Abstract
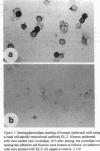
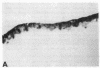
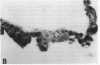
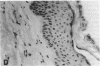
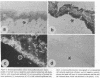
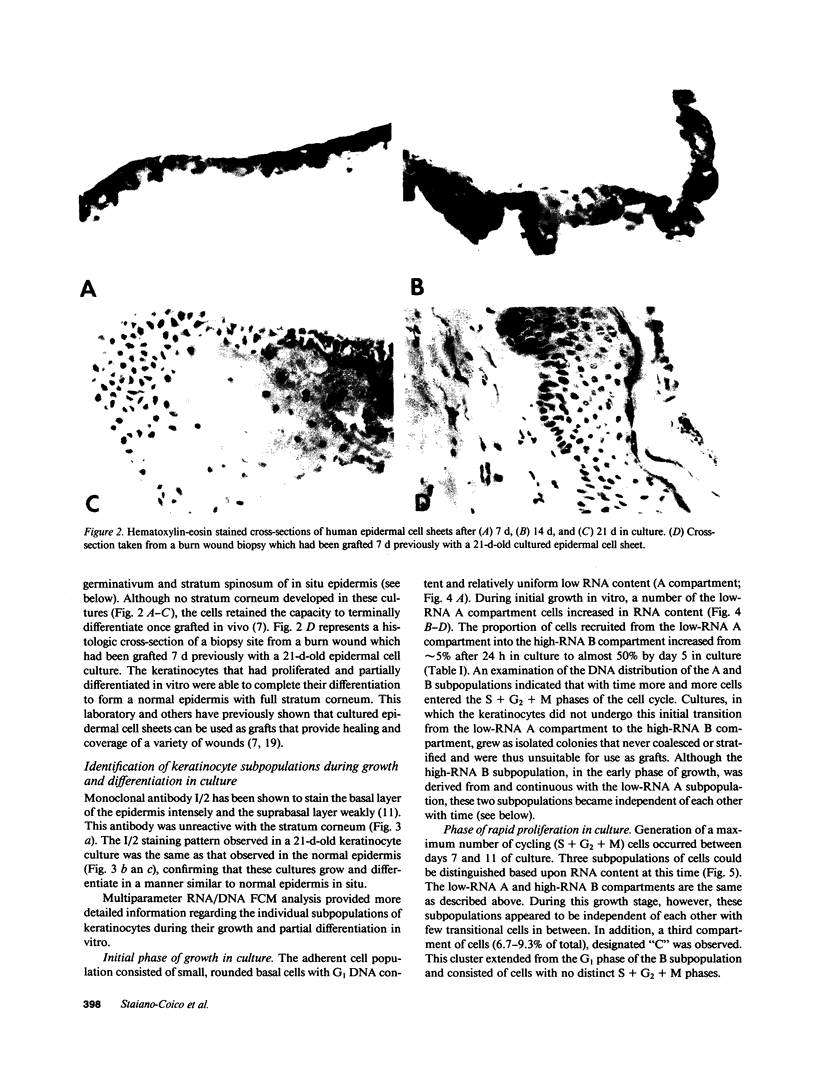
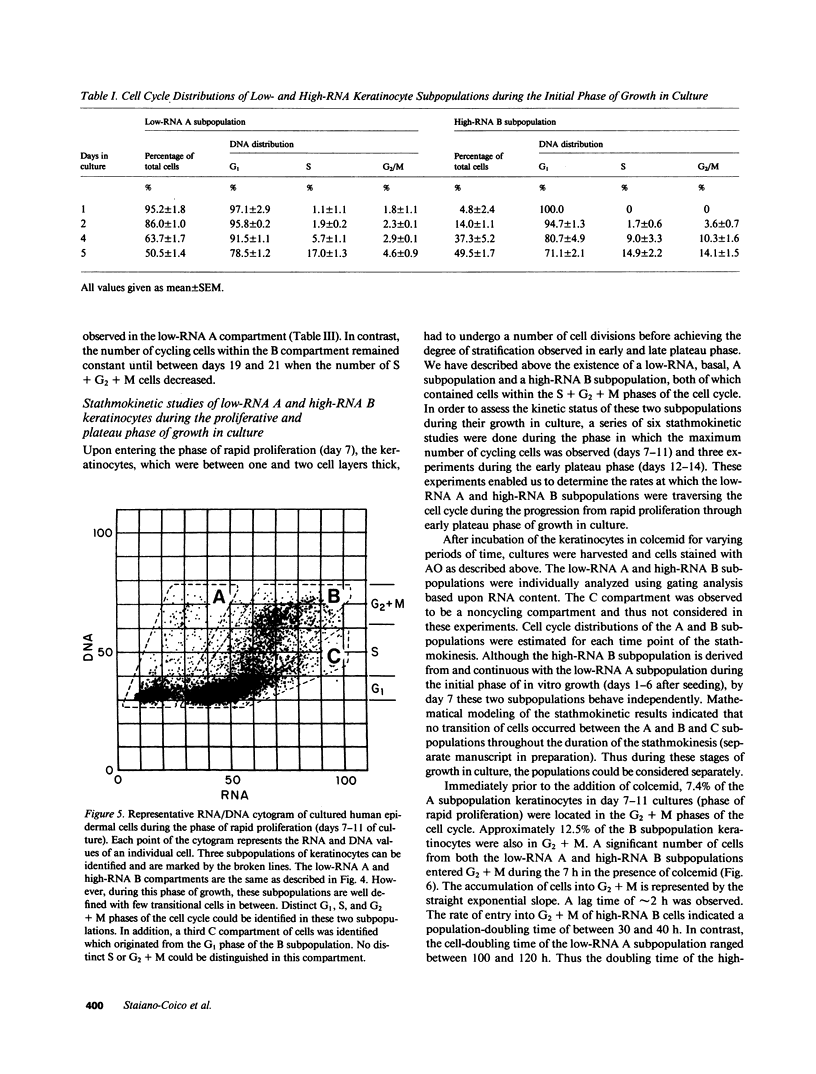
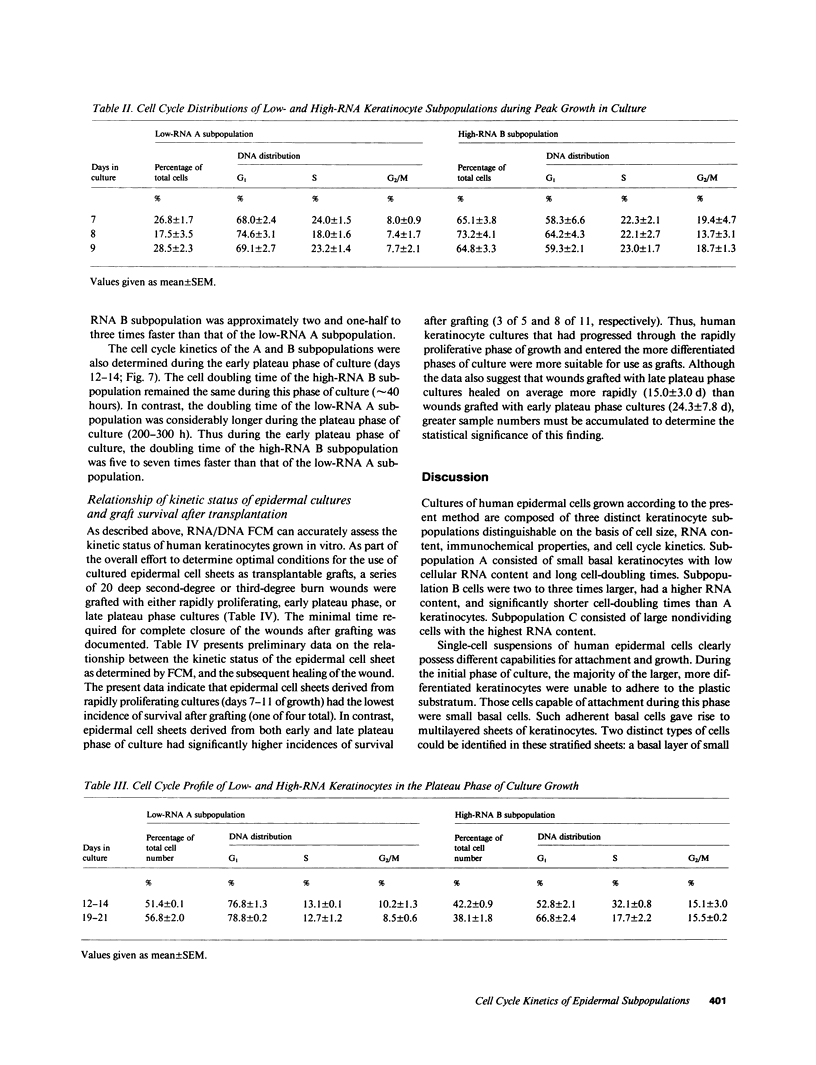
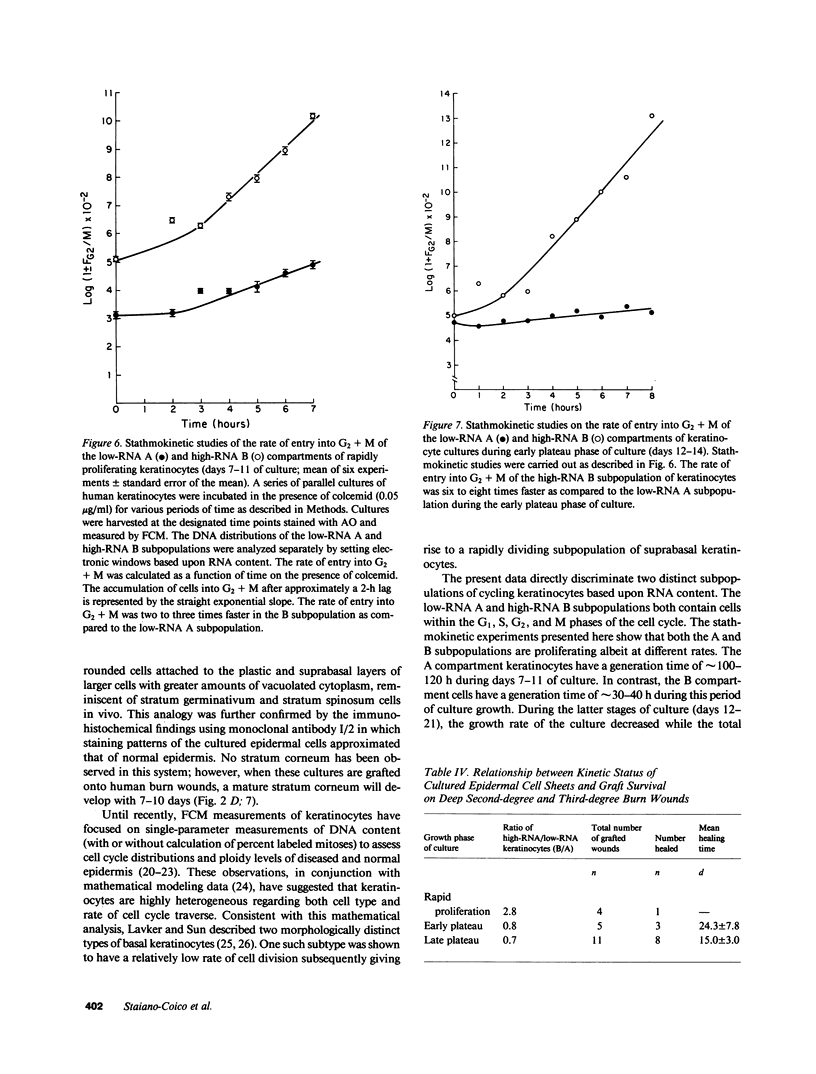
Stratification of human epidermal cells into multilayered sheets composed of basal and suprabasal layers (resembling the stratum germinativum and stratum spinosum of the epidermis) was studied in a dermal component-free culture system. Although no stratum corneum developed in vitro, this culture system provided a method to study early events in human keratinocyte differentiation. Multiparameter flow cytometric analysis of acridine orange-stained epidermal cells from these cultures revealed three distinct subpopulations differing in cell size, RNA content, and cell cycle kinetics. The first subpopulation was composed of small basal keratinocytes with low RNA content and a long generation time. The second subpopulation consisted of larger keratinocytes, having higher RNA content and a significantly shorter generation time. Finally, the third subpopulation contained the largest cells, which did not divide, and represent the more terminally differentiated keratinocytes. This in vitro approach provides discriminating cytochemical parameters by which the maturity of the epidermal cell sheets can be assessed prior to grafting onto human burn patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., REYNOLDS J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br J Plast Surg. 1952 Apr;5(1):25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Drewinko B., Schumann J., Göhde W., Dosik G., Latreille J., Johnston D. A., Freireich E. J. Cellular DNA content as a marker of neoplasia in man. Am J Med. 1980 Aug;69(2):195–203. doi: 10.1016/0002-9343(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Göhde W., Johnston D. A., Smallwood L., Schumann J., Drewinko B., Freireich E. J. Determination of ploidy and proliferative characteristics of human solid tumors by pulse cytophotometry. Cancer Res. 1978 Oct;38(10):3333–3339. [PubMed] [Google Scholar]
- Bauer K. D., Dethlefsen L. A. Total cellular RNA content: correlation between flow cytometry and ultraviolet spectroscopy. J Histochem Cytochem. 1980 Jun;28(6):493–498. doi: 10.1177/28.6.6156196. [DOI] [PubMed] [Google Scholar]
- Clausen O. P., Thorud E., Aarnaes E. Evidence of rapid and slow progression of cells through G2 phase in mouse epidermis: a comparison between phase durations measured by different methods. Cell Tissue Kinet. 1981 May;14(3):227–240. doi: 10.1111/j.1365-2184.1981.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D. P., Staiano-Coico L., Sharpless T. K., Melamed M. L. Correlation between cell cycle duration and RNA content. J Cell Physiol. 1979 Sep;100(3):425–438. doi: 10.1002/jcp.1041000306. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z. Molecular interactions and cellular changes during the cell cycle. Pharmacol Ther. 1983;21(2):143–188. doi: 10.1016/0163-7258(83)90071-2. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Xue S., Staiano-Coico L., Melamed M. R. Rapid analysis of drug effects on the cell cycle. Cytometry. 1981 Jan;1(4):279–286. doi: 10.1002/cyto.990010407. [DOI] [PubMed] [Google Scholar]
- Dover R., Potten C. S. Cell cycle kinetics of cultured human epidermal keratinocytes. J Invest Dermatol. 1983 May;80(5):423–429. doi: 10.1111/1523-1747.ep12555494. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Lee J. S., Hefton J. M., Darzynkiewicz Z., Chiao J. W., de Harven E. Human epidermal cell cultures: growth and differentiation in the absence of differentiation in the absence of dermal components or medium supplements. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5340–5344. doi: 10.1073/pnas.76.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson R. A., Cardon-Cardo C., Higgins P. J. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1984 Nov;8(11):803–820. doi: 10.1097/00000478-198411000-00001. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 Aug 16;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Posnett D. N., Crow M. K., Horikoshi T., Mayer L., Carter D. M. Purification and in vitro growth of human epidermal basal keratinocytes using a monoclonal antibody. J Invest Dermatol. 1985 Oct;85(4):299–303. doi: 10.1111/1523-1747.ep12276866. [DOI] [PubMed] [Google Scholar]
- Hefton J. M., Amberson J. B., Biozes D. G., Weksler M. E. Loss of HLA-DR expression by human epidermal cells after growth in culture. J Invest Dermatol. 1984 Jul;83(1):48–50. doi: 10.1111/1523-1747.ep12261671. [DOI] [PubMed] [Google Scholar]
- Hefton J. M., Madden M. R., Finkelstein J. L., Shires G. T. Grafting of burn patients with allografts of cultured epidermal cells. Lancet. 1983 Aug 20;2(8347):428–430. doi: 10.1016/s0140-6736(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Karasek M. A., Charlton M. E. Growth of postembryonic skin epithelial cells on collagen gels. J Invest Dermatol. 1971 Mar;56(3):205–210. doi: 10.1111/1523-1747.ep12260838. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Epidermal stem cells. J Invest Dermatol. 1983 Jul;81(1 Suppl):121s–127s. doi: 10.1111/1523-1747.ep12540880. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 1982 Mar 5;215(4537):1239–1241. doi: 10.1126/science.7058342. [DOI] [PubMed] [Google Scholar]
- Melbye S. W., Karasek M. A. Some characteristics of a factor stimulating skin epithelial cell growth in vitro. Exp Cell Res. 1973 Jun;79(2):279–286. doi: 10.1016/0014-4827(73)90446-1. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Wichmann H. E., Loeffler M., Dobek K., Major D. Evidence for discrete cell kinetic subpopulations in mouse epidermis based on mathematical analysis. Cell Tissue Kinet. 1982 May;15(3):305–329. doi: 10.1111/j.1365-2184.1982.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Traganos F., Evenson D. P., Staiano-Coico L., Darzynkiewicz Z., Melamed M. R. Action of dihydroxyanthraquinone on cell cycle progression and survival of a variety of cultured mammalian cells. Cancer Res. 1980 Mar;40(3):671–681. [PubMed] [Google Scholar]