenda with increased resource allocation and concerted international action.
Acknowledgements
SDL is funded by the Wellcome Trust, London, UK. GC is partly funded by the Consortium to Respond Effectively to the AIDS and Tuberculosis Epidemics (CREATE).
Abbreviations
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral treatment
- HIV
human immunodeficiency virus
- HIV-TB
HIV-associated TB
- IRD
immune reconstitution disease
- IRIS
immune reconstitution inflammatory syndrome
- MDR-TB
multi-drug resistant tuberculosis
- TB
tuberculosis
- WHO
World Health Organization
- XDR-TB
extensively resistant tuberculosis
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- (1).Rieder HL, Cauthen GM, Comstock GW, Snider DE., Jr Epidemiology of tuberculosis in the United States. Epidemiol Rev. 1989;11:79–98. doi: 10.1093/oxfordjournals.epirev.a036046. [DOI] [PubMed] [Google Scholar]
- (2).Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- (3).World Health Organization . Global tuberculosis control, 2009. Epidemiology, strategy, financing. World Health Organization; Geneva: WHO/HTM/TB/2009.411. [** A comprehensive overview of the burden of TB at global, regional and national levels with data available up to 2007. In this report, increasing availability of data have permitted the estimates of the burden of HIV-TB to be revised. These find almost double the number of cases occur each year than was previously estimated.] [Google Scholar]
- (4).World Health Organization . Global Tuberculosis Control. Surveillance, planning and financing. World Health Organization; Geneva: 2008. WHO/HTM/TB/2008.393. [* A comprehensive annual overview of the burden of tuberculosis at the global, regional and national levels using data available up to 2006.] [Google Scholar]
- (5).Lazarus JV, Olsen M, Ditiu L, Matic S. Tuberculosis-HIV co-infection: policy and epidemiology in 25 countries in the WHO European region. HIV Med. 2008;9:406–14. doi: 10.1111/j.1468-1293.2008.00567.x. [* This paper highlights the rising trends in the burden of HIV-associated tuberculosis in several European countries,] [DOI] [PubMed] [Google Scholar]
- (6).Ahmed AB, Abubakar I, Delpech V, et al. The growing impact of HIV infection on the epidemiology of tuberculosis in England and Wales: 1999 2003. Thorax. 2007;62:672–6. doi: 10.1136/thx.2006.072611. [* This paper highlights the increasing burden of HIV-associated tuberculosis in England and Wales, the majority of which is attributable to people born outside the UK.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Albalak R, O’Brien RJ, Kammerer JS, et al. Trends in tuberculosis/human immunodeficiency virus comorbidity, United States, 1993-2004. Arch Intern Med. 2007;167:2443–52. doi: 10.1001/archinte.167.22.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ciglenecki I, Glynn JR, Mwinga A, et al. Population differences in death rates in HIV-positive patients with tuberculosis. Int J Tuberc Lung Dis. 2007;11:1121–8. [PubMed] [Google Scholar]
- (10).Haar CH, Cobelens FG, Kalisvaart NA, et al. HIV-related mortality among tuberculosis patients in The Netherlands, 1993-2001. Int J Tuberc Lung Dis. 2007;11:1038–41. [PubMed] [Google Scholar]
- (11).Cain KP, Kanara N, Laserson KF, et al. The epidemiology of HIV-associated tuberculosis in rural Cambodia. Int J Tuberc Lung Dis. 2007;11:1008–13. [PubMed] [Google Scholar]
- (12).Zwang J, Garenne M, Kahn K, et al. Trends in mortality from pulmonary tuberculosis and HIV/AIDS co-infection in rural South Africa (Agincourt) Trans R Soc Trop Med Hyg. 2007;101:893–8. doi: 10.1016/j.trstmh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- (13).Duarte EC, Bierrenbach AL, Barbosa da Silva JJ, et al. Associated factors with deaths among pulmonary tuberculosis patients: a case-control study with secondary data. J Epidemiol Community Health. 2009;63:233–238. doi: 10.1136/jech.2008.078972. [DOI] [PubMed] [Google Scholar]
- (14).Saraceni V, King BS, Cavalcante SC, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–72. [* Between 1996 and 2005, tuberculosis was the leading cause of AIDS-related deaths in Rio de Janeiro, Brazil, accounting for 9% of deaths (double the number due to Pneumocystis jiroveci pneumonia) despite free access to antiretroviral therapy.] [PMC free article] [PubMed] [Google Scholar]
- (15).Lopez-Gatell H, Cole SR, Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22:1869–73. doi: 10.1097/QAD.0b013e32830e010c. [* Tuberculosis accounted for a 2.4-fold greater adjusted risk of AIDS-associated death among men in the Multicentre AIDS Cohort Study (MACS) in the USA.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lopez-Gatell H, Cole SR, Hessol NA, et al. Effect of tuberculosis on the survival of women infected with human immunodeficiency virus. Am J Epidemiol. 2007;165:1134–42. doi: 10.1093/aje/kwk116. [DOI] [PubMed] [Google Scholar]
- (17).Martinson NA, Karstaedt A, Venter WD, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–50. doi: 10.1097/QAD.0b013e3282eea47f. [* This study from South African gold mines highlights sepsis as a frequent cause of death in patients with HIV-associated TB.] [DOI] [PubMed] [Google Scholar]
- (18).Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis. 2007;11:828–37. [PubMed] [Google Scholar]
- (19).Golub JE, Durovni B, King BS, et al. Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2008;22:2527–33. doi: 10.1097/QAD.0b013e328311ac4e. [** This retrospective study from Brazil found a high rate of recurrent TB and that recurrence rates were lower among those patients with complete TB treatment, receipt of antiretroviral therapy and CD4 cell counts >200 cells/μL.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Charalambous S, Grant AD, Moloi V, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis. 2008;12:942–8. [* Using molecular epidemiology, this study confirms that approximately two-thirds of recurrent TB episodes in the gold-mines are due to reinfection.] [PubMed] [Google Scholar]
- (21).Nahid P, Gonzalez LC, Rudoy I, et al. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–206. doi: 10.1164/rccm.200509-1529OC. [* This retrospective observational study from San Francisco found that TB recurrence rates were over 9-fold higher among HIV-coinfected patients and that these rates were decreased by greater duration of TB treatment. Antiretroviral therapy was associated decreased the time to smear-conversion and improved survival.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sonnenberg P, Murray J, Glynn JR, et al. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–93. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- (23).Churchyard GJ, Fielding K, Charalambous S, et al. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS. 2003;17:2063–70. doi: 10.1097/00002030-200309260-00007. [DOI] [PubMed] [Google Scholar]
- (24).Lawn SD, Bekker LG, Middelkoop K, et al. Impact of HIV Infection on the Epidemiology of Tuberculosis in a Peri-Urban Community in South Africa: The Need for Age-Specific Interventions. Clin Infect Dis. 2006;42:1040–7. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- (25).Glynn JR, Murray J, Bester A, et al. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS. 2008;22:1859–67. doi: 10.1097/QAD.0b013e3283097cfa. [** This large HIV-seroconverter cohort of South African gold miners was followed long-term in the pre-antiretroviral treatment era. Data reveal the combined impact of advancing immunodeficiency and the effect on increased onward disease transmission.] [DOI] [PubMed] [Google Scholar]
- (26).Middelkoop K, Bekker LG, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47:349–55. doi: 10.1086/589750. [** The very high annual risk of infection (4 – 5%) among school children was documented in a poor South African community. However, data suggested that the HIV-related increases in TB notification rates in the community have not resulted in increased transmission to children.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [* This cross-sectional survey in a high HIV-prevalence South African community found a very high prevalence of undiagnosed tuberculosis in HIV-infected people.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Corbett EL, Charalambous S, Moloi VM, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med. 2004;170:673–9. doi: 10.1164/rccm.200405-590OC. [DOI] [PubMed] [Google Scholar]
- (29).Corbett EL, Bandason T, Cheung YB, et al. Epidemiology of Tuberculosis in a High HIV Prevalence Population Provided with Enhanced Diagnosis of Symptomatic Disease. PLoS Med. 2007;4:e22. doi: 10.1371/journal.pmed.0040022. [** In this work place intervention study, increased access was provided to tuberculosis diagnostic services. After 2 years, the prevalence of TB was found to be comparatively low. The adjusted incidence rate ratio was 18.8 cases/100PYs for HIV-infected subjects with a population attributable fraction of 78%. Most patients with prevalent culture-positive TB had sub-clinical disease when first detected.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lawn SD, Bangani N, Vogt M, et al. Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa. BMC Infect Dis. 2007;7:99. doi: 10.1186/1471-2334-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Rangaka MX, Wilkinson KA, Seldon R, et al. Effect of HIV-1 Infection on T-Cell-based and Skin Test Detection of Tuberculosis Infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- (32).Hanifa Y, Grant AD, Lewis J, et al. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis. 2009;13:39–46. [PubMed] [Google Scholar]
- (33).Corbett EL, Charalambous S, Fielding K, et al. Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV)-negative South African gold miners during a decade of epidemic HIV-associated TB. J Infect Dis. 2003;188:1156–63. doi: 10.1086/378519. [DOI] [PubMed] [Google Scholar]
- (34).Zignol M, Hosseini MS, Wright A, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006;194:479–85. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- (35).Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. [** This article provides a comprehensive review of the overlap between the HIV and MDR-TB epidemics.] [DOI] [PubMed] [Google Scholar]
- (36).Dubrovina I, Miskinis K, Lyepshina S, et al. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics? Int J Tuberc Lung Dis. 2008;12:756–62. [* This study suggests that an independent association (adjusted odds 1.7, 95%CI 1.3-2.3) between HIV and MDR-TB exists in the Ukraine.] [PubMed] [Google Scholar]
- (37).Shah NS, Pratt R, Armstrong L, et al. Extensively drug-resistant tuberculosis in the United States, 1993-2007. JAMA. 2008;300:2153–60. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- (38).Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- (39).Andrews JR, Gandhi NR, Moodley P, et al. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198:1582–9. doi: 10.1086/592991. [* This study used molecular epidemiological techniques to show that reinfection was the predominant source of MDR- and XDR-TB in Tugela Ferry outbreak in South Africa.] [DOI] [PubMed] [Google Scholar]
- (40).Pillay M, Sturm AW. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin Infect Dis. 2007;45:1409–14. doi: 10.1086/522987. [* This article reports on the TB strain that caused the Tugela Ferry outbreak in South Africa and historical samples show this strain has been associated with MDR- TB in the area since 1994 and with XDR-TB since 2001.] [DOI] [PubMed] [Google Scholar]
- (41).Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: no time for denial or complacency. PLoS Med. 2007;4:e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Andrews JR, Shah NS, Gandhi N, Moll T, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and antiretroviral therapy rollout in South Africa. J Infect Dis. 2007;196(Suppl 3):S482–S490. doi: 10.1086/521121. [DOI] [PubMed] [Google Scholar]
- (43).Escombe AR, Oeser C, Gilman RH, et al. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis. 2007;44:1349–57. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Escombe AR, Moore DA, Gilman RH, et al. The Infectiousness of Tuberculosis Patients Coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [** This study from Peru reports on the infectiousness of patients within a TB-HIV hospital ward and found that most transmission was associated with a small number of inadequately treated cases of MDR-TB.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- (46).Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- (47).Lawn SD, Edwards D, Wood R. Tuberculosis transmission from patients with smear-negative pulmonary tuberculosis in sub-Saharan Africa. Clin Infect Dis. 2009;48:496–97. doi: 10.1086/596550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Corbett EL, Muzangwa J, Chaka K, et al. Nursing and community rates of Mycobacterium tuberculosis infection among students in Harare, Zimbabwe. Clin Infect Dis. 2007;44:317–23. doi: 10.1086/509926. [* This study demonstrated a very high rate of tuberculin skin test conversions among nursing students working in hospitals in Harare. Here a substantial proportion of health care workers are HIV-infected.] [DOI] [PubMed] [Google Scholar]
- (50).Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11:593–605. [PubMed] [Google Scholar]
- (51).Galgalo T, Dalal S, Cain KP, et al. Tuberculosis risk among staff of a large public hospital in Kenya. Int J Tuberc Lung Dis. 2008;12:949–54. [** This study found that HIV-infected health care workers in a large hospital in Kenya were at very high risk of developing TB (adjusted odds = 29.1, 95%CI 5.1-167).] [PubMed] [Google Scholar]
- (52).Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis. 2007;196(Suppl 1):S108–S113. doi: 10.1086/518661. [* This is a useful review of TB infection control in HIV care and treatment services.] [DOI] [PubMed] [Google Scholar]
- (53).Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [* This study shows that natural ventilation can be used to effect high rates of air exchange in hospitals in resource-limited settings.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Escombe AR, Moore DA, Gilman RH, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [** This study finds that upper-room ultraviolet light and negative air ionization may substantially reduce nosocomial TB transmission.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–7. doi: 10.1016/S0140-6736(07)61636-5. [** This study models the Tugela Ferry nosocomial outbreak of MDR- and XDR-TB and found that a combination of administrative, environmental and personal infection control measures would halve the projected number of cases by 2012.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Akksilp S, Karnkawinpong O, Wattanaamornkiat W, et al. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13:1001–7. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Sanguanwongse N, Cain KP, Suriya P, et al. Antiretroviral therapy for HIV- infected tuberculosis patients saves lives but needs to be used more frequently in Thailand. J Acquir Immune Defic Syndr. 2008;48:181–9. doi: 10.1097/QAI.0b013e318177594e. [DOI] [PubMed] [Google Scholar]
- (58).Velasco M, Castilla V, Sanz J, et al. Effect of Simultaneous Use of Highly Active Antiretroviral Therapy on Survival of HIV Patients With Tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- (59).Bong CN, Chen SC, Jong YJ, et al. Outcomes of HIV-infected children with tuberculosis who are started on antiretroviral therapy in Malawi. Int J Tuberc Lung Dis. 2007;11:534–8. [PubMed] [Google Scholar]
- (60).Makombe SD, Harries AD, Yu JK, et al. Outcomes of tuberculosis patients who start antiretroviral therapy under routine programme conditions in Malawi. Int J Tuberc Lung Dis. 2007;11:412–6. [PubMed] [Google Scholar]
- (61).Zachariah R, Fitzgerald M, Massaquoi M, et al. Does antiretroviral treatment reduce case fatality among HIV-positive patients with tuberculosis in Malawi? Int J Tuberc Lung Dis. 2007;11:848–53. [PubMed] [Google Scholar]
- (62).Lawn SD, Wood R. When should antiretroviral treatment be started in patients with HIV-associated tuberculosis in South Africa? S Afr Med J. 2007;97:412, 414–2, 415. [PubMed] [Google Scholar]
- (63).Yu JK, Bong CN, Chen SC, et al. Outcomes in HIV-infected patients who develop tuberculosis after starting antiretroviral treatment in Malawi. Int J Tuberc Lung Dis. 2008;12:692–4. [PubMed] [Google Scholar]
- (64).Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- (65).Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- (67).Westreich D, MacPhail P, Van Rie A, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23:707–715. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Blanc FX, Havlir DV, Onyebujoh PC, Thim S, Goldfeld AE, Delfraissy JF. Treatment strategies for HIV-infected patients with tuberculosis: ongoing and planned clinical trials. J Infect Dis. 2007;196(Suppl 1):S46–S51. doi: 10.1086/518658. [DOI] [PubMed] [Google Scholar]
- (69).Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS. 2005;19:1113–24. doi: 10.1097/01.aids.0000176211.08581.5a. [DOI] [PubMed] [Google Scholar]
- (70).Muga R, Ferreros I, Langohr K, et al. Changes in the incidence of tuberculosis in a cohort of HIV-seroconverters before and after the introduction of HAART. AIDS. 2007;21:2521–7. doi: 10.1097/QAD.0b013e3282f1c933. [DOI] [PubMed] [Google Scholar]
- (71).Moreno S, Jarrin I, Iribarren JA, et al. Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status. Int J Tuberc Lung Dis. 2008;12:1393–400. [PubMed] [Google Scholar]
- (72).Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–8. doi: 10.1097/QAD.0b013e328216f441. [** These retrospective observational data from Brazil are the first to indicate that isoniazid and antiretroviral therapy may have an additive effect in preventing TB.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995-2001. PLoS ONE. 2007;2:e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti- retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [* Few data exist on the impact of ART on TB prevention in children. In this South African paediatric cohort, the rate decreased from 53.3 to 6.4 cases per 100 person-years during ART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Brinkhof MW, Egger M, Boulle A, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45:1518–21. doi: 10.1086/522986. [* This study compared the impact of ART on TB rates in cohorts in high- and low- income settings and found a similar proportionate risk reduction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–7. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- (77).Pacheco AG, Durovni B, Cavalcante SC, et al. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS ONE. 2008;3:e3132. doi: 10.1371/journal.pone.0003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Grant AD, Charalambous S, Fielding KL, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA. 2005;293:2719–25. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- (79).Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- (80).Atun RA, Lebcir RM, Drobniewski F, et al. High coverage with HAART is required to substantially reduce the number of deaths from tuberculosis: system dynamics simulation. Int J STD AIDS. 2007;18:267–73. doi: 10.1258/095646207780659024. [DOI] [PubMed] [Google Scholar]
- (81).Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- (82).Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–10. doi: 10.1097/QAD.0b013e3282f4a607. [* TB accounted for 41% of immune reconstitution disease events in this prospective South African cohort.] [DOI] [PubMed] [Google Scholar]
- (83).Lawn SD, Lipman MC, Easterbrook PJ. Immune reconstitution disease associated with mycobacterial infections. Curr Opin HIV AIDS. 2008;3:425–31. doi: 10.1097/COH.0b013e3282fe99dc. [* A recent review af TB immune reconstitution disease.] [DOI] [PubMed] [Google Scholar]
- (84).Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [* These consensus case definitions will permit standardisation of data collected in studies of TB immune reconstitution disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [** This was the first study to report on rates, risk factors and clinical presentations of TB immune reconstitution disease in an African cohort. The study highlighted the major importance of the timing of ART initiation; those initiating ART in the first month of TB treatment having a 70-fold higher risk than those initiating ART after at least 3 months of TB treatment.] [DOI] [PubMed] [Google Scholar]
- (86).Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11:1282–9. [PubMed] [Google Scholar]
- (87).Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune Reconstitution and “Unmasking” of Tuberculosis during Antiretroviral Therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [* This paper proposes a conceptual framework to understand the temporal clustering of TB cases in the initial months of ART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Manabe YC, Breen R, Perti T, et al. Unmasked Tuberculosis and Tuberculosis Immune Reconstitution Inflammatory Disease: A Disease Spectrum after Initiation of Antiretroviral Therapy. J Infect Dis. 2009;199:437–44. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- (89).Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–5. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- (90).Lonnroth K, Raviglione M. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med. 2008;29:481–91. doi: 10.1055/s-0028-1085700. [* A useful overview of approaches to control of the epidemic highlighting the need for scale-up of comprehensive prevention strategies.] [DOI] [PubMed] [Google Scholar]