Abstract
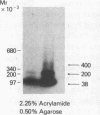
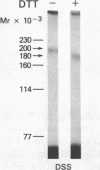
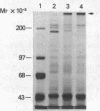
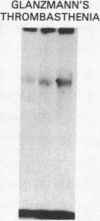
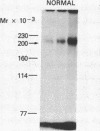
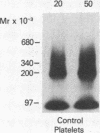
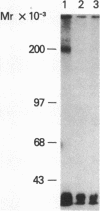
To identify the molecular site of thrombin binding to the platelet membrane, we covalently linked 125I-thrombin to platelets by using the bifunctional chemical cross-linking agents disuccinimidyl suberate and dithiobis(succinimidyl propionate). The proteins cross-linked to 125I-thrombin by this method were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and followed by autoradiography. Two radiolabeled thrombin complexes were identified, a major species of Mr approximately 200,000 and a minor one of Mr approximately 400,000. Hirudin prevented the formation of both complexes. The radioactivity of the approximately 200,000-Mr complex was always 7-10-fold greater than the radioactivity of the approximately 400,000-Mr complex regardless of the thrombin concentration to which the platelets were exposed (0.1-29 nM). Although 125I-thrombin complexes generated with thrombasthenic platelets (lacking glycoprotein IIb/IIIa) were indistinguishable from normal, no complexes appeared when Bernard-Soulier platelets (lacking glycoprotein Ib [GPIb]) were used. Complex formation was blocked by rabbit antiglycocalicin antiserum, but not by the monoclonal antibody 6D1, which is directed against the site on GPIb where von Willebrand factor (vWf) binds in the presence of ristocetin. Although cross-linking studies suggested that vWf might partially inhibit thrombin binding to platelets, this was not confirmed by equilibrium binding studies in the presence of vWf and ristocetin. The data suggest, therefore, that at all thrombin concentrations binding occurs at the same membrane site, despite evidence from equilibrium studies for high and low affinity classes of receptors, and that the approximately 400,000-Mr complex is simply a dimer of the approximately 200,000-Mr species. We conclude that the membrane site to which thrombin binds is the glycocalicin portion of platelet GPIb at a site remote from the point of ristocetin-dependent vWf binding.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemetson K. J., McGregor J. L., James E., Dechavanne M., Lüscher E. F. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982 Aug;70(2):304–311. doi: 10.1172/JCI110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S. Effects of tertiary amine local anesthetics on von Willebrand factor-dependent platelet function: alteration of membrane reactivity and degradation of GPIb by a calcium-dependent protease(s). Blood. 1982 Sep;60(3):731–743. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Corash L., Tan H., Gralnick H. R. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977 Jan;49(1):71–87. [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Degos L., Dautigny A., Brouet J. C., Colombani M., Ardaillou N., Caen J. P., Colombani J. A molecular defect in thrombasthenic platelets. J Clin Invest. 1975 Jul;56(1):236–240. doi: 10.1172/JCI108074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin specificity. Ann N Y Acad Sci. 1981;370:468–495. doi: 10.1111/j.1749-6632.1981.tb29757.x. [DOI] [PubMed] [Google Scholar]
- Ganguly P., Gould N. L. Receptor aggregation: a possible mechanism of platelet stimulation by thrombin. Thromb Res. 1979;15(5-6):879–884. doi: 10.1016/0049-3848(79)90196-8. [DOI] [PubMed] [Google Scholar]
- Ganguly P., Gould N. L. Thrombin receptors of human platelets: thrombin binding and antithrombin properties of glycoprotein I. Br J Haematol. 1979 May;42(1):137–145. doi: 10.1111/j.1365-2141.1979.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Ganguly P., Sonnichsen W. J. Binding of thrombin to human platelets and its possible significance. Br J Haematol. 1976 Oct;34(2):291–301. doi: 10.1111/j.1365-2141.1976.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Glenn K., Bowen-Pope D. F., Ross R. Platelet-derived growth factor. III. Identification of a platelet-derived growth factor receptor by affinity labeling. J Biol Chem. 1982 May 10;257(9):5172–5176. [PubMed] [Google Scholar]
- Grainick H. R., Williams S. B., Coller B. S. Asialo von Willebrand factor interactions with platelets. Interdependence of glycoproteins Ib and IIb/IIIa for binding and aggregation. J Clin Invest. 1985 Jan;75(1):19–25. doi: 10.1172/JCI111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon J. T., Jamieson G. A. Thrombin binds to a high-affinity approximately 900 000-dalton site on human platelets. Biochemistry. 1985 Jan 1;24(1):58–64. doi: 10.1021/bi00322a010. [DOI] [PubMed] [Google Scholar]
- Horne M. K., 3rd The adsorption of thrombin to polypropylene tubes: the effect of polyethylene glycol and bovine serum albumin. Thromb Res. 1985 Jan 1;37(1):201–212. doi: 10.1016/0049-3848(85)90047-7. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest. 1978 Mar;61(3):861–864. doi: 10.1172/JCI109000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao K. J., Pizzo S. V., McKee P. A. Demonstration and characterization of specific binding sites for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1979 Apr;63(4):656–664. doi: 10.1172/JCI109348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen N. E., Simons E. R. Preparation and application of a photoreactive thrombin analogue: binding to human platelets. Biochemistry. 1981 Jul 7;20(14):4141–4147. doi: 10.1021/bi00517a030. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Jaffe E. A., Weksler B. B. Immunoinhibition of ristocetin-induced platelet aggregation. J Clin Invest. 1977 Jan;59(1):143–148. doi: 10.1172/JCI108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T., Hasitz M., Jamieson G. A. Platelet glycocalicin. Interaction with thrombin and role as thrombin receptor of the platelet surface. J Biol Chem. 1978 May 25;253(10):3435–3443. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Filion-Myklebust C., Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980 Apr 10;597(2):235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Fenton J. W., 2nd, Detwiler T. C. Dissociation of thrombin from platelets by hirudin. Evidence for receptor processing. J Biol Chem. 1979 Sep 25;254(18):8723–8725. [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Evidence for a single class of thrombin-binding sites of human platelets. Biochemistry. 1976 May 18;15(10):2144–2149. doi: 10.1021/bi00655a018. [DOI] [PubMed] [Google Scholar]
- White G. C., 2nd, Workman E. F., Jr, Lundblad R. L. Thrombin binding to thrombasthenic platelets. J Lab Clin Med. 1978 Jan;91(1):76–82. [PubMed] [Google Scholar]