Abstract
Background
Kidney function, expressed as glomerular filtration rate (GFR), is commonly estimated from serum creatinine (Scr) and, when decreased, may serve as a nonclassical risk factor for incident cardiovascular disease (CVD). The ability of estimated GFR (eGFR) to predict CVD events during 5–10 years of follow-up is assessed using data from the Strong Heart Study (SHS), a large cohort with a high prevalence of diabetes.
Methods
eGFRs were calculated with the abbreviated Modification of Diet in Renal Disease study (MDRD) and the Cockcroft-Gault (CG) equations. These estimates were compared in participants with normal and abnormal Scr. The association between eGFR and incident CVD was assessed.
Results
More subjects were labeled as having low eGFR (<60 ml/min per 1.73 m2) by the MDRD or CG equation, than by Scr alone. When Scr was in the normal range, both equations labeled similar numbers of participants as having low eGFRs, although concordance between the equations was poor. However, when Scr was elevated, the MDRD equation labeled more subjects as having low eGFR. Persons with low eGFR had increased risk of CVD.
Conclusions
The MDRD and CG equations labeled more participants as having decreased GFR than did Scr alone. Decreased eGFR was predictive of CVD in this American Indian population with a high prevalence of obesity and type 2 diabetes mellitus.
Keywords: Cockcroft-Gault, Concordance, Cox proportional hazard model, Glomerular filtration rate, Kidney disease, MDRD, Serum creatinine
Introduction
Chronic kidney disease (CKD), even when glomerular filtration rate (GFR) is only mildly or moderately decreased, increases risk for all-cause and cardiovascular disease (CVD) mortality (1–5). Although an adverse CVD risk factor profile is associated with declining kidney function (6), CKD is independently associated with higher rates of CVD even after adjusting for CVD risk factors (7, 8), suggesting contributions from nonclassical CVD risk factors associated with decreased GFR. Accurate assessment of kidney function is, therefore, important for assessing cardiovascular risk. Normal GFR in adults aged 20–30 years is approximately 125 ml/min per 1.73 m2 and declines each year by approximately 1 ml/min per 1.73 m2 thereafter (9, 10). GFR is only rarely measured in clinical practice or in observational cohort studies, because of the technical challenges associated with infusion clearance techniques, resistance to the use of exogenous radiolabeled clearance markers and well-described inaccuracies in the collection of 24-hour urine samples. Absent these gold standard measures, GFR is usually estimated either by inspection of serum creatinine (Scr) values or, because of the insensitivity of Scr to early CKD (11), by the use of Scr-based estimating equations for GFR or creatinine clearance. These equations use Scr along with other demographic, clinical and laboratory data. While an emerging literature continues to explore alternative endogenous filtration markers which might improve the estimation of GFR, most strategies depend upon measurement of Scr, because it is easily, cheaply and reproducibly measured and serves as a relatively good filtration marker, due to proximal tubular secretion (12). These considerations led to the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines, which recommend use of prediction equations, such as the Modification of Diet in Renal Disease (MDRD) study equation and the Cockcroft-Gault (CG) equation, to estimate GFR (13). These prediction formulae approximate unmeasured physiologic factors that affect serum creatinine level by using various demographic and clinical variables. Neither of these equations were developed in populations that included American Indians, and they have not been validated in an American Indian population (14).
In this article, we examine the concordance of estimated GFR (eGFR) measures as calculated with both the MDRD and the CG equations in a cohort characterized by high levels of obesity, diabetes and kidney abnormalities. We hypothesize low concordance between the MDRD and CG estimating equations among those with reduced kidney function as defined by Scr. These differences would depend on factors, such as age and sex. We used the MDRD equation to generate the thresholds for the normal and abnormal Scr groups. Therefore, we expect the MDRD eGFR to label subjects in concordance with their Scr levels.
Subjects and methods
Study population
The Strong Heart Study (SHS) was initiated in 1988 to investigate CVD and its risk factors in geographically diverse groups of American Indians. The SHS design, survey methods and laboratory techniques have been published (15, 16). The SHS cohort of 4,549 American Indians includes men and women aged 45 to 74 years who were seen at the first examination (1989–1991). The second and third examinations were conducted in 1993–1995 and 1998–2000, respectively. This population has a high prevalence of diabetes and kidney abnormalities (17, 18).
Clinical examination and laboratory determinations
Baseline and follow-up examinations consisted of a personal interview and a physical examination (15). Weight, height, blood pressure and waist and hip circumferences were measured (15, 16). Fasting blood samples were obtained for measures of lipids, insulin, plasma creatinine, plasma fibrinogen and glycated hemoglobin (A1c), and a 75-g oral glucose tolerance test was performed (19–24). Prevalent diabetes was identified by use of hypoglycemic agents, fasting glucose ≥126 mg/dL (25) or self-report.
Surveillance for cardiovascular events
CVD surveillance for nonfatal and fatal clinical events occurred throughout the follow-up period and is complete through December 31, 2002 (26). Criteria used to define definite fatal myocardial infarction (MI), stroke, coronary heart disease and nonfatal CVD have been published (27), as have methods for ascertaining incident CVD events (15, 28, 29).
Kidney function measures
Serum creatinine measures were performed by a single core laboratory and determined using automated alkaline picrate methodology run on a rapid flow analyzer (15, 28). We defined abnormal GFR as eGFR <60 ml/min per 1.73 m2 (30, 31). The SHS median age of 45 years and eGFR of 60 ml/min per 1.73 m2 were used to retrospectively define the corresponding Scr levels for the normal versus the abnormal groups. This definition estimates sex-specific thresholds of abnormal Scr (1.4 mg/dL in men and 1.1 mg/dL in women). Estimated GFR was calculated using 2 equations. The abbreviated MDRD equation is: , where Scr is measured in mg/dL and age in years (32).
Because SHS includes only American Indians, the MDRD equation factor for ethnicity was dropped for all participants. We note that other researchers have handled American Indian data similarly or have used a constant that was midway between those for whites and blacks (33). The CG equation to estimate creatinine clearance (CCr) is as follows:
where age is measured in years, weight in kilograms and Scr in mg/dL (32). This result was then adjusted for body surface area (BSA) (34):
BSA was calculated as , where weight is measured in kilograms and height in centimeters.
Using the definitions and stages of kidney disease as defined by the US National Kidney Foundation, 4 groups were defined based on GFR as estimated by the MDRD and CG formulae: group 1 consisted of persons with normal eGFR as estimated by both the MDRD and the CG equations; group 2 consisted of persons defined as having normal eGFR by MDRD but abnormal eGFR by CG; group 3 consisted of persons having abnormal eGFR by MDRD and normal eGFR by CG; and group 4 consisted of persons defined as having abnormal eGFR as estimated by both MDRD and CG. Commentary is provided on results of supplementary analyses using the CG equation without normalization for body surface area.
Statistical analysis
Descriptive statistics of the subgroups are summarized with respect to age, sex, diabetes and CVD status. Mean Scr with corresponding 95% confidence intervals are presented. Mean eGFR was calculated using the 2 estimating equations, stratified by age categories and compared at baseline. Normal and abnormal kidney function groups were contrasted to study concordance between Scr and both of the GFR estimates and to study the characteristics of cases with abnormal kidney function that were not identified by Scr. The Cox proportional hazard model was used to determine the association between eGFR categories and CVD risk. A paired t-test was used to study mean differences among continuous GFR measures.
Results
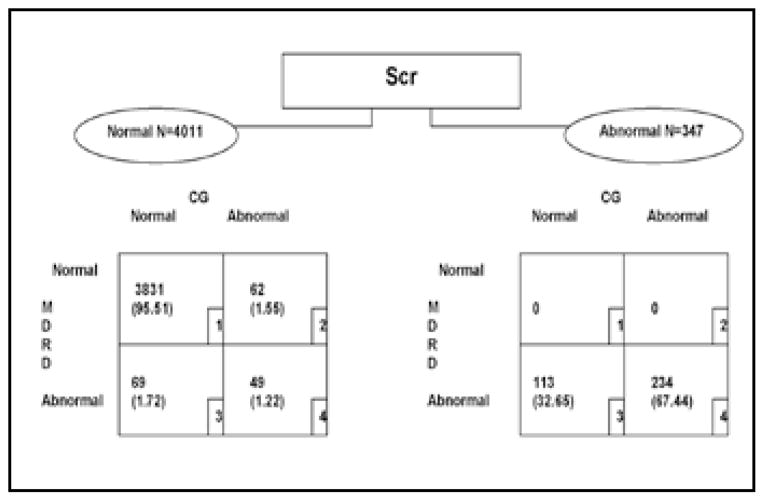
Figure 1 shows the distribution of participants with complete data to allow calculation of GFR with the CG and MDRD equations. Figure 1 shows that 95.5% of the SHS subjects who had normal Scr levels also had normal eGFR using either estimating equation. Discordance between the 2 eGFR measures was present in groups 2 and 3, with 62 and 69 subjects, respectively. In addition to the 131 subjects who showed at least 1 abnormal eGFR measure, 49 subjects were identified as having abnormal kidney function by both eGFR measures but not by Scr.
Fig. 1.
Schematic of estimated glomerular filtration rate distribution stratified by normal versus abnormal serum creatinine (Scr) levels. CG = Cockcroft-Gault equation; MDRD = Modification of Diet in Renal Disease study equation.
Table I shows selected baseline characteristics of the 4,011 participants with normal baseline Scr. As expected, mean Scr was lowest in group 1 and highest in group 4, with intermediate values in groups 2 and 3. Mean age followed a similar pattern. Those in group 3 were more likely to be female than those in group 2 (88.4% vs. 43.6%).
TABLE I.
BASELINE CHARACTERISTICS OF STRONG HEART STUDY PARTICIPANTS WITH COMPLETE KIDNEY DATA, WITH NORMAL Scr SCORES, 1988–2002 (n=4,011)
| All | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| Number (%) | 4,011 | 3,831 (95.51) | 62 (1.55) | 69 (1.72) | 49 (1.22) |
| Scr, mean ± SD | 0.86 ± 0.15 | 0.85 ± 0.15 | 1.04 ± 0.14 | 1.03 ± 0.10 | 1.11 ± 0.15 |
| Age, mean ± SD | 56.05 ± 7.99 | 55.40 ± 7.66 | 68.95 ± 4.95 | 66.16 ± 3.85 | 68.94 ± 3.93 |
| Females (%) | 2,425 (57.77) | 2,187 (57.09) | 27 (43.55) | 61 (88.41) | 32 (65.31) |
| Diabetes (%) | 1,901 (46.19) | 1,734 (45.37) | 19 (30.65) | 30 (44.12) | 19 (38.78) |
| CVD (%) | 155 (3.69) | 129 (3.37) | 6 (9.68) | 4 (5.80) | 4 (8.16) |
Group 1: persons with normal eGFR as calculated by both MDRD and CG.
Group 2: persons with normal eGFR as calculated by MDRD but abnormal as calculated by CG.
Group 3: persons with abnormal eGFR as calculated by MDRD and normal as calculated by CG.
Group 4: persons with abnormal eGFR as calculated by both MDRD or CG.
CG = Cockcroft-Gault equation; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; MDRD = Modification of Diet in Renal Disease study equation; Scr = serum creatinine.
Of 347 subjects (Fig. 1) with abnormal baseline Scr, eGFR calculated with the CG formula indicated that 113 had normal kidney function, while the eGFR calculated with the MDRD formula was, by design, consistent with the Scr results. Furthermore, analysis conducted using the CG equation without normalizing it to body surface area overestimated renal insufficiency, with 710 subjects labeled as having decreased estimated clearance (data not shown).
Tables II and III show diabetes-adjusted and multivariable-adjusted (with covariates for diabetes, age and sex) hazard ratios for the relationship between baseline eGFR and incident CVD over 5 and 10 years, respectively. Compared with participants whose eGFR was >90 ml/min per 1.73 m2 at baseline, increased CVD risk was observed with reduced kidney function, even after adjustment for the effect of age, sex and diabetes. This relationship was observed for 5 and 10 years of follow-up with the same incremental increase in CVD risk with deteriorating kidney function.
TABLE II.
COX PROPORTIONAL HAZARD MODELS SHOWING INCIDENCE OF CVD EVENTS IN SHS 5-YEAR FOLLOW-UP, BY CATEGORY OF BASELINE KIDNEY FUNCTION
| Reference: eGFR ≥90, number (event %) | eGFR 60–89, number (event %) HR (95% CI) |
eGFR 30–59, number (event %) HR (95% CI) |
eGFR <30, number (event %) HR (95% CI) |
|
|---|---|---|---|---|
| Diabetes-adjusted analysis | ||||
| MDRD | 99 (7.94) | 184 (8.88) | 39 (13.13) | 6 (14.63) |
| 1.0 | 1.21 (0.95–1.54) | 2.08 (1.44–3.10)* | 4.10 (1.80–9.34)* | |
| CG | 161 (8.11) | 136 (9.56) | 25 (12.32) | 5 (15.63) |
| 1.0 | 1.35 (1.07–1.70)* | 2.39 (1.57–3.64)* | 4.39 (1.80–10.68)* | |
| Multivariate adjusted analysis† | ||||
| MDRD | 99 (7.94) | 184 (8.88) | 39 (13.13) | 6 (14.63) |
| 1.0 | 1.20 (0.93–1.54) | 1.94 (1.30–2.89)* | 3.73 (1.63–8.53)* | |
| CG | 161 (8.11) | 136 (9.56) | 25 (12.32) | 5 (15.63) |
| 1.0 | 1.08 (0.84–1.40) | 1.69 (1.06–2.71)* | 3.67 (1.50–8.98)* | |
CG = Cockcroft-Gault equation; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HR = hazard ratio; MDRD = Modification of Diet in Renal Disease study equation; 95% CI = 95% confidence interval; SHS = Strong Heart Study.
Significant at the 5% level of significance.
Adjusted for diabetes, age and sex.
TABLE III.
COX PROPORTIONAL HAZARD MODELS SHOWING INCIDENCE OF CVD EVENTS IN SHS 10-YEAR FOLLOW UP, BY CATEGORY OF BASELINE KIDNEY FUNCTION
| eGFR ≥90, number (event %) | eGFR 60–89, number (event %) HR (95% CI) |
eGFR 30–59, number (event %) HR (95% CI) |
eGFR <30, number (event %) HR (95% CI) |
|
|---|---|---|---|---|
| Diabetes-adjusted analysis | ||||
| MDRD | 268 (18.93) | 464 (19.74) | 122 (32.11) | 24 (40.86) |
| 1.0 | 1.12 (0.97–1.30) | 2.08 (1.68–2.56)* | 4.10 (2.70–6.22)* | |
| CG | 398 (17.91) | 366 (22.14) | 94 (34.56) | 16 (37.21) |
| 1.0 | 1.45 (1.26–1.67)* | 2.89 (2.31–3.62)* | 4.26 (2.58–7.02)* | |
| Multivariate adjusted analysis† | ||||
| MDRD | 268 (18.93) | 464 (19.74) | 122 (32.11) | 24 (40.86) |
| 1.0 | 1.08 (0.93–1.26) | 1.84 (1.47–2.32)* | 3.87 (2.54–5.88)* | |
| CG | 398 (17.91) | 366 (22.14) | 94 (34.56) | 16 (37.21) |
| 1.0 | 1.12 (0.96–1.31) | 2.01 (1.56–2.59)* | 3.77 (2.29–6.23)* | |
CG = Cockcroft-Gault equation; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HR = hazard ratio; MDRD = Modification of Diet in Renal Disease study equation; 95% CI = 95% confidence interval; SHS = Strong Heart Study.
Significant at the 5% level of significance.
Adjusted for diabetes, age and sex.
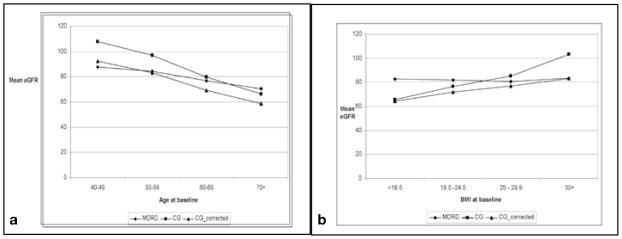
Figure 2a, b shows mean eGFR by age and body mass index (BMI), respectively, as calculated by the equations (i) MDRD, (ii) CG and (iii) CG corrected for obesity (35). We adopted the Saracino correction (corrected CG = CG [1.25 - 0.012 BMI]) in part to account for some of the observed variability in the performance of the CG equation when estimating GFR, especially among obese subjects. Use of the Saracino correction decreased the divergence between MDRD and CG GFR estimates in subjects with BMI ≥30 and led to similar estimates for the slope of age-related GFR decline in our cohort.
Fig. 2.
a) Mean estimated glomerular filtration rate (eGFR) at baseline calculated by the MDRD, CG and corrected CG, versus age category. b) Mean eGFR at baseline calculated by the MDRD, CG, and corrected CG, versus body mass index (BMI) category. CG = Cockcroft-Gault equation; MDRD = Modification of Diet in Renal Disease study equation.
Discussion
We compared 2 estimates of GFR in a population with a high prevalence of obesity and diabetes and at high risk for CKD and CVD. Among the 4,011 SHS participants with normal Scr, 4.5% had abnormal eGFR at baseline as assessed with either the MDRD or CG equations. Among persons with a normal Scr, the MDRD and CG equations identified about the same number of people with abnormal kidney function (n=111 for CG, vs. n=118 for MDRD), although only 49 individuals were common to both groups, suggesting poor concordance between these estimating equations.
Among the 347 individuals with an initially elevated Scr, nearly one third had normal kidney function (eGFR ≥60 ml/min per 1.73 m2) as estimated with the CG equation, but none had normal function as estimated with the MDRD equation. It is worth emphasizing that this inequality did not persist at later study visits. At the third SHS exam, 334 persons had abnormal Scr. Among those, 334 persons had abnormal eGFR as indicated by the MDRD equation, and all but 56 persons had abnormal eGFR as indicated by the CG equation (data not shown). Further, the age-stratified data showed that eGFR calculated with the MDRD and CG equations differed most in the youngest (aged 40–59 years) subjects and in those at extremes of BMI (<18.5 and >30). The improved agreement in eGFR between MDRD and the Saracino-corrected CG suggests that prevalent obesity in younger subjects may have significantly contributed to differences in eGFR.
Our major focus was on the ability of eGFR as calculated by MDRD and CG to predict new cases of CVD. Our data suggest that, compared with persons whose eGFR was ≥90 at baseline, those in the <30, 30–59 and 60–89 categories, using either estimating equation, had increased risk of incident CVD. The risk increased as kidney function decreased. These findings were consistent in both adjusted and unadjusted analyses. When we conducted the same analyses using a dichotomy of eGFR ≥60 as the reference, where the 60–89 eGFR group was pooled with those with eGFR >90 (Tabs. II and III use >90 as the reference), we observed no association between eGFR <60 and incident CVD for either the MDRD or CG estimates. We suspect that this latter result is due both to misclassification of subjects with eGFR around 60 ml/min per 1.73 m2 and to our high-risk study population with its multiplicity of CVD risk factors as compared with most other white or multiethnic populations. Indeed, while Go et al (36) identified increased CVD as a function of eGFR with hazard ratios similar to ours, but with a reference of ≥60 ml/min per 1.73 m2 in the Kaiser Permanente Northern California registry, the effect of eGFR on CVD was only apparent in the National Health and Nutrition Examination Survey (37), the Atherosclerosis Risk in Communities study (38) and the Cardiovascular Health Study (39) using a reference eGFR of ≥ 90 ml/min per 1.73 m2.
Few epidemiologic studies include direct measures of GFR, because techniques such as kidney clearance of 125I-iothalamate are expensive and difficult to perform (40), thus, this and most other large studies of kidney disease depend on Scr and eGFR. The objective of this report was not to evaluate the performance of these measures with respect to a gold standard but to assess the concordance of these creatinine-based GFR estimating equations and their relative utility in identifying progressively decreased eGFR as a CVD risk factor. In this respect, when applied to our American Indian population, the MDRD and CG equations identified similar numbers of subjects as having CKD, although with poor agreement at an individual level. However, both measures identified progressive CKD as an independent predictor of incident CVD in this population with a high prevalence of obesity and diabetes. Whether better estimating equations, based on Scr, cystatin C or some other marker could be derived from specific validation studies in diverse groups of American Indians remains a subject for future investigation but should not impede further efforts to elucidate contributions of progressive CKD to cardiovascular morbidity and mortality in American Indians (33, 41, 42).
Acknowledgments
We thank Rachel Schaperow, MedStar Research Institute, Hyattsville, Maryland, USA, for editing the manuscript.
Financial support: This work was supported by a Kirschstein National Research Service Award (F32 DK075204), by U01HL041642 (from the National Institute of Diabetes and Digestive and Kidney Disease) and by a grant from the Robert Wood Johnson Foundation.
Footnotes
The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the US Indian Health Service.
Conflicts of interest statement: B.V.H. has served on the advisory boards of Merck, Shering Plough and the Egg Nutrition Council, and has received research support from Merck and Pfizer. The other authors have nothing to declare.
References
- 1.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 2.Gibson CM, Pinto DS, Murphy SA, et al. TIMI Study Group. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535–1543. doi: 10.1016/j.jacc.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Januzzi JL, Jr, Snapinn SM, DiBattiste PM, Jang IK, Theroux P. Benefits and safety of tirofiban among acute coronary syndrome patients with mild to moderate renal insufficiency: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (Prism-Plus) Trial. Circulation. 2002;105:2361–2366. doi: 10.1161/01.cir.0000016359.94919.16. [DOI] [PubMed] [Google Scholar]
- 4.Newby LK, Bhapkar MV, White HD, et al. SYMPHONY and 2nd SYMPHONY Investigators. Predictors of 90-day outcome in patients stabilized after acute coronary syndromes. Heart J. 2003;24:172–181. doi: 10.1016/s0195-668x(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003;41:718–724. doi: 10.1016/s0735-1097(02)02956-x. [DOI] [PubMed] [Google Scholar]
- 6.Anavekar NS, Pfeffer MA. Cardiovascular risk in chronic kidney disease. Kidney Int. 2004;66 (Suppl 92):S11–S15. doi: 10.1111/j.1523-1755.2004.09203.x. [DOI] [PubMed] [Google Scholar]
- 7.Al Suwaidi J, Reddan DN, Williams K, et al. GUSTO-IIb, GUSTO-III, PURSUIT. Global Use of Strategies to Open Occluded Coronary Arteries. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy; PARAGON-A Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 8.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol. 2003;92:509–514. doi: 10.1016/s0002-9149(03)00716-1. [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The National High Blood Pressure Education Program Coordinating Committee. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 11.Duncan L, Heathcote J, Djurdjev O, Levin A. Screening for renal disease using serum creatinine: who are we missing? Nephrol Dial Transplant. 2001;16:1042–1046. doi: 10.1093/ndt/16.5.1042. [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147:19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 14.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 16.Howard BV, Welty TK, Fabsitz RR, et al. Risk factors for coronary heart disease in diabetic and nondiabetic American Indians. The Strong Heart Study. Diabetes. 1992;41(Suppl 2):4–11. doi: 10.2337/diab.41.2.s4. [DOI] [PubMed] [Google Scholar]
- 17.Lee ET, Welty TK, Cowan LD, et al. Incidence of diabetes in American Indians of three geographic areas: The Strong Heart Study. Diabetes Care. 2002;25:49–54. doi: 10.2337/diacare.25.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Robbins DC, Knowler WC, Lee ET, et al. Regional differences in albuminuria among American Indians: an epidemic of renal disease. Kidney Int. 1996;492:557–563. doi: 10.1038/ki.1996.79. [DOI] [PubMed] [Google Scholar]
- 19.Lipid Research Clinics Program. Manual of laboratory operations. Vol. 1. Washington, DC: Department of Health, Education, and Welfare; 1994. DHEW publication no. 75–628. [Google Scholar]
- 20.Morgan C, Lazarow A. Immunoassay of insulin: two antibody system: plasma insulin levels in normal, subdiabetic and diabetic rats. Diabetes. 1963;12:115–126. [Google Scholar]
- 21.Von Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 22.Little RR, England JD, Wiedmeyer HM, et al. Interlaboratory standardization of glycated hemoglobin determinations. Clin Chem. 1986;32:358–360. [PubMed] [Google Scholar]
- 23.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin dependent) diabetes following diet-induced reduction of hyperglycemia. Diabetologia. 1984;26:127–133. doi: 10.1007/BF00281119. [DOI] [PubMed] [Google Scholar]
- 24.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 25.Resnick HE, Jones K, Ruotolo G, et al. Strong Heart Study. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care. 2003;26:861–867. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 26.Lee ET, Cowan LD, Welty TK, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations aged 45–74 years, 1984–88. The Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 27.Lee ET, Devereux RB, Yeh JL, Waung W, Go O. Selected mortality rates in the Strong Heart Study population. J Invest Med. 1995;43:510A. [Google Scholar]
- 28.Howard BV, Lee ET, Cowan LD, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1995;142:254–268. doi: 10.1093/oxfordjournals.aje.a117632. [DOI] [PubMed] [Google Scholar]
- 29.Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians: the Strong Heart Study. Circulation. 1999;99:2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- 32.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Part 5: evaluation of laboratory measurements for clinical assessment of kidney disease. Guideline 4: estimation of GFR. Am J Kidney Dis. 2002;39 (Suppl 1):S76–S110. [Google Scholar]
- 33.Scavini M, Stidley CA, Paine SS, et al. The burden of chronic kidney disease among the Zuni Indians: the Zuni Kidney Project. Clin J Am Soc Nephrol. 2007;2:509–516. doi: 10.2215/CJN.02780806. [DOI] [PubMed] [Google Scholar]
- 34.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 35.Saracino A, Morrone LF, Suriano V, et al. A simple method for correcting overestimated glomerular filtration rate in obese subjects evaluated by the Cockcroft and Gault formula: a comparison with 51Cr EDTA clearance. Clin Nephrol. 2004;62:97–103. doi: 10.5414/cnp62097. [DOI] [PubMed] [Google Scholar]
- 36.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 37.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 38.Heiss G, Wharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C and the ARIC Investigators. Case-control analysis of atherosclerosis and established risk factors. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 39.Mittalhenkle A, Stehman-Breen CO, Shlipak MG, et al. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol. 2008;3:450–456. doi: 10.2215/CJN.02610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 41.Pavkov ME, Bennett PH, Sievers ML, et al. Predominant effect of kidney disease on mortality in Pima Indians with or without type 2 diabetes. Kidney Int. 2005;68:1267–1274. doi: 10.1111/j.1523-1755.2005.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Knowler WC, Devereux RB, et al. Albuminuria within the “normal” range and risk of cardiovascular disease and death in American Indians: the Strong Heart Study. Am J Kidney Dis. 2007;49:208–216. doi: 10.1053/j.ajkd.2006.10.017. [DOI] [PubMed] [Google Scholar]