Abstract
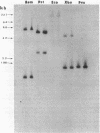
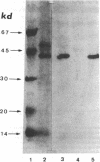
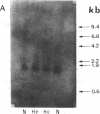
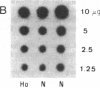
In order to determine the molecular basis of uroporphyrinogen (URO) decarboxylase deficiency responsible for hepatoerythropoietic porphyria (HEP) and familial porphyria cutanea tarda, we used a human URO decarboxylase cDNA to analyze the organization and expression of the URO decarboxylase gene in lymphoblastoid cells from normal individuals and from two patients with HEP. We could detect neither deletions nor rearrangements in the URO decarboxylase gene. Synthesis, processing, and cell-free translation of the specific transcripts appeared to be normal. The half-life of the abnormal protein was 12 times shorter than that of the normal enzyme. The results indicate that the enzyme defect is due to a rapid degradation of the protein in vivo. This study is the first to provide information regarding the molecular mechanism responsible for the URO decarboxylase deficiency in HEP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Hutton J. J. Adenosine deaminase messenger RNAs in lymphoblast cell lines derived from leukemic patients and patients with hereditary adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1649–1660. doi: 10.1172/JCI110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguadé J. P., Castells A., Indacochea A., Rodés J. A case of biochemically unclassifiable hepatic porphyria. Br J Dermatol. 1969 Apr;81(4):270–275. doi: 10.1111/j.1365-2133.1969.tb13979.x. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Davidson B. L., Perignon J. L., Kelley W. N. Genetic expression in partial adenosine deaminase deficiency. mRNA levels and protein turnover for the enzyme variants in human B-lymphoblast cell lines. J Biol Chem. 1985 Mar 25;260(6):3875–3880. [PubMed] [Google Scholar]
- De Verneuil H., Sassa S., Kappas A. Effects of polychlorinated biphenyl compounds, 2,3,7,8-tetrachlorodibenzo-p-dioxin, phenobarbital and iron on hepatic uroporphyrinogen decarboxylase. Implications for the pathogenesis of porphyria. Biochem J. 1983 Jul 15;214(1):145–151. doi: 10.1042/bj2140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Lee G. B., Tovey J. A. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978 Aug 10;299(6):274–278. doi: 10.1056/NEJM197808102990603. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Smith S. G., Herrero C., Lecha M., Mascaro J. M., Muniesa A. M., Czarnecki D. B., Brenan J., Poulos V., DE Salamanca R. E. Hepatoerythropoietic porphyria: a new uroporphyrinogen decarboxylase defect or homozygous porphyria cutanea tarda? Lancet. 1981 Apr 25;1(8226):916–919. doi: 10.1016/s0140-6736(81)91615-9. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Tovey J. A., Sheppard D. M. Purification of uroporphyrinogen decarboxylase from human erythrocytes. Immunochemical evidence for a single protein with decarboxylase activity in human erythrocytes and liver. Biochem J. 1983 Oct 1;215(1):45–55. doi: 10.1042/bj2150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Seki Y., Sano S. Uroporphyrinogen decarboxylase. Purification, properties, and inhibition by polychlorinated biphenyl isomers. J Biol Chem. 1983 Apr 10;258(7):4285–4292. [PubMed] [Google Scholar]
- Kidd V. J., Golbus M. S., Wallace R. B., Itakura K., Woo S. L. Prenatal diagnosis of alpha 1-antitrypsin deficiency by direct analysis of the mutation site in the gene. N Engl J Med. 1984 Mar 8;310(10):639–642. doi: 10.1056/NEJM198403083101007. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazaro P., de Salamanca R. E., Elder G. H., Villaseca M. L., Chinarro S., Jaqueti G. Is hepatoerythropoietic porphyria a homozygous form of porphyria cutanea tarda? Inheritance of uroporphyrinogen decarboxylase deficiency in a Spanish family. Br J Dermatol. 1984 May;110(5):613–617. doi: 10.1111/j.1365-2133.1984.tb04687.x. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Dubart A., Grandchamp B., de Verneuil H., Rosa J., Nordmann Y., Goossens M. Isolation and identification of a cDNA clone coding for rat uroporphyrinogen decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3346–3350. doi: 10.1073/pnas.81.11.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka J. G., Kushner J. P. Purification and characterization of bovine hepatic uroporphyrinogen decarboxylase. Biochemistry. 1983 Sep 27;22(20):4664–4672. doi: 10.1021/bi00289a009. [DOI] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., van Ormondt H., Meera Khan P., van der Eb A. J. Adenosine deaminase (ADA) deficiency in cells derived from humans with severe combined immunodeficiency is due to an aberration of the ADA protein. Nucleic Acids Res. 1984 Jan 25;12(2):1015–1024. doi: 10.1093/nar/12.2.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L. DNA in medicine. Human genetics. Lancet. 1984 Dec 1;2(8414):1257–1262. doi: 10.1016/s0140-6736(84)92806-x. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Mattes P. M., Daddona P. E., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Demonstration of structural variants in lymphoblastoid cells derived from patients with a deficiency of the enzyme. J Clin Invest. 1982 Mar;69(3):706–715. doi: 10.1172/JCI110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Aitken G., Nordmann Y. Familial and sporadic porphyria cutanea: two different diseases. Hum Genet. 1978 Oct 31;44(2):145–151. doi: 10.1007/BF00295407. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Beaumont C., Deybach J. C., Nordmann Y., Sfar Z., Kastally R. Enzymatic and immunological studies of uroporphyrinogen decarboxylase in familial porphyria cutanea tarda and hepatoerythropoietic porphyria. Am J Hum Genet. 1984 May;36(3):613–622. [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Sassa S., Kappas A. Purification and properties of uroporphyrinogen decarboxylase from human erythrocytes. A single enzyme catalyzing the four sequential decarboxylations of uroporphyrinogens I and III. J Biol Chem. 1983 Feb 25;258(4):2454–2460. [PubMed] [Google Scholar]