Abstract
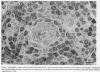
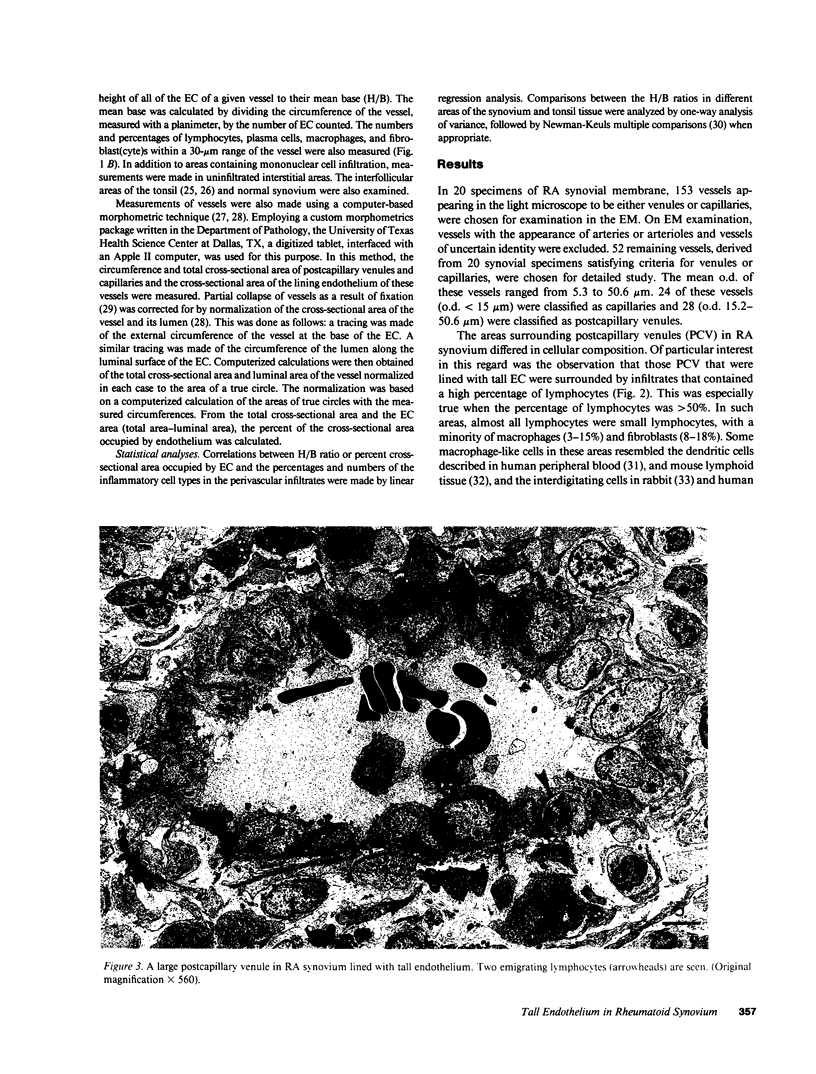
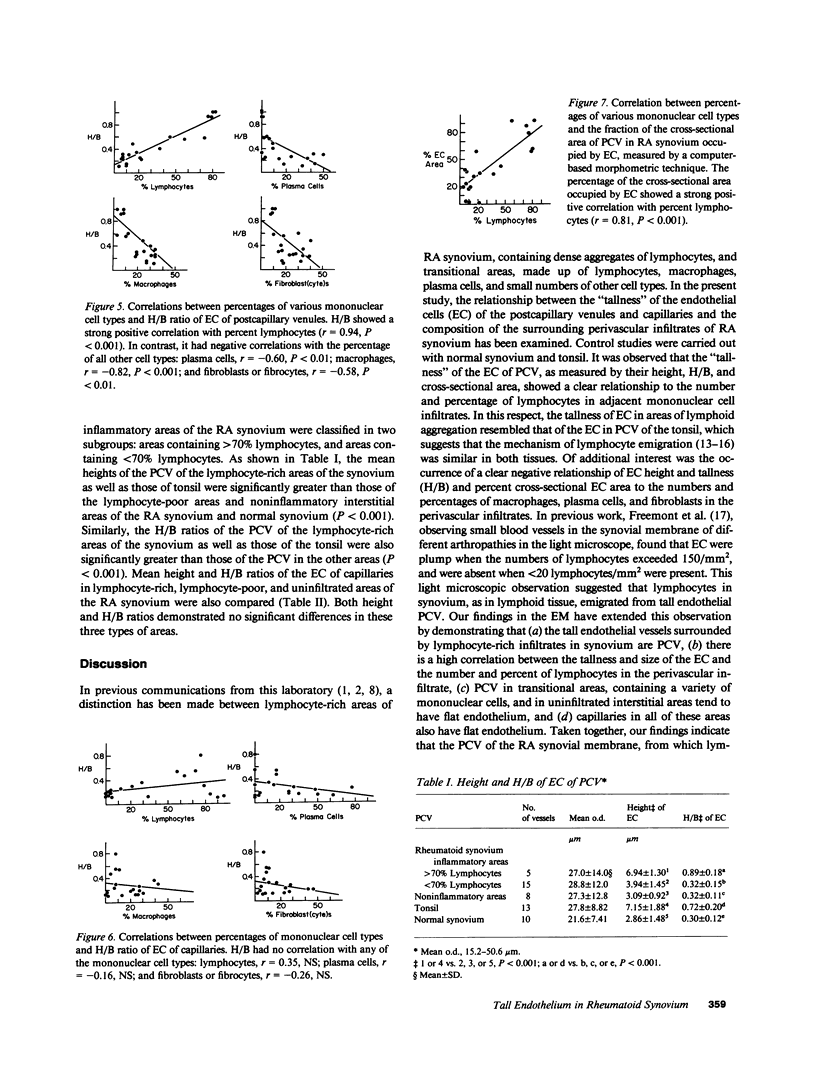
The relationship between (a) "tallness" and (b) cross-sectional area of the endothelial cells (EC) of postcapillary venules (PCV) and capillaries and the cellular composition of adjacent perivascular mononuclear cell infiltrates in rheumatoid (RA) synovial membrane has been examined by electron microscopy. "Tallness" of the EC was measured as the ratio of the height of the EC to its base (H/B). H/B showed a strong positive correlation with the number and percent of perivascular lymphocytes, i.e., the denser the lymphoid aggregation, the taller the EC. In contrast, H/B showed negative correlations with percent perivascular plasma cells, macrophages, and fibroblast(cyte)s. No such correlations were observed with pericapillary infiltrates. A computer-based morphometric technique yielded similar relationships between the cross-sectional area of the EC and the composition of the perivascular infiltrates. These results indicate that the EC of PCV in lymphocyte-rich areas of synovium tend to be tall and to occupy an increased fraction of the cross-sectional area of the vessel. In contrast, in areas rich in macrophages and plasma cells, EC tend to be flat and to occupy a smaller fraction of the cross-sectional area. PCV in uninfiltrated interstitial areas and in normal synovium had flat EC, and capillaries had flat EC regardless of the character of the surrounding infiltrate. Finally, PCV in lymphocyte-rich areas closely resembled those of tonsil in appearance. Our findings indicate that the PCV of the RA synovial membrane from which lymphocytes emigrate to form perivascular lymphoid aggregates resemble those of lymphoid tissue. They suggest that chronic inflammatory tissue and normal lymphoid tissue share mechanisms of lymphocyte emigration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. O., Anderson N. D. Lymphocyte emigration from high endothelial venules in rat lymph nodes. Immunology. 1976 Nov;31(5):731–748. [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Bélisle C., Sainte-Marie G. The narrowing of high endothelial venules of the rat lymph node. Anat Rec. 1985 Feb;211(2):184–191. doi: 10.1002/ar.1092110210. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Espinoza L. R., Vasey F. B., Espinoza C. G., Bocanegra T. S., Germain B. F. Vascular changes in psoriatic synovium. A light and electron microscopic study. Arthritis Rheum. 1982 Jun;25(6):677–684. doi: 10.1002/art.1780250611. [DOI] [PubMed] [Google Scholar]
- FERNANDO N. V., MOVAT H. Z. THE FINE STRUCTURE OF THE TERMINAL VASCULAR BED. III. THE CAPILLARIES. Exp Mol Pathol. 1964 Apr;34:87–97. doi: 10.1016/0014-4800(64)90043-7. [DOI] [PubMed] [Google Scholar]
- Freemont A. J., Jones C. J., Bromley M., Andrews P. Changes in vascular endothelium related to lymphocyte collections in diseased synovia. Arthritis Rheum. 1983 Dec;26(12):1427–1433. doi: 10.1002/art.1780261203. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Migration of lymphocytes and thymocytes in the rat. I. The route of migration from blood to spleen and lymph nodes. J Exp Med. 1968 Jan 1;127(1):155–168. doi: 10.1084/jem.127.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Shannon S. L. Peroxidase arthritis. II. Lymphoid cell-endothelial interactions during a developing immunologic inflammatory response. Am J Pathol. 1972 Oct;69(1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Heusermann U., Stutte H. J., Müller-Hermelink H. K. Interdigitating cells in the white pulp of the human spleen. Cell Tissue Res. 1974;153(3):415–417. [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Wigren A., Wigzell H. Relationship between HLA-DR-expressing cells and T lymphocytes of different subsets in rheumatoid synovial tissue. Scand J Immunol. 1981 May;15(5):501–507. doi: 10.1111/j.1365-3083.1982.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHESI V. T., GOWANS J. L. THE MIGRATION OF LYMPHOCYTES THROUGH THE ENDOTHELIUM OF VENULES IN LYMPH NODES: AN ELECTRON MICROSCOPE STUDY. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE TERMINAL VASCULAR BED. IV. THE VENULES AND THEIR PERIVASCULAR CELLS (PERICYTES, ADVENTITIAL CELLS). Exp Mol Pathol. 1964 Apr;34:98–114. doi: 10.1016/0014-4800(64)90044-9. [DOI] [PubMed] [Google Scholar]
- McMillan E. M., Wasik R., Everett M. A. Identification of T-lymphocytes and T-subsets in human tonsil using monoclonal antibodies and the immunoperoxidase technic. Am J Clin Pathol. 1981 Dec;76(6):737–744. doi: 10.1093/ajcp/76.6.737. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. Human endothelial cell cultures: phenotypic modulation by leukocyte interleukins. J Cell Physiol. 1985 Mar;122(3):424–434. doi: 10.1002/jcp.1041220313. [DOI] [PubMed] [Google Scholar]
- Muntz K. H., Hagler H. K., Boulas H. J., Buja L. M. Fluorescence microscopic morphometry of functioning blood vessels and adrenergic nerves in myocardium. Anat Rec. 1984 Jan;208(1):65–68. doi: 10.1002/ar.1092080108. [DOI] [PubMed] [Google Scholar]
- Norton W. L., Lewis D., Ziff M. Light and electron microscopic observations on the synovitis of Reiter's disease. Arthritis Rheum. 1966 Dec;9(6):747–757. doi: 10.1002/art.1780090602. [DOI] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin J. A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968 Dec;25(5):452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Schoefl G. I. The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med. 1972 Sep 1;136(3):568–588. doi: 10.1084/jem.136.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si L., Roscoe G., Whiteside T. L. Selective distribution and quantitation of T-lymphocyte subsets in germinal centers of human tonsils. Definition by use of monoclonal antibodies. Arch Pathol Lab Med. 1983 May;107(5):228–231. [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. I. Quantitative analysis of inflammatory cells in situ. J Immunol. 1984 May;132(5):2393–2401. [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Burmester G. R. Demonstration of Ia antigens on certain dendritic cells and on a novel elongate cell found in human synovial tissue. Scand J Immunol. 1981 Oct;14(4):439–444. doi: 10.1111/j.1365-3083.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]